化工学报 ›› 2025, Vol. 76 ›› Issue (11): 6086-6099.DOI: 10.11949/0438-1157.20250285
石美玉1( ), 赵波琛1, 束远1, 牛强2(
), 赵波琛1, 束远1, 牛强2( ), 张鹏飞1,3(
), 张鹏飞1,3( )
)
收稿日期:2025-03-24
修回日期:2025-05-08
出版日期:2025-11-25
发布日期:2025-12-19
通讯作者:
牛强,张鹏飞
作者简介:石美玉(1996—),女,博士研究生,Smy1464632131@163.com
基金资助:
Meiyu SHI1( ), Bochen ZHAO1, Yuan SHU1, Qiang NIU2(
), Bochen ZHAO1, Yuan SHU1, Qiang NIU2( ), Pengfei ZHANG1,3(
), Pengfei ZHANG1,3( )
)
Received:2025-03-24
Revised:2025-05-08
Online:2025-11-25
Published:2025-12-19
Contact:
Qiang NIU, Pengfei ZHANG
摘要:
CO2作为温室气体之一,对全球气候变化产生了显著影响。开发高效、经济的CO2捕获技术至关重要。利用CaCO3吸附CO2是一种具有潜力的方法,其原料来源广泛、成本低廉且可通过调控形貌和比表面积优化吸附性能。本研究以KCl、NaCl和LiCl为盐模板制备了CaCO3材料,并系统评估了其对CO2的吸附性能。结果表明,CaCO3-KCl的比表面积显著高于CaCO3-NaCl和CaCO3-LiCl,分别为72.6、40.2和18.9 m2/g。且CaCO3-KCl呈现多孔纳米片结构。CO2-TPD显示,CaCO3-KCl表面具有较多的吸附位点。重要的是,CaCO3-KCl、CaCO3-NaCl和CaCO3-LiCl的CO2吸附量分别为0.39、0.32和0.31 mmol/g。CaCO3-KCl在10次穿透循环实验中的CO2吸附量仅降低了7%,并对CO2/N2有着较好的分离效果。本研究揭示了盐模板剂对CaCO3形貌、比表面积及CO2吸附性能的影响,为高效CO2吸附材料的设计提供了重要参考。
中图分类号:
石美玉, 赵波琛, 束远, 牛强, 张鹏飞. 盐模板法制备多孔碳酸钙及其CO2吸附特性研究[J]. 化工学报, 2025, 76(11): 6086-6099.
Meiyu SHI, Bochen ZHAO, Yuan SHU, Qiang NIU, Pengfei ZHANG. Preparation of porous calcium carbonate by salt template method and its CO2 adsorption characteristics[J]. CIESC Journal, 2025, 76(11): 6086-6099.
| 吸附剂 | 比表面积/ (m2/g) | 孔容(BJH)/ (cm3/g) | 孔径/nm |
|---|---|---|---|
| CaCO3-KCl | 72.6 | 0.25 | 14.04 |
| CaCO3-NaCl | 40.2 | 0.19 | 18.50 |
| CaCO3-LiCl | 13.7 | 0.06 | 26.87 |
| CaCO3-KCl(循环10次后) | 55.2 | 0.23 | 13.04 |
表1 CaCO3-XCl的比表面积、孔容及孔径
Table 1 Specific surface area, pore volume and pore size of CaCO3-XCl
| 吸附剂 | 比表面积/ (m2/g) | 孔容(BJH)/ (cm3/g) | 孔径/nm |
|---|---|---|---|
| CaCO3-KCl | 72.6 | 0.25 | 14.04 |
| CaCO3-NaCl | 40.2 | 0.19 | 18.50 |
| CaCO3-LiCl | 13.7 | 0.06 | 26.87 |
| CaCO3-KCl(循环10次后) | 55.2 | 0.23 | 13.04 |
| 吸附剂 | 吸附温度/℃ | 比表面积/(m2/g) | 循环次数(吸附下降量) |
|---|---|---|---|
| CaCO3-es[ | 30 | 97.4 | 7次(6.0%) |
| ESCE-Ca9Y1[ | 700 | 16.69 | 5次(30.3%) |
CaCO3-MgO[ AMS-MgCa5[ | 280 340 | 230 177.2 | 10次(35.0%) 10次(28.8%) |
| CaCO3-KCl(本研究) | 30 | 72.6 | 10次(7%) |
表2 本研究中的吸附剂与同类典型Ca基吸附剂的循环稳定性比较
Table 2 Comparison of cyclic stability of the adsorbents in this study with similar typical Ca-based adsorbents
