• •
收稿日期:2021-10-08
修回日期:2022-01-08
通讯作者:
石德智
作者简介:童海航(1998—),男,硕士研究生,基金资助:
Haihang TONG( ),Dezhi SHI(
),Dezhi SHI( ),Jiayu LIU,Huayi CAI,Dan LUO,Fei CHEN
),Jiayu LIU,Huayi CAI,Dan LUO,Fei CHEN
Received:2021-10-08
Revised:2022-01-08
Contact:
Dezhi SHI
摘要:
通过文献计量学分析表明暗发酵制氢是目前研究最热门的生物制氢方法,Fe、Ni、Co、Ag等金属纳米颗粒作为该领域研究热点可改善暗发酵制氢存在底物转化率与产氢效率均有待提高的难题。介绍了金属纳米颗粒的特点、生物相容性及其与酶、微生物细胞的作用机理,进一步从促进木质纤维素水解影响产氢、对水解酶的固定化影响产氢、提高氢化酶活性影响产氢、调控发酵微生物细胞代谢和促进细胞电子传递影响产氢、改善微生物群落结构影响多菌群协同产氢等几个方面对典型金属纳米颗粒辅助木质纤维素暗发酵产氢的研究现状进行综述,并对金属纳米颗粒应用于暗发酵产氢存在的难点及前景方向进行了展望。
中图分类号:
童海航,石德智,刘嘉宇,蔡桦伊,罗丹,陈飞. 金属纳米颗粒辅助木质纤维素暗发酵生物制氢的研究进展[J]. 化工学报, DOI: 10.11949/0438-1157.S20211412.
Haihang TONG,Dezhi SHI,Jiayu LIU,Huayi CAI,Dan LUO,Fei CHEN. Research progress on dark fermentative bio-hydrogen production from lignocellulose assisted by metal nanoparticles[J]. CIESC Journal, DOI: 10.11949/0438-1157.S20211412.
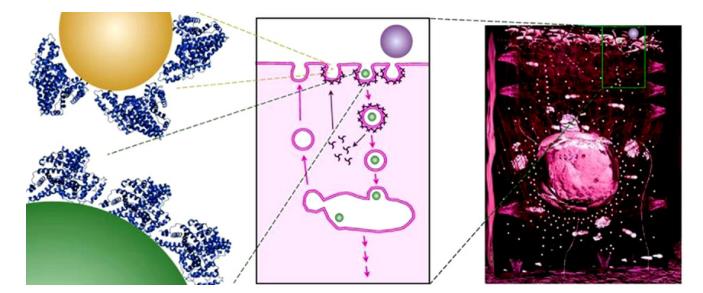
图2 纳米颗粒与细胞的作用(受体介导的内吞作用:纳米颗粒被蛋白质冠层包裹)[35]
Fig.2 Interaction between nanoparticles and cell(receptor mediated endocytosis: nanoparticles are coated by a protein corona)
| 序号 | 纳米材料 | 固定/结合模式 | 条件参数 |
|---|---|---|---|
| 1 | Fe3O4 NPs [ | 戊二醛表面修饰共价结合 | T=60℃, pH=4.5 |
| 2 | Fe3O4 NPs [ | 通过半胱氨酸基团的表面功能化卵清蛋白结合 | T=80℃, pH=4.5 |
| 3 | Fe3O4@SiO2[ | 物理吸附 | T=70℃, pH=4.0 |
| 4 | Fe3O4 NPs @SiO2[ | APTES共价结合 | T=25℃, pH=4 |
| 5 | Fe3O4 NPs @SiO2[ | 甲基丙烯酸缩水甘油酯表面功能化共价结合 | T=50℃, pH=5.0 |
| 6 | Fe3O4@SiO2@KIT-6NPs[ | APTES共价结合 | T=35℃, pH=4.5 |
| 7 | Fe3O4 NPs /壳聚糖[ | 戊二醛表面修饰共价结合 | T=60℃, pH=5.5 |
| 8 | Fe3O4 NPs/壳聚糖[ | 戊二醛表面修饰共价结合 | T=60℃, pH=5.0 |
| 9 | Cu/Fe3O4 NPs[ | APTES共价结合 | T=80℃, pH=5 |
| 10 | Cu2+ modified Fe3O4-NH2NPs[ | 亲和吸附 | T=35℃, pH=4.5 |
| 11 | Fe3O4@Au NPs[ | 通过聚乙二醇和L-天门冬氨酸共价结合 | T=50℃, pH=4.8 |
| 12 | Fe2O3 NPs[ | 戊二醛表面修饰共价结合 | T=50℃, pH=4.8 |
| 13 | CoFe2O4 NPs [ | 基于EDS & NHS的戊二醛结合 | T=50℃, pH=5.0 |
| 14 | MnO2 NPs [ | 戊二醛表面改性卵圆结合 | T=70℃, pH=5.0 |
表1 各种金属纳米颗粒在纤维素酶/漆酶固定化中的应用
Table 1 Application of MNPs on cellulose or laccase immobilization
| 序号 | 纳米材料 | 固定/结合模式 | 条件参数 |
|---|---|---|---|
| 1 | Fe3O4 NPs [ | 戊二醛表面修饰共价结合 | T=60℃, pH=4.5 |
| 2 | Fe3O4 NPs [ | 通过半胱氨酸基团的表面功能化卵清蛋白结合 | T=80℃, pH=4.5 |
| 3 | Fe3O4@SiO2[ | 物理吸附 | T=70℃, pH=4.0 |
| 4 | Fe3O4 NPs @SiO2[ | APTES共价结合 | T=25℃, pH=4 |
| 5 | Fe3O4 NPs @SiO2[ | 甲基丙烯酸缩水甘油酯表面功能化共价结合 | T=50℃, pH=5.0 |
| 6 | Fe3O4@SiO2@KIT-6NPs[ | APTES共价结合 | T=35℃, pH=4.5 |
| 7 | Fe3O4 NPs /壳聚糖[ | 戊二醛表面修饰共价结合 | T=60℃, pH=5.5 |
| 8 | Fe3O4 NPs/壳聚糖[ | 戊二醛表面修饰共价结合 | T=60℃, pH=5.0 |
| 9 | Cu/Fe3O4 NPs[ | APTES共价结合 | T=80℃, pH=5 |
| 10 | Cu2+ modified Fe3O4-NH2NPs[ | 亲和吸附 | T=35℃, pH=4.5 |
| 11 | Fe3O4@Au NPs[ | 通过聚乙二醇和L-天门冬氨酸共价结合 | T=50℃, pH=4.8 |
| 12 | Fe2O3 NPs[ | 戊二醛表面修饰共价结合 | T=50℃, pH=4.8 |
| 13 | CoFe2O4 NPs [ | 基于EDS & NHS的戊二醛结合 | T=50℃, pH=5.0 |
| 14 | MnO2 NPs [ | 戊二醛表面改性卵圆结合 | T=70℃, pH=5.0 |
| 序号 | 微生物种类 | 基质 | 条件参数 | 纳米材料 | H2产量提高效果a |
|---|---|---|---|---|---|
| 1 | Enterobacter | Straw | pH 7,37℃ | Fe0NPs | 提高73.1%[ |
| 2 | Enterobacter cloacae DH-89 | Glucose | pH 7,37℃ | FeNPs | 提高230%[ |
| 3 | Clostridium pasteurianum | Glucose | 35℃ | Fe2O3 NPs | 提高52.5%[ |
| 4 | Clostridium butyricum | Sucrose | pH 7,35℃ | α-Fe2O3 NPs | 提高32.64%[ |
| 5 | Enterobacter aerogenes | Glucose | pH 6.0,37℃ | γ- Fe2O3 NPs | 提高17%[ |
| 6 | Mixed culture bacteria | Inorganic salt | pH 6,37℃ | Iron oxide NPs | 提高81.4%[ |
| 7 | Heat pretreated sludge | Sugarcane bagasse | pH 5.0,30℃ | Fe3O4 NPs | 提高69.6%[ |
| 8 | Anaerobic sludge | Paper mill waste water | pH 7.5 | Fe3O4 NPs | 提高127.4%[ |
| 9 | Parageobacillus thermoglucosidasius KCTC 33548 | Glucose, Starch | pH 6.5,55℃ | Fe3O4 NPs | 提高315%[ |
| 10 | Heat pretreated sludge | Grass | pH 7,37℃ | Fe0 NPs / biochar | 提高89.8%[ |
| 11 | Anaerobic bacteria | Glucose | pH 7,30℃ | Fe0 NPs / activated carbon | 提高50.2%[ |
| 12 | Enterobacter aerogenes | Glucose | pH 6.8,37℃ | Fe0 NPs /chitosan | 提高30%[ |
| 13 | Clostridium pasteurianum | Glucose | pH 7,35℃ | α-Fe2O3&TiO2 NPs | 提高24.9%[ |
| 14 | Anaerobic mixed bacteria | Glucose | pH 7,37℃ | Fe2O3-Fe3O4 NPs /carbon | 提高33.7%[ |
| 15 | Enterobacter aerogenes | Fruit waste | pH 6.5,37℃ | Fe3O4 NPs /DSAC | 提高204.5%[ |
| 16 | Mixed culture bacteria | Gelatinaceous wastewater | pH 6,35℃ | Fe3O4/graphene oxide | 提高41.9%[ |
| 17 | Enterobacter aerogenes | Glucose | pH 6,37℃ | Ferric citrate NPs | 提高50.45%[ |
| 18 | Mesophilic bacteria | Starch | pH 5.0-6.0,37℃ | Fe0 NPs, Ni0 NPs | 提高37%[ |
| 19 | Clostridium butyricum | Glucose, Starch | pH 6.8,37℃ | Fe0 NPs&Ni0 NPs | 提高28%[ |
| 20 | Hydrogen-producing bacteria | Anaerobic sludge | pH 5.0,37℃ | Fe2O3 NPs, NiO Nps | 分别提高24%和16%[ |
| 21 | Thermophilic mixed bacteria | Glucose | pH 5.5,60℃ | α-Fe2O3 NPs, NiO NPs | 分别提高34.38%和5.47%[ |
| 22 | Mixed consortia | Glucose | pH 5.6,35℃ | Ni NPs | 提高22.71%[ |
| 23 | Clostridium butyricum | Glucose | pH6.9,37℃,55℃ | NiFe2O4 NPs | 分别提高38.6%(37℃),28.3%(55℃)[ |
