化工学报 ›› 2023, Vol. 74 ›› Issue (1): 45-59.DOI: 10.11949/0438-1157.20221075
谭卓涛( ), 齐思雨, 许梦蛟, 戴杰, 朱晨杰(
), 齐思雨, 许梦蛟, 戴杰, 朱晨杰( ), 应汉杰(
), 应汉杰( )
)
收稿日期:2022-08-01
修回日期:2022-10-28
出版日期:2023-01-05
发布日期:2023-03-20
通讯作者:
朱晨杰,应汉杰
作者简介:谭卓涛(1991—),男,副研究员,joh_yy@njtech.edu.cn
基金资助:
Zhuotao TAN( ), Siyu QI, Mengjiao XU, Jie DAI, Chenjie ZHU(
), Siyu QI, Mengjiao XU, Jie DAI, Chenjie ZHU( ), Hanjie YING(
), Hanjie YING( )
)
Received:2022-08-01
Revised:2022-10-28
Online:2023-01-05
Published:2023-03-20
Contact:
Chenjie ZHU, Hanjie YING
摘要:
氧化还原酶是生物催化过程中应用最为广泛的一类酶,其可以在温和条件下高选择性进行催化,在化工、医药、农业等领域发挥着重要作用。大部分氧化还原酶催化的反应需要烟酰胺辅因子参与。烟酰胺辅因子价格昂贵,大量外源添加会加重成本,无法满足工业生产的要求,因此开发高效经济的辅因子再生策略对氧化还原酶的工业应用具有重要意义。在常用的辅因子再生方法中,酶法因其高效绿色的特点得到了广泛的应用。其中,辅酶自循环的氧化还原级联体系是一种特殊的酶法再生烟酰胺辅因子策略,它能结合多酶级联催化,在不添加任何共底物的情况下,完成烟酰胺辅因子内部的自给自足循环再生。