| 吸附剂 | 吸附温度/℃ | 比表面积/(m2/g) | 循环次数(吸附下降量) |
|---|---|---|---|
| CaCO3-es[ | 30 | 97.4 | 7次(6.0%) |
| ESCE-Ca9Y1[ | 700 | 16.69 | 5次(30.3%) |
CaCO3-MgO[ AMS-MgCa5[ | 280 340 | 230 177.2 | 10次(35.0%) 10次(28.8%) |
| CaCO3-KCl(本研究) | 30 | 72.6 | 10次(7%) |
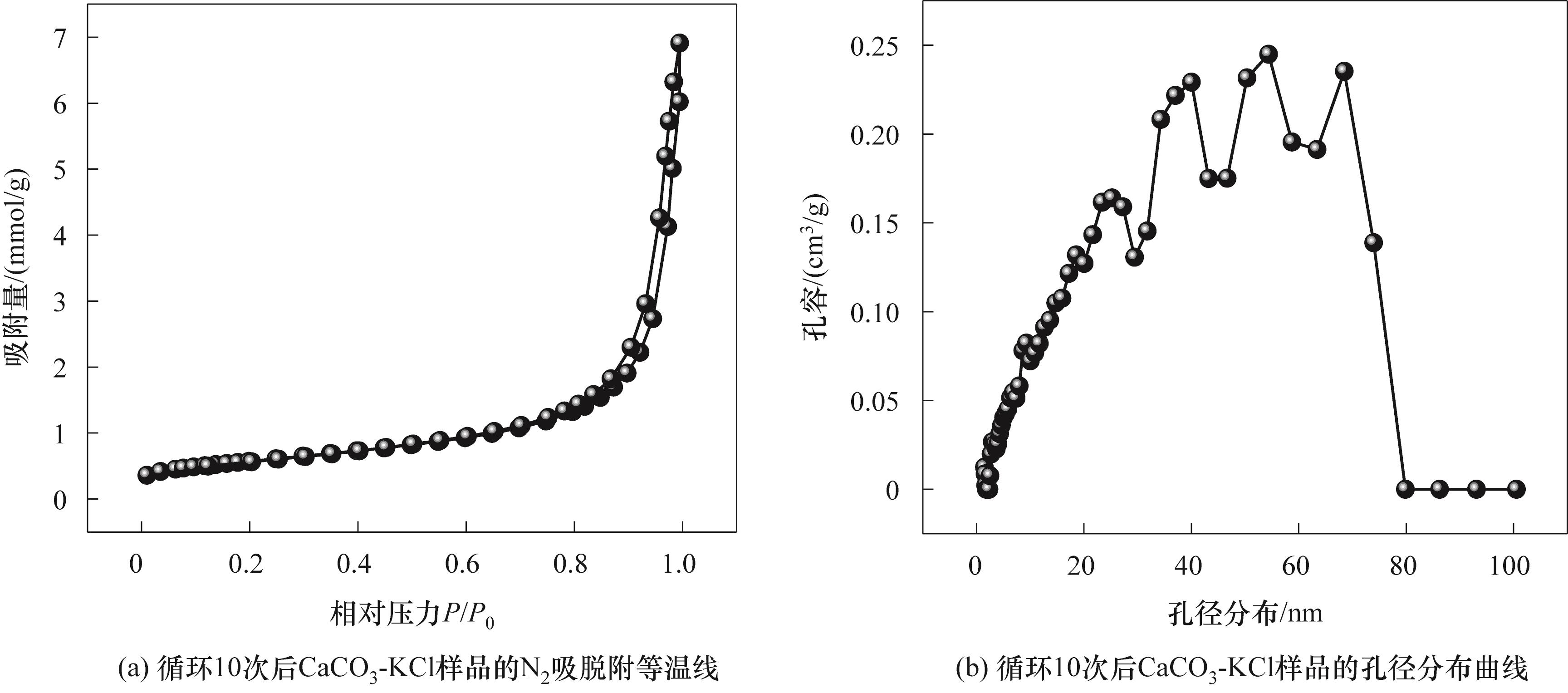
图14 循环10次后CaCO3-XCl的N2物理吸脱附曲线和BJH粒径分布
Fig.14 N2 physical adsorption-desorption curves and BJH particle size distribution for CaCO3-XCl after cycling for 10 times
| [1] | 虎雅荣, 李三秀, 彭娟. 海参状Cu2O/Cu@N-C的制备及电化学还原CO2制甲酸盐[J]. 宁夏大学学报(自然科学版), 2024, 45(4): 379-388. |
| Hu Y R, Li S X, Peng J. Preparation of sea cucumber-like Cu2O/Cu@N-C and electrochemical reduction of CO2 to produce formate[J]. Journal of Ningxia University (Natural Science Edition), 2024, 45(4): 379-388. | |
| [2] | Zhao C B, Wang L M, Huang L, et al. Recent advances in intermediate-temperature CO2 capture: materials, technologies and applications[J]. Journal of Energy Chemistry, 2024, 90: 435-452. |
| [3] | 赵俊德, 周爱国, 陈彦霖, 等. 吸附法CO2直接空气捕集技术能耗现状[J]. 化工学报, 2025, 76(4): 1375-1390. |
| Zhao J D, Zhou A G, Chen Y L, et al. Energy consumption of adsorption CO2 direct air capture[J]. China Industrial Economics, 2025, 76(4): 1375-1390. | |
| [4] | 王炳杰, 解强, 沙雨桐, 等. CO2吸附用竹基活性炭制备研究进展[J]. 新型炭材料, 2025, 40(2): 317-322. |
| Wang B J, Xie Q, Sha Y T, et al. Research progress on the preparation of bamboo-based activated carbon for CO2 adsorption[J]. New Carbon Materials, 2025, 40(2): 1-17. | |
| [5] | Lu W, Li J, Qi G S, et al. Preparation and properties of zeolite-fly ash-slag composite porous materials: CO2 adsorption performance and mechanical property[J]. Environmental Science and Pollution Research, 2023, 30(10): 27303-27314. |
| [6] | 杜盛郁, 牛强, 张鹏飞. 反应性挤出技术制备碳基乙炔氢氯化催化剂[J]. 宁夏大学学报(自然科学版), 2024, 45(3): 273-281. |
| Du S Y, Niu Q, Zhang P F. Synthesis of carbon-based catalysts for acetylene hydrochlorination using reactive extrusion technology[J]. Journal of Ningxia University (Natural Science Edition), 2024, 45(3): 273-281. | |
| [7] | 王恩民, 李文翠, 雷成, 等. 碱式碳酸镁催化酚醛聚合制备多孔炭及其CO2吸附性能[J]. 化工学报, 2015, 66(7): 2565-2572. |