| 24 | Bacillus anthracis | Palm oil | pH 7,37℃ | NiO NPs, CoO NPs | 分别提高151%和167%[ |
| 25 | Clostridium beijerinckii | Rice mill wastewater | pH 7,37℃ | NiO NPs, CoO NPs | 分别提高109%和90.4%[ |
| 26 | Clostridiumbutyricum | Glucose | pH 7.6,30℃ | Fe NPs@SiO2, Pd NPs@SiO2, Ag NPs@SiO2, Cu NPs/SiO2 | 提高38%[ |
| 27 | Mixed consortia | Glucose | pH 5.5,50℃ | ZnO NPs | 提高29%[ |
| 28 | Clostridiumbutyricum | Sucrose | pH 7.2,35℃ | Au NPs | 提高61.7%[ |
| 29 | Enterobacter cloacae | Glucose | pH 7,37℃ | Pd(II) NPs | 在单一菌种和混合菌种培养条件下分别提高1.5%和9%[ |
| 30 | Mixed culture dominated by Clostridium species | Glucose | pH 8.0-9.4,35℃ | Ag NPs | 提高67.3%[ |
表2 不同类型金属纳米材料在暗发酵生物制氢中的应用
Table 2 Application of different MNPs in dark fermentative hydrogen production
| 序号 | 微生物种类 | 基质 | 条件参数 | 纳米材料 | H2产量提高效果a |
|---|---|---|---|---|---|
| 1 | Enterobacter | Straw | pH 7,37℃ | Fe0NPs | 提高73.1%[ |
| 2 | Enterobacter cloacae DH-89 | Glucose | pH 7,37℃ | FeNPs | 提高230%[ |
| 3 | Clostridium pasteurianum | Glucose | 35℃ | Fe2O3 NPs | 提高52.5%[ |
| 4 | Clostridium butyricum | Sucrose | pH 7,35℃ | α-Fe2O3 NPs | 提高32.64%[ |
| 5 | Enterobacter aerogenes | Glucose | pH 6.0,37℃ | γ- Fe2O3 NPs | 提高17%[ |
| 6 | Mixed culture bacteria | Inorganic salt | pH 6,37℃ | Iron oxide NPs | 提高81.4%[ |
| 7 | Heat pretreated sludge | Sugarcane bagasse | pH 5.0,30℃ | Fe3O4 NPs | 提高69.6%[ |
| 8 | Anaerobic sludge | Paper mill waste water | pH 7.5 | Fe3O4 NPs | 提高127.4%[ |
| 9 | Parageobacillus thermoglucosidasius KCTC 33548 | Glucose, Starch | pH 6.5,55℃ | Fe3O4 NPs | 提高315%[ |
| 10 | Heat pretreated sludge | Grass | pH 7,37℃ | Fe0 NPs / biochar | 提高89.8%[ |
| 11 | Anaerobic bacteria | Glucose | pH 7,30℃ | Fe0 NPs / activated carbon | 提高50.2%[ |
| 12 | Enterobacter aerogenes | Glucose | pH 6.8,37℃ | Fe0 NPs /chitosan | 提高30%[ |
| 13 | Clostridium pasteurianum | Glucose | pH 7,35℃ | α-Fe2O3&TiO2 NPs | 提高24.9%[ |
| 14 | Anaerobic mixed bacteria | Glucose | pH 7,37℃ | Fe2O3-Fe3O4 NPs /carbon | 提高33.7%[ |
| 15 | Enterobacter aerogenes | Fruit waste | pH 6.5,37℃ | Fe3O4 NPs /DSAC | 提高204.5%[ |
| 16 | Mixed culture bacteria | Gelatinaceous wastewater | pH 6,35℃ | Fe3O4/graphene oxide | 提高41.9%[ |
| 17 | Enterobacter aerogenes | Glucose | pH 6,37℃ | Ferric citrate NPs | 提高50.45%[ |
| 18 | Mesophilic bacteria | Starch | pH 5.0-6.0,37℃ | Fe0 NPs, Ni0 NPs | 提高37%[ |
| 19 | Clostridium butyricum | Glucose, Starch | pH 6.8,37℃ | Fe0 NPs&Ni0 NPs | 提高28%[ |
| 20 | Hydrogen-producing bacteria | Anaerobic sludge | pH 5.0,37℃ | Fe2O3 NPs, NiO Nps | 分别提高24%和16%[ |
| 21 | Thermophilic mixed bacteria | Glucose | pH 5.5,60℃ | α-Fe2O3 NPs, NiO NPs | 分别提高34.38%和5.47%[ |
| 22 | Mixed consortia | Glucose | pH 5.6,35℃ | Ni NPs | 提高22.71%[ |
| 23 | Clostridium butyricum | Glucose | pH6.9,37℃,55℃ | NiFe2O4 NPs | 分别提高38.6%(37℃),28.3%(55℃)[ |
| 24 | Bacillus anthracis | Palm oil | pH 7,37℃ | NiO NPs, CoO NPs | 分别提高151%和167%[ |
| 25 | Clostridium beijerinckii | Rice mill wastewater | pH 7,37℃ | NiO NPs, CoO NPs | 分别提高109%和90.4%[ |
| 26 | Clostridiumbutyricum | Glucose | pH 7.6,30℃ | Fe NPs@SiO2, Pd NPs@SiO2, Ag NPs@SiO2, Cu NPs/SiO2 | 提高38%[ |
| 27 | Mixed consortia | Glucose | pH 5.5,50℃ | ZnO NPs | 提高29%[ |
| 28 | Clostridiumbutyricum | Sucrose | pH 7.2,35℃ | Au NPs | 提高61.7%[ |
| 29 | Enterobacter cloacae | Glucose | pH 7,37℃ | Pd(II) NPs | 在单一菌种和混合菌种培养条件下分别提高1.5%和9%[ |
| 30 | Mixed culture dominated by Clostridium species | Glucose | pH 8.0-9.4,35℃ | Ag NPs | 提高67.3%[ |

图7 金属纳米颗粒浓度与木质纤维素暗发酵氢气产量之间的关系,[91, 97, 98, 102, 109, 117]
Fig.7 Relation between addition of MNPs and H2 production in dark fermentation from lignocellulose
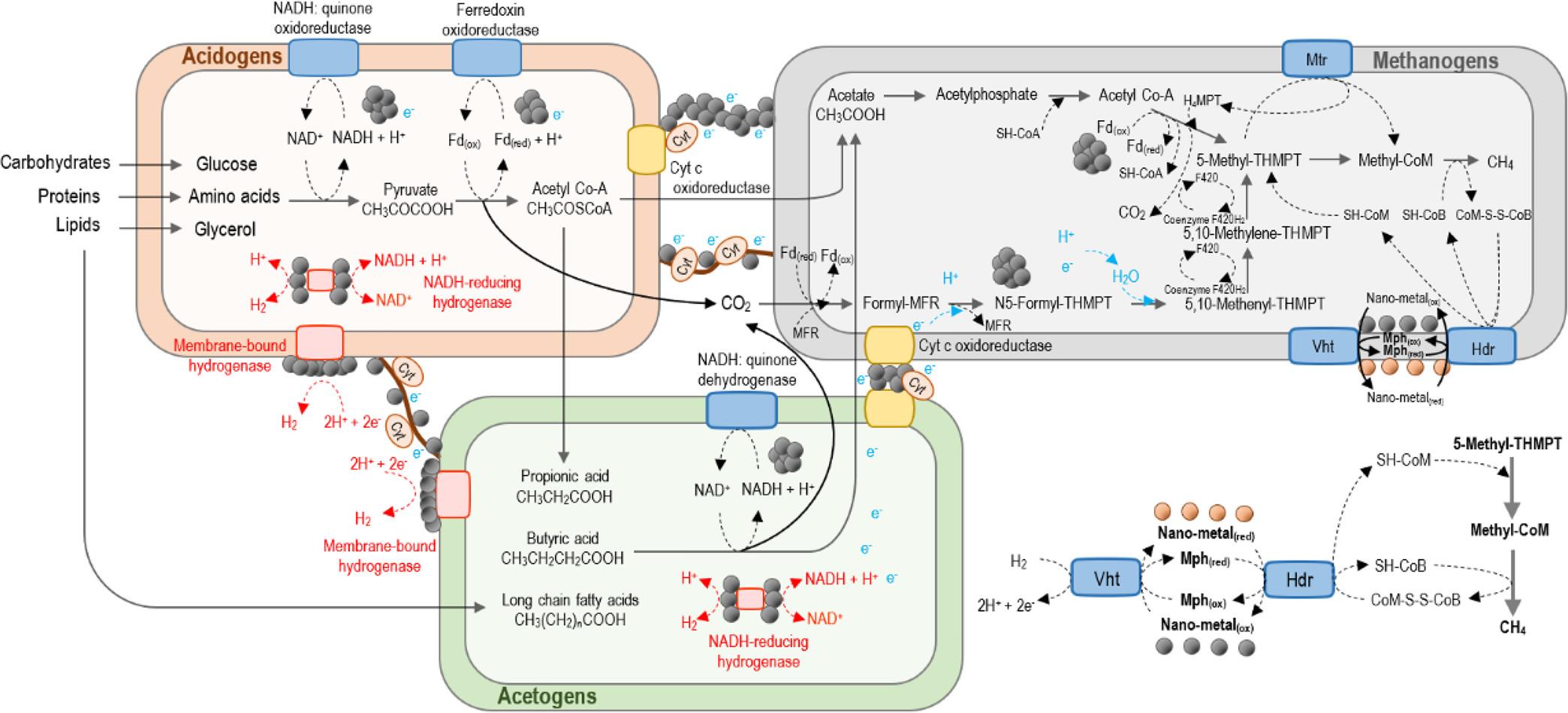
图8 金属纳米颗粒在产氢和产甲烷过程中参与微生物种间电子传递[120]
Fig.8 MNPs involve in intracellular and extracellular electron transportation for H2 and CH4 production via IET interaction

图10 扫描电镜下观察到的肠杆菌细胞表面蛋白与Fe2O3NPs响应产生的生物纳米导线[24]
Fig.10 SEM image of bacterial nanowire produced by response of surface protein of E. aerogenes cells and Fe2O3NPs
| 1 | Ladole M R, Mevada J S, Pandit A B. Ultrasonic hyperactivation of cellulase immobilized on magnetic nanoparticles[J]. Bioresource Technology, 2017, 239: 117-126. |
| 2 | Taherdanak M, Zilouei H, Karimi K. The effects of Fe0 and Ni0 nanoparticles versus Fe2+ and Ni2+ ions on dark hydrogen fermentation[J]. International Journal of Hydrogen Energy, 2016, 41(1): 167-173. |
| 3 | Lang Y Z, Arnepalli R R, Tiwari A. A review on hydrogen production: methods, materials and nanotechnology[J]. Journal of Nanoscience and Nanotechnology, 2011, 11(5): 3719-3739. |
| 4 | Engliman N S, Abdul P M, Wu S Y, et al. Influence of iron (II) oxide nanoparticle on biohydrogen production in thermophilic mixed fermentation[J]. International Journal of Hydrogen Energy, 2017, 42(45): 27482-27493. |