相比其他酶法再生策略,其具有副产物少、原子经济性高等突出优势。本文按照酶促反应进行的顺序将辅酶自循环的氧化还原级联体系分为四大类进行讨论,并以醇/醛脱氢酶为基础,综述了该体系应用于生物催化过程的机遇与挑战,为进一步开发更高效的辅酶自循环的氧化还原级联体系提供思路。
中图分类号:
谭卓涛, 齐思雨, 许梦蛟, 戴杰, 朱晨杰, 应汉杰. 辅酶自循环的氧化还原级联体系在生物催化过程中的应用:机遇与挑战[J]. 化工学报, 2023, 74(1): 45-59.
Zhuotao TAN, Siyu QI, Mengjiao XU, Jie DAI, Chenjie ZHU, Hanjie YING. Application of the redox cascade systems with coenzyme self-cycling in biocatalytic processes: opportunities and challenges[J]. CIESC Journal, 2023, 74(1): 45-59.
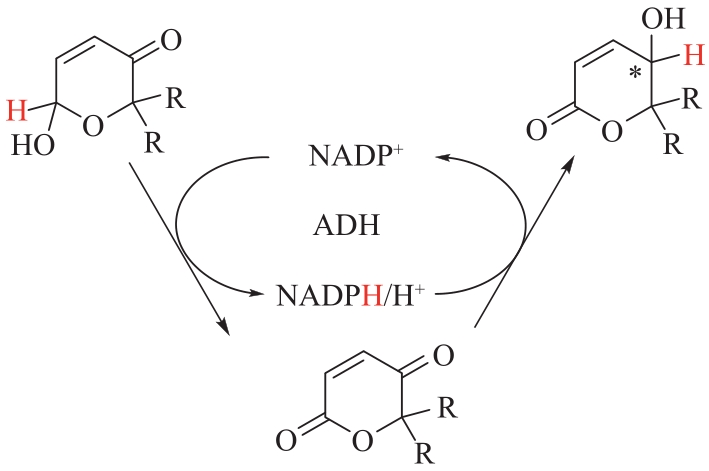
图3 醇脱氢酶催化吡喃酮类化合物生成对映体γ-羟基-δ-内酯的氧化还原异构化反应
Fig.3 Oxidation-reduction isomerization of pyranone compounds to enantiomer γ-hydroxy-δ-lactone catalyzed by alcohol dehydrogenase
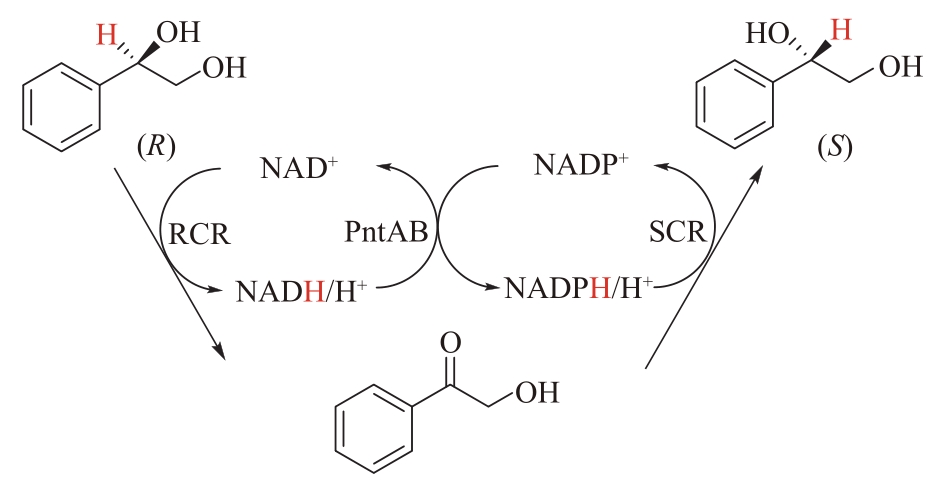