| Wang E M, Li W C, Lei C, et al. Synthesis of porous carbon through polymerization catalyzed by basic magnesium carbonate and their CO2 adsorption performance[J]. CIESC Journal, 2015, 66(7): 2565-2572. | |
| [8] | Boyjoo Y, Merigot, Lamonier J F, et al. Synthesis of CaCO3@C yolk-shell particles for CO2 adsorption[J]. RSC Advances, 2015, 5(32): 24872-24876. |
| [9] | 冯嘉琪. 多壳层碳酸钙空心微球吸附剂的制备及其CO2吸附性能研究[D]. 天津: 天津大学, 2017. |
| Feng J Q. Preparation and CO2 adsorption capacity of multi-shelled hollow CaCO3 microspheres[D]. Tianjin: Tianjin University, 2017. | |
| [10] | 师琦, 吴素芳. 沉淀法SiO2包覆纳米CaCO3吸附剂性能[J]. 化工学报, 2009, 60(2): 507-513. |
| Shi Q, Wu S F. Properties of SiO2 coated nano SiO2/CaCO3 sorbents by precipitation method[J]. Journal of the Chemical Industry and Engineering Society of China, 2009, 60(2): 507-513. | |
| [11] | Feng J Q, Guo H X, Wang S P, et al. Fabrication of multi-shelled hollow Mg-modified CaCO3 microspheres and their improved CO2 adsorption performance[J]. Chemical Engineering Journal, 2017, 321: 401-411. |
| [12] | Li S, Jiang T, Xu Z H, et al. The Mn-promoted double-shelled CaCO3 hollow microspheres as high efficient CO2 adsorbents[J]. Chemical Engineering Journal, 2019, 372: 53-64. |
| [13] | Liu X, Zhang J, Liu K, et al. Regulation of the pore structure of carbon nanosheets based electrocatalyst for efficient polysulfides phase conversions[J]. Journal of Materials Science & Technology, 2024, 171: 37-46. |
| [14] | Shi J S, Xu J G, Cui H M, et al. NaCl template synthesis of N-doped porous carbon from magnesium gluconate for efficient CO2 adsorption[J]. Separation and Purification Technology, 2025, 355: 129756. |
| [15] | Dong H, Fu X L, Wang J, et al. In-situ construction of porous Si@C composites with LiCl template to provide silicon anode expansion buffer[J]. Carbon, 2021, 173: 687-695. |
| [16] | Wu M, Yan D J, Tang X N, et al. Synthesis of potassium-modified graphitic carbon nitride with high photocatalytic activity for hydrogen evolution[J]. ChemSusChem, 2014, 7(9): 2654-2658. |
| [17] | Qiu D, Cao T F, Zhang J, et al. Precise carbon structure control by salt template for high performance sodium-ion storage[J]. Journal of Energy Chemistry, 2019, 31: 101-106. |