| 5 | Srivastava N, Srivastava M, Gupta V K, et al. A novel strategy to enhance biohydrogen production using graphene oxide treated thermostable crude cellulase and sugarcane bagasse hydrolyzate under co-culture system[J]. Bioresource Technology, 2018, 270: 337-345. |
| 6 | Patel S K S, Lee J K, Kalia V C. Beyond the theoretical yields of dark-fermentative biohydrogen[J]. Indian Journal of Microbiology, 2018, 58(4): 529-530. |
| 7 | Sekoai P T, Ouma C N M, du Preez S P, et al. Application of nanoparticles in biofuels: an overview[J]. Fuel, 2019, 237: 380-397. |
| 8 | Srivastava N, Srivastava M, Malhotra B D, et al. Nanoengineered cellulosic biohydrogen production via dark fermentation: a novel approach[J]. Biotechnology Advances, 2019, 37(6): 107384. |
| 9 | Srivastava N, Srivastava M, Mishra P K, et al. Advances in nanomaterials induced biohydrogen production using waste biomass[J]. Bioresource Technology, 2020, 307: 123094. |
| 10 | Manish S, Banerjee R. Comparison of biohydrogen production processes[J]. International Journal of Hydrogen Energy, 2008, 33(1): 279-286. |
| 11 | Nikolaidis P, Poullikkas A. A comparative overview of hydrogen production processes[J]. Renewable and Sustainable Energy Reviews, 2017, 67: 597-611. |
| 12 | Azman N F, Abdeshahian P, Kadier A, et al. Biohydrogen production from de-oiled rice bran as sustainable feedstock in fermentative process[J]. International Journal of Hydrogen Energy, 2016, 41(1): 145-156. |
| 13 | Ding J, Wang X, Zhou X F, et al. CFD optimization of continuous stirred-tank (CSTR) reactor for biohydrogen production[J]. Bioresource Technology, 2010, 101(18): 7005-7013. |
| 14 | Venkata Mohan S. Harnessing of biohydrogen from wastewater treatment using mixed fermentative consortia: Process evaluation towards optimization[J]. International Journal of Hydrogen Energy, 2009, 34(17): 7460-7474. |
| 15 | Kim M, Day D F. Composition of sugar cane, energy cane, and sweet sorghum suitable for ethanol production at Louisiana sugar mills[J]. Journal of Industrial Microbiology & Biotechnology, 2011, 38(7): 803-807. |
| 16 | Hu F, Ragauskas A. Pretreatment and lignocellulosic chemistry[J]. Bioenergy Research, 2012, 5(4): 1043-1066. |
| 17 | Rocha G J M, Martín C, Da Silva V F N, et al. Mass balance of pilot-scale pretreatment of sugarcane bagasse by steam explosion followed by alkaline delignification[J]. Bioresource Technology, 2012, 111: 447-452. |
| 18 | Pandey A, Nigam P, Soccol C R, et al. Advances in microbial amylases[J]. Biotechnology and Applied Biochemistry, 2000, 31 (2): 135-152. |
| 19 | Furlan F F, Filho R T, Pinto F H, et al. Bioelectricity versus bioethanol from sugarcane bagasse: is it worth being flexible?[J]. Biotechnology for Biofuels, 2013, 6(1): 142. |
| 20 | Sharma M, Joshi M, Nigam S, et al. ZnO tetrapods and activated carbon based hybrid composite: Adsorbents for enhanced decontamination of hexavalent chromium from aqueous solution[J]. Chemical Engineering Journal, 2019, 358: 540-551. |
| 21 | Mishra P, Thakur S, Mahapatra D M, et al. Impacts of nano-metal oxides on hydrogen production in anaerobic digestion of palm oil mill effluent - A novel approach[J]. International Journal of Hydrogen Energy, 2018, 43(5): 2666-2676. |
| 22 | Grieger K D, Fjordbøge A, Hartmann N B, et al. Environmental benefits and risks of zero-valent iron nanoparticles (nZVI) for in situ remediation: Risk mitigation or trade-off?[J]. Journal of Contaminant Hydrology, 2010, 118(3-4): 165-183. |
| 23 | Lin H N, Hu B B, Zhu M J. Enhanced hydrogen production and sugar accumulation from spent mushroom compost by Clostridium thermocellum supplemented with PEG8000 and JFC-E[J]. International Journal of Hydrogen Energy, 2016, 41(4): 2383-2390. |
| 24 | Lin R C, Cheng J, Ding L K, et al. Enhanced dark hydrogen fermentation by addition of ferric oxide nanoparticles using Enterobacteraerogenes[J]. Bioresource Technology, 2016, 207: 213-219. |
| 25 | Pugazhendhi A, Shobana S, Nguyen D D, et al. Application of nanotechnology (nanoparticles) in dark fermentative hydrogen production[J]. International Journal of Hydrogen Energy, 2019, 44(3): 1431-1440. |
| 26 | Taherdanak M, Zilouei H, Karimi K. Investigating the effects of iron and nickel nanoparticles on dark hydrogen fermentation from starch using central composite design[J]. International Journal of Hydrogen Energy, 2015, 40(38): 12956-12963. |
| 27 | Hokkanen S, Bhatnagar A, Sillanpää M. A review on modification methods to cellulose-based adsorbents to improve adsorption capacity[J]. Water Research, 2016, 91: 156-173. |
| 28 | Srivastava N, Srivastava M, Kushwaha D, et al. Efficient dark fermentative hydrogen production from enzyme hydrolyzed rice straw by Clostridium pasteurianum (MTCC116)[J]. Bioresource Technology, 2017, 238: 552-558. |