图6 一对选择性互补的ADHs催化(R)-1-苯基-1,2-乙二醇的立体转化RCR:NAD+依赖的(R)-ADH;SCR:NADPH依赖的(S)-ADH
Fig.6 A pair of selectively complementary ADHs catalyze the stereotransformation of (R)-1-phenyl-1,2-ethanediol RCR: NAD+-dependent (R)-ADH; SCR: NADPH-dependent (S)-ADH
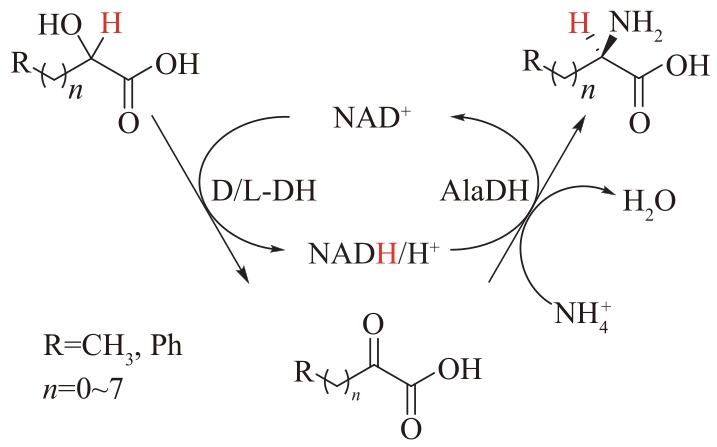
图7 丙氨酸脱氢酶(AlaDH)偶联乳酸脱氢酶(LDH)制备L-丙氨酸及其衍生物
Fig.7 Preparing L-alanine and its derivatives by alanine dehydrogenase (AlaDH) coupled with lactate dehydrogenase (LDH)
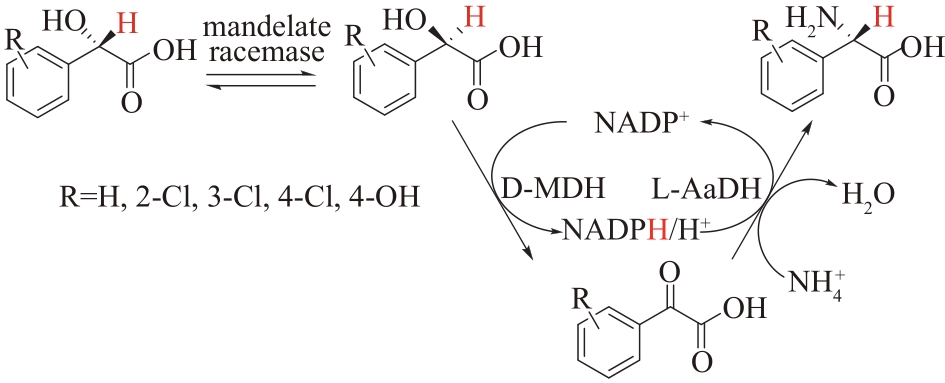
图8 扁桃酸外消旋酶偶联D-扁桃酸脱氢酶(D-MDH)和L-氨基酸脱氢酶(L-AADH)合成L-苯甘氨酸及其衍生物
Fig.8 Coupling of mandelate racemase, D-mandelate dehydrogenase (D-MDH) and L-amino acid dehydrogenase (L-AADH) to synthesize L-phenylglycine and its derivatives
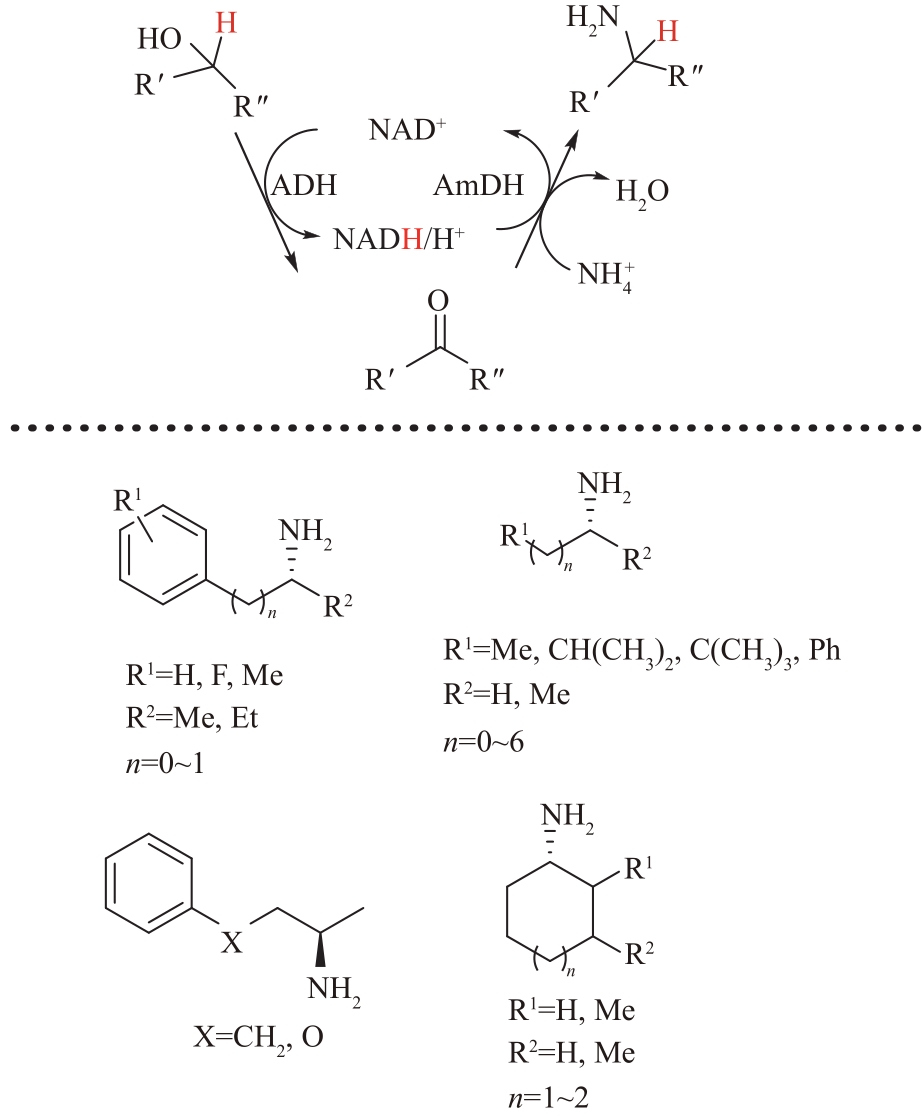
图9 醇脱氢酶(ADH)偶联胺脱氢酶(AmDH)催化醇生成手性胺