| [18] | Cao Y H, Wang X M, Gu Z R, et al. Potassium chloride templated carbon preparation for supercapacitor[J]. Journal of Power Sources, 2018, 384: 360-366. |
| [19] | Durmus F C, Jordá J M M. Silver foams with hierarchical porous structures: from manufacturing to antibacterial activity[J]. ACS Applied Materials & Interfaces, 2021, 13(30): 35865-35877. |
| [20] | Huang M Z, Zeng W Y, Zhu Z W. Facile synthesis of porous Mo2C/C composites by using luffa sponge-derived carbon template in molten salt media[J]. Royal Society Open Science, 2019, 6(6). 190547-190552. |
| [21] | Song P, Li C C, Zhao N M, et al. Molten salt-confined pyrolysis towards heteroatom-doped porous carbon nanosheets for high-energy-density Zn-ion hybrid supercapacitors[J]. Journal of Colloid and Interface Science, 2023, 633: 362-373. |
| [22] | Rehman A, Park S J. Environmental remediation by microporous carbon: an efficient contender for CO2 and methylene blue adsorption[J]. Journal of CO2 Utilization, 2019, 34: 656-667. |
| [23] | Chui S Y, Kao C Y, Huang T T, et al. Microalgal biomass production and on-site bioremediation of carbon dioxide, nitrogen oxide and sulfur dioxide from flue gas using Chlorella sp. cultures[J]. Bioresource Technology, 2011, 102(19): 9135-9142. |
| [24] | Liu C B, Wang Y T, Zhang B. Synthesis of ammonia via an electroreduction removal of NO from exhausted gas: an upgrading to N2 fixation[J]. Science China Chemistry, 2020, 63(9): 1173-1174. |
| [25] | Shu Y, Liu Q, Shi M, et al. Surfactant-free synthesis of crystalline mesoporous metal oxides by a seeds/NaCl-mediated growth strategy[J]. Advanced Science, 2024, 11(1): 2304533-230462. |
| [26] | Sotelo A, Rasekh S, Torres M A, et al. Effect of synthesis methods on the Ca3Co4O9 thermoelectric ceramic performances[J]. Journal of Solid State Chemistry, 2015, 221: 247-254. |
| [27] | Nahar A, Sahadat Hossain M, Akbor M A, et al. Evaluation of anti-microbial activity of biotite and biotite composite based on crystallographic parameters: estimation of crystallite size employing X-ray diffraction data[J]. Results in Engineering, 2024, 23: 102595. |
| [28] | Cao Z, An Y T, Wang X L, et al. Characterization of corrosion behavior of CLF-1 in liquid lithium using calibration-free laser-induced breakdown spectroscopy in depth profile analysis[J]. Materials, 2020, 13(1): 240-252. |