| 29 | Sewwandi K A H S, Nitisoravut R. Nano zero valent iron embedded on chitosan for enhancement of biohydrogen production in dark fermentation[J]. Energy Reports, 2020, 6: 392-396. |
| 30 | Zhang J S, Zhao W Q, Yang J W, et al. Comparison of mesophilic and thermophilic dark fermentation with nickel ferrite nanoparticles supplementation for biohydrogen production[J]. Bioresource Technology, 2021, 329: 124853. |
| 31 | Wei Z, Zeng G M, Huang F, et al. Bioconversion of oxygen-pretreated Kraft lignin to microbial lipid with oleaginous Rhodococcus opacus DSM 1069[J]. Green Chemistry, 2015, 17(5): 2784-2789. |
| 32 | Bilal S, Ali L, Khan A L, et al. Endophytic fungus Paecilomyces formosus LHL10 produces sester-terpenoid YW3548 and cyclic peptide that inhibit urease and α-glucosidase enzyme activities[J]. Archives of Microbiology, 2018, 200(10): 1493-1502. |
| 33 | Srivastava N, Srivastava M, Manikanta A, et al. Nanomaterials for biofuel production using lignocellulosic waste[J]. Environmental Chemistry Letters, 2017, 15(2): 179-184. |
| 34 | Lynch I, Cedervall T, Lundqvist M, et al. The nanoparticle-protein complex as a biological entity; a complex fluids and surface science challenge for the 21st century[J]. Advances in Colloid and Interface Science, 2007, 134-135: 167-174. |
| 35 | Gao H, Shi W, Freund L B. Mechanics of receptor-mediated endocytosis[J]. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(27): 9469-9474. |
| 36 | Gadhe A, Sonawane S S, Varma M N. Influence of nickel and hematite nanoparticle powder on the production of biohydrogen from complex distillery wastewater in batch fermentation[J]. International Journal of Hydrogen Energy, 2015, 40(34): 10734-10743. |
| 37 | Wang J L, Wan W. Influence of Ni2+ concentration on biohydrogen production[J]. Bioresource Technology, 2008, 99(18): 8864-8868. |
| 38 | Zhang Y F, Shen J Q. Enhancement effect of gold nanoparticles on biohydrogen production from artificial wastewater[J]. International Journal of Hydrogen Energy, 2007, 32(1): 17-23. |
| 39 | Zheng X J, Yu H Q. Biological hydrogen production by enriched anaerobic cultures in the presence of copper and zinc[J]. Journal of Environmental Science and Health Part A-Toxic/Hazardous Substances and Environmental Engineering, 2004, 39(1): 89-101. |
| 40 | Thota S P, Badiya P K, Yerram S, et al. Macro-micro fungal cultures synergy for innovative cellulase enzymes production and biomass structural analyses[J]. Renewable Energy, 2017, 103: 766-773. |
| 41 | Lukowiak A, Kedziora A, Strek W. Antimicrobial graphene family materials: Progress, advances, hopes and fears[J]. Advances in Colloid and Interface Science, 2016, 236: 101-112. |
| 42 | Wang D, Ikenberry M, Peña L, et al. Acid-functionalized nanoparticles for pretreatment of wheat straw[J]. Journal of Biomaterials and Nanobiotechnolog, 2012, 3(3): 342-352. |
| 43 | Singh R K, Tiwari M K, Singh R, et al. From protein engineering to immobilization: promising strategies for the upgrade of industrial enzymes[J]. International Journal of Molecular Sciences, 2013, 14(1): 1232-1277. |
| 44 | Kumar A, Singh S, Nain L. Magnetic nanoparticle immobilized cellulase enzyme for saccharification of paddy straw[J]. International Journal of Current Microbiology and Applied Sciences, 2018, 7(4): 881-893. |
| 45 | Srivastava N, Singh J, Ramteke P W, et al. Improved production of reducing sugars from rice straw using crude cellulase activated with Fe3O4/Alginate nanocomposite[J]. Bioresource Technology, 2015, 183: 262-266. |
| 46 | Cherian E, Dharmendirakumar M, Baskar G. Immobilization of cellulase onto MnO2 nanoparticles for bioethanol production by enhanced hydrolysis of agricultural waste[J]. Chinese Journal of Catalysis, 2015, 36(8): 1223-1229. |
| 47 | Ingle A P, Chandel A K, Antunes F A F, et al. New trends in application of nanotechnology for the pretreatment of lignocellulosic biomass[J]. Biofuels Bioproducts and Biorefining, 2019, 13(3): 776-788. |
| 48 | Guo F, Fang Z, Xu C C, et al. Solid acid mediated hydrolysis of biomass for producing biofuels[J]. Progress in Energy and Combustion Science, 2012, 38(5): 672-690. |
| 49 | Gill C S, Price B A, Jones C W. Sulfonic acid-functionalized silica-coated magnetic nanoparticle catalysts[J]. Journal of Catalysis, 2007, 251(1): 145-152. |
| 50 | Peña L, Ikenberry M, Ware B, et al. Cellobiose hydrolysis using acid-functionalized nanoparticles[J]. Biotechnology and Bioprocess Engineering, 2011, 16(6): 1214-1222. |
| 51 | Su T C, Fang Z, Zhang F, et al. Hydrolysis of selected tropical plant wastes catalyzed by a magnetic carbonaceous acid with microwave[J]. Scientific Reports, 2015, 5: 17538. |
| 52 | Ingle A P, Philippini R R, Souza Melo Y C, et al. Acid-functionalized magnetic nanocatalysts mediated pretreatment of sugarcane straw: an eco-friendly and cost-effective approach[J]. Cellulose, 2020, 27(12): 7067-7078. |