| [29] | Chen Y y, Hu Z P, Xu Y F, et al. Microstructure evolution and interface structure of Al-40 wt% Si composites produced by high-energy ball milling[J]. Journal of Materials Science & Technology, 2019, 35(4): 512-519. |
| [30] | Mähler J, Persson I. A study of the hydration of the alkali metal ions in aqueous solution[J]. Inorganic Chemistry, 2012, 51(1): 425-438. |
| [31] | Chen L, Shi G S, Shen J, et al. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing[J]. Nature, 2017, 550(7676): 380-383. |
| [32] | Horikawa T, Muguruma T, Do D D, et al. Scanning curves of water adsorption on graphitized thermal carbon black and ordered mesoporous carbon[J]. Carbon, 2015, 95: 137-143. |
| [33] | Williams S F, Wan H, Chittock J, et al. Characterization of skin barrier defects using infrared spectroscopy in patients with atopic dermatitis[J]. Clinical and Experimental Dermatology, 2024, 49(5): 466-477. |
| [34] | Shi B N, Zhang L F, Yang Z D, et al. Photothermal conversion-enabled temperature modulation for the growth of complex polymorphic architectures of calcium carbonate[J]. Journal of Materials Chemistry A, 2024, 12(25): 15090-15098. |
| [35] | Sun H J, Meng X X, Luo C F, et al. Quantifying crystallinity in poly(p-phenylene terephthalamide) by Raman spectroscopy[J]. Macromolecules, 2024, 57(15): 7390-7397. |
| [36] | Marshall C P, Stockdale G, Carr C A. Raman spectroscopy of geological varieties of hematite of varying crystallinity and morphology[J]. Journal of Raman Spectroscopy, 2025, 56(7): 590-597. |
| [37] | Wang P P, Shi M Y, Shu Y, et al. Porous CaCO3 fabricated by Ca-containing solid wastes for effective CO2 adsorption[J]. Journal of Environmental Chemical Engineering, 2025, 13(2): 115815. |
| [38] | 司康乐, 匡盛铎, 罗长新, 等. 鸡蛋壳源Ca基吸附剂制备及其CO2吸附性能的研究[J]. 电力科技与环保, 2024, 40(5): 513-523. |
| Si K L, Kuang S D, Luo C X, et al. Preparation of eggshell-derived calcium-based adsorbent and its CO2 adsorption performance[J]. Electric Power Technology and Environmental Protection, 2024, 40(5): 513-523. | |
| [39] | Teixeira P, Correia P, Pinheiro C I C. CO2 capture by CaCO3-MgO and CeO2-MgO sorbents promoted by ternary alkali metal salts in a fixed bed reactor[J]. Chemical Engineering Science, 2024, 289: 119856. |
| [40] | Song J B, Qu T, Zhang J P, et al. Enhancing cyclic stability and anti-sintering capacity of CaCO3-MgO sorbents for CO2 capture[J]. Chemical Engineering Science, 2025, 309: 121433. |
| [41] | Yao S L, Zhang H H, Chen Z Z, et al. Promotion of graphitic carbon oxidation via stimulating CO2 desorption by calcium carbonate[J]. Journal of Hazardous Materials, 2019, 363: 10-15. |
| [42] | Finnie K S, Cassidy D J, Bartlett J R, et al. IR spectroscopy of surface water and hydroxyl species on nanocrystalline TiO2 films[J]. Langmuir, 2001, 17(3): 816-820. |
| [1] | 吴梓航, 徐震原, 游锦方, 潘权稳, 王如竹. 基于吸附式储冷技术的深井钻探设备冷却系统[J]. 化工学报, 2025, 76(S1): 309-317. |
| [2] | 田宇红, 杜壮壮, 徐慧芳, 祝自强, 王宇聪. ZIF-8基多孔液体制备及其SO2吸附性能[J]. 化工学报, 2025, 76(8): 4284-4296. |
| [3] | 张建伟, 刘玉成, 董鑫, 冯颖. 气泡扰动强化撞击流共沉淀法合成碳酸钙粉体[J]. 化工学报, 2025, 76(8): 4052-4060. |
| [4] | 史松伟, 赵诚, 刘帅, 应雨轩, 严密. 富铁飞灰耦合Fe-Zn/Al2O3脱除沼气H2S研究[J]. 化工学报, 2025, 76(8): 4239-4247. |
| [5] | 郭彭涛, 王婷, 薛波, 应允攀, 刘大欢. 用于CH4/N2分离的多吸附位点超微孔MOF[J]. 化工学报, 2025, 76(5): 2304-2312. |
| [6] | 巴雅琪, 吴涛, 邸安頔, 陆安慧. 多孔炭材料用于低碳烃分离的研究进展[J]. 化工学报, 2025, 76(5): 2136-2157. |
| [7] | 谈朋, 李雪梅, 刘晓勤, 孙林兵. 基于柔性MOFs的磁响应复合材料及其丙烯吸附性能研究[J]. 化工学报, 2025, 76(5): 2230-2240. |
| [8] | 贾晶宇, 孔德齐, 沈圆辉, 张东辉, 李文彬, 唐忠利. 合成氨反应器尾气变压吸附氨分离工艺的模拟与分析[J]. 化工学报, 2025, 76(2): 718-730. |
| [9] | 邹立, 马砺, 张鹏宇, 魏高明, 郭睿智, 赵钦新. 煅烧电石渣强化生物质气化制氢特性及其反应动力学研究[J]. 化工学报, 2025, 76(11): 6040-6057. |
| [10] | 陈彦霖, 周爱国, 郑家乐, 杨川箬, 葛天舒. 载体对于胺浸渍类DAC吸附剂性能的影响[J]. 化工学报, 2024, 75(S1): 217-222. |
| [11] | 唐宇昊, 张迎迎, 赵智伟, 鲁梦悦, 张飞飞, 王小青, 杨江峰. 弱极性超微孔Sc/In-CPM-66A用于CH4/N2吸附分离性能[J]. 化工学报, 2024, 75(9): 3210-3220. |
| [12] | 王涛虹, 王超, 李政, 刘莹, 田歌, 常刚刚, 阳晓宇, 鲍宗必. 固载Cu(Ⅰ)的π络合MOF吸附剂用于乙烷/乙烯的选择性分离[J]. 化工学报, 2024, 75(7): 2565-2573. |
| [13] | 王岩, 周佳文, 孙培亮, 陈勇, 齐元红, 彭冲. 磁性聚氨基噻唑吸附剂脱除水体Hg2+性能[J]. 化工学报, 2024, 75(6): 2283-2298. |
| [14] | 冀钟, 赵彦玲, 陈雨濛, 高林霞, 王翼鹏, 刘欢. ZSM-5分子筛对典型涂装VOCs的吸附性能及机理研究[J]. 化工学报, 2024, 75(6): 2332-2343. |
| [15] | 刘莹, 郑芳, 杨启炜, 张治国, 任其龙, 鲍宗必. 二甲苯异构体吸附分离研究进展[J]. 化工学报, 2024, 75(4): 1081-1095. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号