| 53 | Antunes F A F, Chandel A K, Terán-Hilares R, et al. Overcoming challenges in lignocellulosic biomass pretreatment for second-generation (2G) sugar production: emerging role of nano, biotechnological and promising approaches[J]. 3 Biotech, 2019, 9(6): 1-17. |
| 54 | Amin R, Khorshidi A, Shojaei A F, et al. Immobilization of laccase on modified Fe3O4@SiO2@Kit-6 magnetite nanoparticles for enhanced delignification of olive pomace bio-waste[J]. International Journal of Biological Macromolecules, 2018, 114: 106-113. |
| 55 | Ibarra-Gonzalez P, Rong B G. A review of the current state of biofuels production from lignocellulosic biomass using thermochemical conversion routes[J]. Chinese Journal of Chemical Engineering, 2019, 27(7): 1523-1535. |
| 56 | Riva S. Laccases: blue enzymes for green chemistry[J]. Trends in Biotechnology, 2006, 24(5): 219-226. |
| 57 | Sukumaran R K, Singhania R R, Mathew G M, et al. Cellulase production using biomass feed stock and its application in lignocellulose saccharification for bio-ethanol production[J]. Renewable Energy, 2009, 34(2): 421-424. |
| 58 | Khoshnevisan K, Poorakbar E, Baharifar H, et al. Recent advances of cellulase immobilization onto magnetic nanoparticles: an update review[J]. Magnetochemistry, 2019, 5(2): 36. |
| 59 | Rajnish K N, Samuel M S, John J A, et al. Immobilization of cellulase enzymes on nano and micro-materials for breakdown of cellulose for biofuel production-a narrative review[J]. International Journal of Biological Macromolecules, 2021, 182: 1793-1802. |
| 60 | Han H L, Cui M J, Wei L L, et al. Enhancement effect of hematite nanoparticles on fermentative hydrogen production[J]. Bioresource Technology, 2011, 102(17): 7903-7909. |
| 61 | Ramrez W G. Plaza Central de Mercado de Bogotá: Las variaciones de un paradigma 1849-1953[M]. Plaza Central de Mercado de Bogotá: Las variaciones de un paradigma1849-1953, 2017. |
| 62 | Cao X H, Tan C L, Zhang X, et al. Solution-processed two-dimensional metal dichalcogenide-based nanomaterials for energy storage and conversion[J]. Advanced Materials, 2016, 28(29): 6167-6196. |
| 63 | Vaghari H, Jafarizadeh-Malmiri H, Mohammadlou M, et al. Application of magnetic nanoparticles in smart enzyme immobilization[J]. Biotechnology Letters, 2016, 38(2): 223-233. |
| 64 | Chen F, Fan G Q, Zhang Z P, et al. Encapsulation of omega-3 fatty acids in nanoemulsions and microgels: Impact of delivery system type and protein addition on gastrointestinal fate[J]. Food Research International, 2017, 100: 387-395. |
| 65 | Lynch I, Dawson K A. Protein-nanoparticle interactions[J]. Nano Today, 2008, 3(1-2): 40-47. |
| 66 | Brady D, Jordaan J. Advances in enzyme immobilisation[J]. Biotechnology Letters, 2009, 31(11): 1639-1650. |
| 67 | Poorakbar E, Saboury A A, Laame Rad B, et al. Immobilization of cellulase onto core-shell magnetic gold nanoparticles functionalized by aspartic acid and determination of its activity[J]. The Protein Journal, 2020, 39(4): 328-336. |
| 68 | Xu J L, Huo S H, Yuan Z H, et al. Characterization of direct cellulase immobilization with superparamagnetic nanoparticles[J]. Biocatalysis and Biotransformation, 2011, 29(2-3):71-76. |
| 69 | Srivastava N, Rawat R, Sharma R, et al. Effect of nickel-cobaltite nanoparticles on production and thermostability of cellulases from newly isolated thermotolerant aspergillus fumigatus NS (class: eurotiomycetes)[J]. Applied Biochemistry and Biotechnology, 2014, 174(3): 1092-1103. |
| 70 | Salem A H, Mietzel T, Brunstermann R, et al. Effect of cell immobilization, hematite nanoparticles and formation of hydrogen-producing granules on biohydrogen production from sucrose wastewater[J]. International Journal of Hydrogen Energy, 2017, 42(40): 25225-25233. |
| 71 | Gou Z C, Ma N L, Zhang W Q, et al. Innovative hydrolysis of corn stover biowaste by modified magnetite laccase immobilized nanoparticles[J]. Environmental Research, 2020, 188: 109829. |
| 72 | Han J, Rong J H, Wang Y, et al. Immobilization of cellulase on thermo-sensitive magnetic microspheres: improved stability and reproducibility[J]. Bioprocess and Biosystems Engineering, 2018, 41(7): 1051-1060. |
| 73 | Li Y, Wang X Y, Zhang R Z, et al. Molecular imprinting and immobilization of cellulase onto magnetic Fe3O4@SiO2 nanoparticles[J]. Journal of Nanoscience and Nanotechnology, 2014, 14(4): 2931-2936. |
| 74 | Hu J P, Yuan B N, Zhang Y M, et al. Immobilization of laccase on magnetic silica nanoparticles and its application in the oxidation of guaiacol, a phenolic lignin model compound[J]. RSC Advances, 2015, 5(120): 99439-99447. |
| 75 | Tao Q L, Li Y, Shi Y, et al. Application of molecular imprinted magnetic Fe3O4@SiO2 nanoparticles for selective immobilization of cellulase[J]. Journal of Nanoscience and Nanotechnology, 2016, 16(6): 6055-6060. |
| 76 | Sánchez-Ramírez J, Martínez-Hernández J L, Segura-Ceniceros P, et al. Cellulases immobilization on chitosan-coated magnetic nanoparticles: application for agave atrovirens lignocellulosic biomass hydrolysis[J]. Bioprocess and Biosystems Engineering, 2017, 40(1): 9-22. |
| 77 | Zang L M, Qiu J H, Wu X L, et al. Preparation of magnetic chitosan nanoparticles as support for cellulase immobilization[J]. Industrial & Engineering Chemistry Research, 2014, 53(9): 3448-3454. |
| 78 | Abbaszadeh M, Hejazi P. Metal affinity immobilization of cellulase on Fe3O4 nanoparticles with copper as ligand for biocatalytic applications[J]. Food Chemistry, 2019, 290: 47-55. |
| 79 | Alahakoon T, Koh J W, Chong X W C, et al. Immobilization of cellulases on amine and aldehyde functionalized Fe2O3 magnetic nanoparticles[J]. Preparative Biochemistry and Biotechnology, 2012, 42(3): 234-248. |
| 80 | Bohara R A, Thorat N D, Pawar S H. Immobilization of cellulase on functionalized cobalt ferrite nanoparticles[J]. Korean Journal of Chemical Engineering, 2016, 33(1): 216-222. |
| 81 | Poorakbar E, Shafiee A, Saboury A A, et al. Synthesis of magnetic gold mesoporous silica nanoparticles core shell for cellulase enzyme immobilization: Improvement of enzymatic activity and thermal stability[J]. Process Biochemistry, 2018, 71: 92-100. |
| 82 | Manasa P, Saroj P, Korrapati N, et al. Immobilization of cellulase enzyme on zinc ferrite nanoparticles in increasing enzymatic hydrolysis on ultrasound-assisted alkaline pretreated crotalaria juncea biomass[J]. Indian Journal of Science and Technology, 2017, 10(24): 1-7. |
| 83 | Logan, B E. Peer Reviewed: extracting hydrogen and electricity from renewable resources[J]. Environmental Science & Technology, 2004, 38(9): 160A-167A. |
| 84 | Kapdan I K, Kargi F. Bio-hydrogen production from waste materials[J]. Enzyme and Microbial Technology, 2006, 38(5): 569-582. |
| 85 | Akhtar M K, Jones P R. Construction of a synthetic YdbK-dependent pyruvate: H2 pathway in Escherichia coli BL21(DE3)[J]. Metabolic Engineering, 2009, 11(3): 139-147. |
| 86 | Adams M W, Stiefel E I. Organometallic iron: the key to biological hydrogen metabolism[J]. Current Opinion in Chemical Biology, 2000, 4(2): 214-220. |
| 87 | Latifi A, Avilan L, Brugna M. Clostridial whole cell and enzyme systems for hydrogen production: current state and perspectives[J]. Applied Microbiology and Biotechnology, 2019, 103(2): 567-575. |
| 88 | Broderick J B, Byer A S, Duschene K S, et al. H-Cluster assembly during maturation of the [FeFe]-hydrogenase[J]. Journal of Biological Inorganic Chemistry, 2014, 19(6): 747-757. |
| 89 | Lee D Y, Li Y Y, Oh Y K, et al. Effect of iron concentration on continuous H2 production using membrane bioreactor[J]. International Journal of Hydrogen Energy, 2009, 34(3): 1244-1252. |
| 90 | Van Ginkel S W, Oh S E, Logan B E. Biohydrogen gas production from food processing and domestic wastewaters[J]. International Journal of Hydrogen Energy, 2005, 30(15): 1535-1542. |
| 91 | Yang G, Wang J L. Improving mechanisms of biohydrogen production from grass using zero-valent iron nanoparticles[J]. Bioresource Technology, 2018, 266: 413-420. |
| 92 | Chen K F, Li S L, Zhang W X. Renewable hydrogen generation by bimetallic zero valent iron nanoparticles[J]. Chemical Engineering Journal, 2011, 170(2-3): 562-567. |
| 93 | Reardon E J. Anaerobic corrosion of granular iron: measurement and interpretation of hydrogen evolution rates[J]. Environmental Science &Technology, 1995, 29(12): 2936-2945. |
| 94 | Beckers L, Hiligsmann S, Lambert S D, et al. Improving effect of metal and oxide nanoparticles encapsulated in porous silica on fermentative biohydrogen production by Clostridium butyricum[J]. Bioresource Technology, 2013, 133: 109-117. |
| 95 | Gadhe A, Sonawane S S, Varma M N. Enhancement effect of hematite and nickel nanoparticles on biohydrogen production from dairy wastewater[J]. International Journal of Hydrogen Energy, 2015, 40(13): 4502-4511. |
| 96 | Soni D, Bafana A, Gandhi D, et al. Stress response of pseudomonas species to silver nanoparticles at the molecular level[J]. Environmental Toxicology and Chemistry, 2014, 33(9): 2126-2132. |
| 97 | Zhang J S, Fan C F, Zhang H W, et al. Ferric oxide/carbon nanoparticles enhanced bio-hydrogen production from glucose[J]. International Journal of Hydrogen Energy, 2018, 43(18): 8729-8738. |
| 98 | Rambabu K, Bharath G, Banat F, et al. Ferric oxide/date seed activated carbon nanocomposites mediated dark fermentation of date fruit wastes for enriched biohydrogen production[J]. International Journal of Hydrogen Energy, 2021, 46(31): 16631-16643. |
| 99 | Rambabu K, Show P L, Bharath G, et al. Enhanced biohydrogen production from date seeds by Clostridium thermocellum ATCC 27405[J]. International Journal of Hydrogen Energy, 2020, 45(42): 22271-22280. |
| 100 | Kumar G, Mathimani T, Rene E R, et al. Application of nanotechnology in dark fermentation for enhanced biohydrogen production using inorganic nanoparticles[J]. International Journal of Hydrogen Energy, 2019, 44(26): 13106-13113. |
| 101 | Nath D, Manhar A K, Gupta K, et al. Phytosynthesized iron nanoparticles: effects on fermentative hydrogen production by Enterobactercloacae DH-89[J]. Bulletin of Materials Science, 2015, 38(6): 1533-1538. |
| 102 | Malik S N, Pugalenthi V, Vaidya A N, et al. Kinetics of nano-catalysed dark fermentative hydrogen production from distillery wastewater[J]. Energy Procedia, 2014, 54: 417-430. |
| 103 | Reddy K, Nasr M, Kumari S, et al. Biohydrogen production from sugarcane bagasse hydrolysate: Effects of pH, S/X, Fe2+, and magnetite nanoparticles[J]. Environmental Science and Pollution Research, 2017, 24(9): 8790-8804. |
| 104 | Nasr M, Tawfik A, Awad H M, et al. Dual production of hydrogen and biochar from industrial effluent containing phenolic compounds[J]. Fuel, 2021, 301: 121087. |
| 105 | Singhvi M, Maharjan A, Thapa A, et al. Nanoparticle-associated single step hydrogen fermentation for the conversion of starch potato waste biomass by thermophilic Parageobacillus thermoglucosidasius[J]. Bioresource Technology, 2021, 337: 125490. |
| 106 | Yang G, Wang J L. Synergistic enhancement of biohydrogen production from grass fermentation using biochar combined with zero-valent iron nanoparticles[J]. Fuel, 2019, 251: 420-427. |
| 107 | Zhang L, Zhang L X, Li D P. Enhanced dark fermentative hydrogen production by zero-valent iron activated carbon micro-electrolysis[J]. International Journal of Hydrogen Energy, 2015, 40(36): 12201-12208. |
| 108 | Hsieh P H, Lai Y C, Chen K Y, et al. Explore the possible effect of TiO2 and magnetic hematite nanoparticle addition on biohydrogen production by Clostridium pasteurianum based on gene expression measurements[J]. International Journal of Hydrogen Energy, 2016, 41(46): 21685-21691. |
| 109 | Mostafa A, El-Dissouky A, Fawzy A, et al. Magnetite/graphene oxide nano-composite for enhancement of hydrogen production from gelatinaceous wastewater[J]. Bioresource Technology, 2016, 216: 520-528. |
| 110 | Sarma S J, Brar S K, Reigner J, et al. Enriched hydrogen production by bioconversion of biodiesel waste supplemented with ferric citrate and its nano-spray dried particles[J]. RSC Advances, 2014, 4(91): 49588-49594. |
| 111 | Moura A G L, Rabelo C A B S, Okino C H, et al. Enhancement of Clostridium butyricum hydrogen production by iron and nickel nanoparticles: Effects on hydA expression[J]. International Journal of Hydrogen Energy, 2020, 45(53): 28447-28461. |
| 112 | Mullai P, Yogeswari M K, Sridevi K. Optimisation and enhancement of biohydrogen production using nickel nanoparticles - A novel approach[J]. Bioresource Technology, 2013, 141: 212-219. |
| 113 | Rambabu K, Bharath G, Thanigaivelan A, et al. Augmented biohydrogen production from rice mill wastewater through nano-metal oxides assisted dark fermentation[J]. Bioresource Technology, 2021, 319: 124243. |
| 114 | Elreedy A, Fujii M, Koyama M, et al. Enhanced fermentative hydrogen production from industrial wastewater using mixed culture bacteria incorporated with iron, nickel, and zinc-based nanoparticles[J]. Water Research, 2019, 151: 349-361. |
| 115 | Mohanraj S, Anbalagan K, Kodhaiyolii S, et al. Comparative evaluation of fermentative hydrogen production using Enterobacter cloacae and mixed culture: Effect of Pd (II) ion and phytogenic palladium nanoparticles[J]. Journal of Biotechnology, 2014, 192: 87-95. |
| 116 | Zhao W, Zhang Y F, Du B, et al. Enhancement effect of silver nanoparticles on fermentative biohydrogen production using mixed bacteria[J]. Bioresource Technology, 2013, 142: 240-245. |
| 117 | Li Y M, Zhang Z P, Lee D J, et al. Role of L-cysteine and iron oxide nanoparticle in affecting hydrogen yield potential and electronic distribution in biohydrogen production from dark fermentation effluents by photo-fermentation[J]. Journal of Cleaner Production, 2020, 276: 123193. |
| 118 | Cao X Y, Zhao L, Dong W F, et al. Revealing the mechanisms of alkali-based magnetic nanosheets enhanced hydrogen production from dark fermentation: Comparison between mesophilic and thermophilic conditions[J]. Bioresource Technology, 2022, 343: 126141. |
| 119 | Cheng J, Li H, Ding L K, et al. Improving hydrogen and methane co-generation in cascading dark fermentation and anaerobic digestion: The effect of magnetite nanoparticles on microbial electron transfer and syntrophism[J]. Chemical Engineering Journal, 2020, 397: 125394. |
| 120 | Elsamadony M, Elreedy A, Mostafa A, et al. Perspectives on potential applications of nanometal derivatives in gaseous bioenergy pathways: mechanisms, life cycle, and toxicity[J]. ACS Sustainable Chemistry and Engineering, 2021, 9(29): 9563-9589. |
| 121 | Nozhevnikova A N, Russkova Y I, Litti Y V, et al. Syntrophy and interspecies electron transfer in methanogenic microbial communities[J]. Microbiology, 2020, 89(2): 129-147. |
| 122 | Saha S, Basak B, Hwang J H, et al. Microbial symbiosis: a network towards biomethanation[J]. Trends in Microbiology, 2020, 28(12): 968-984. |
| 123 | Rotaru A E, Shrestha P M, Liu F H, et al. A new model for electron flow during anaerobic digestion: direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane[J]. Energy and Environmental Science, 2014, 7(1): 408-415. |
| 124 | Kato S, Hashimoto K, Watanabe K. Methanogenesis facilitated by electric syntrophy via (semi)conductive iron-oxide minerals[J]. Environmental Microbiology, 2012, 14(7): 1646-1654. |
| 125 | Liu F H, Rotaru A E, Shrestha P M, et al. Magnetite compensates for the lack of a pilin-associated c-type cytochrome in extracellular electron exchange[J]. Environmental Microbiology, 2015, 17(3): 648-655. |
| 126 | El-Naggar M Y, Wanger G, Leung K M, et al. Electrical transport along bacterial nanowires from Shewanella oneidensis MR-1[J]. Proceedings of the National Academy of Sciences, 2010, 107(42): 18127-18131. |
| 127 | Zhao J, Wang Z Y, Dai Y H, et al. Mitigation of CuO nanoparticle-induced bacterial membrane damage by dissolved organic matter[J]. Water Research, 2013, 47(12): 4169-4178. |
| 128 | Gagliano M C, Ismail S B, Stams A J M, et al. Biofilm formation and granule properties in anaerobic digestion at high salinity[J]. Water Research, 2017, 121: 61-71. |
| 129 | Fang H H P, Zhang T, Liu H. Microbial diversity of a mesophilic hydrogen-producing sludge[J]. Applied Microbiology and Biotechnology, 2002, 58(1): 112-118. |
| 130 | Castelló E, Ferraz A D N, Andreani C, et al. Stability problems in the hydrogen production by dark fermentation: Possible causes and solutions[J]. Renewable and Sustainable Energy Reviews, 2020, 119: 109602. |
| 131 | Whiteley M, Diggle S P, Greenberg E P. Progress in and promise of bacterial quorum sensing research[J]. Nature, 2017, 551(7680): 313-320. |
| 132 | Kalia V C. Quorum sensing inhibitors: an overview[J]. Biotechnology Advances, 2013, 31(2): 224-245. |
| 133 | Kalia V C, Purohit H J. Quenching the quorum sensing system: potential antibacterial drug targets[J]. Critical Reviews in Microbiology, 2011, 37(2): 121-140. |
| 134 | Kumar P, Patel S K S, Lee J K, et al. Extending the limits of Bacillus for novel biotechnological applications[J]. Biotechnology Advances, 2013, 31(8): 1543-1561. |
| 135 | Wang L H, Weng L X, Dong Y H, et al. Specificity and enzyme kinetics of the quorum-quenching N-Acyl homoserine lactone lactonase (AHL-lactonase)[J]. Journal of Biological Chemistry, 2004, 279(14): 13645-13651. |
| 136 | Chen R D, Zhou Z G, Cao Y N, et al. High yield expression of an AHL-lactonase from bacillus sp. B546 in Pichia pastoris and its application to reduce Aeromonas hydrophila mortality in aquaculture[J]. Microb Cell Factories, 2010, 9(1): 1-10. |
| 137 | Song J X, An D, Ren N Q, et al. Effects of pH and ORP on microbial ecology and kinetics for hydrogen production in continuously dark fermentation[J]. Bioresource Technology, 2011, 102(23): 10875-10880. |
| [1] | 孟令玎, 崇汝青, 孙菲雪, 孟子晖, 刘文芳. 改性聚乙烯膜和氧化硅固定化碳酸酐酶[J]. 化工学报, 2023, 74(8): 3472-3484. |
| [2] | 段重达, 姚小伟, 朱家华, 孙静, 胡南, 李广悦. 环境因素对克雷白氏杆菌诱导碳酸钙沉淀的影响[J]. 化工学报, 2023, 74(8): 3543-3553. |
| [3] | 陈雅鑫, 袁航, 刘冠章, 毛磊, 杨纯, 张瑞芳, 张光亚. 蛋白质纳米笼介导的酶自固定化研究进展[J]. 化工学报, 2023, 74(7): 2773-2782. |
| [4] | 汤晓玲, 王嘉瑞, 朱玄烨, 郑仁朝. 基于Pickering乳液的卤醇脱卤酶催化合成手性环氧氯丙烷[J]. 化工学报, 2023, 74(7): 2926-2934. |
| [5] | 朱理想, 罗默也, 张晓东, 龙涛, 余冉. 醌指纹法指示三氯乙烯污染土功能微生物活性应用研究[J]. 化工学报, 2023, 74(6): 2647-2654. |
| [6] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [7] | 张兰河, 赖青燚, 王铁铮, 关潇卓, 张明爽, 程欣, 徐小惠, 贾艳萍. H2O2对SBR脱氮效率和污泥性能的影响[J]. 化工学报, 2023, 74(5): 2186-2196. |
| [8] | 刘瑞琪, 周栖桐, 张悦, 贺莹, 高静, 马丽. 基于金纳米颗粒修饰二氧化硅纳米花的生物传感器构建及应用[J]. 化工学报, 2023, 74(3): 1247-1259. |
| [9] | 贾露凡, 王艺颖, 董钰漫, 李沁园, 谢鑫, 苑昊, 孟涛. 微流控双水相贴壁液滴流动强化酶促反应研究[J]. 化工学报, 2023, 74(3): 1239-1246. |
| [10] | 苏伟怡, 丁佳慧, 李春利, 王洪海, 姜艳军. 酶促反应结晶研究进展[J]. 化工学报, 2023, 74(2): 617-629. |
| [11] | 毕浩然, 张洋, 王凯, 徐晨晨, 霍奕影, 陈必强, 谭天伟. 微生物制造绿色化学品研究进展[J]. 化工学报, 2023, 74(1): 1-13. |
| [12] | 刘昕, 戈钧, 李春. 光驱动微生物杂合系统提高生物制造水平[J]. 化工学报, 2023, 74(1): 330-341. |
| [13] | 谭卓涛, 齐思雨, 许梦蛟, 戴杰, 朱晨杰, 应汉杰. 辅酶自循环的氧化还原级联体系在生物催化过程中的应用:机遇与挑战[J]. 化工学报, 2023, 74(1): 45-59. |
| [14] | 胡阳, 孙彦. 酶分子的自驱动及其介导的微纳马达[J]. 化工学报, 2023, 74(1): 116-132. |
| [15] | 李彩风, 王晓, 李岗建, 林军章, 汪卫东, 束青林, 曹嫣镔, 肖盟. 嗜烃乳化菌SL-1与内源菌协同驱油的菌群作用关系研究[J]. 化工学报, 2022, 73(9): 4095-4102. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号