Fig.9 The formation of chiral amines from alcohols catalyzed by alcohol dehydrogenase (ADH) which coupled with amine dehydrogenase (AmDH)
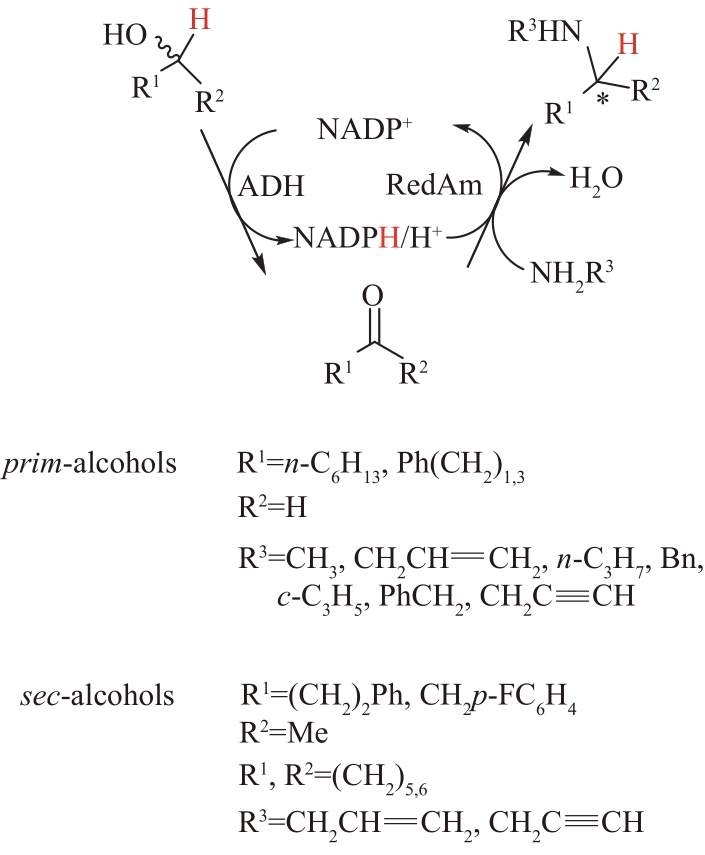
图10 醇脱氢酶(ADH)偶联还原胺化酶(AspRedAm)催化醇生成手性胺
Fig.10 The formation of chiral amines from alcohols catalyzed by alcohol dehydrogenase (ADH) which coupled with reductive aminase (AspRedAm)
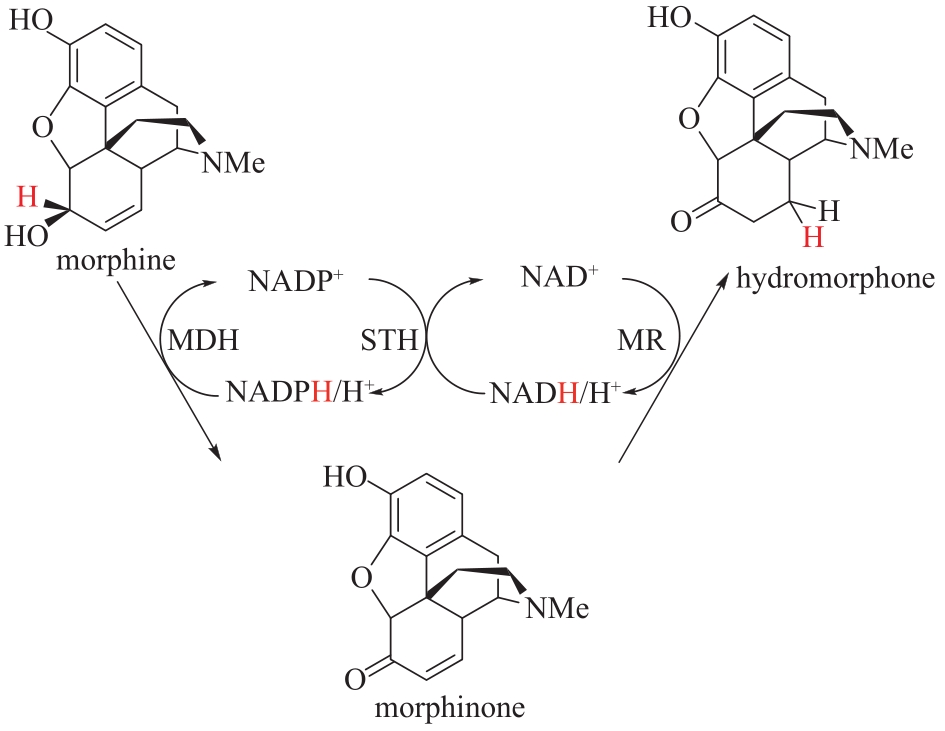
图12 吗啡脱氢酶(MDH)偶联吡啶核苷酸转氢酶(STH)和吗啡酮还原酶(MR)催化吗啡合成氢吗啡酮
Fig.12 Synthesis of dihydromorphine from morphine catalyzed by morphine dehydrogenase (MDH) coupled with pyridine nucleotide transhydrogenase (STH) and morphone reductase(MR)

图13 醇脱氢酶(ADH)偶联烯还原酶(ER)催化环己烯醇转化为环己酮
Fig.13 The conversion of cyclohexenol to cyclohexanone catalyzed by alcohol dehydrogenase (ADH) coupled with ene reductase (ER)
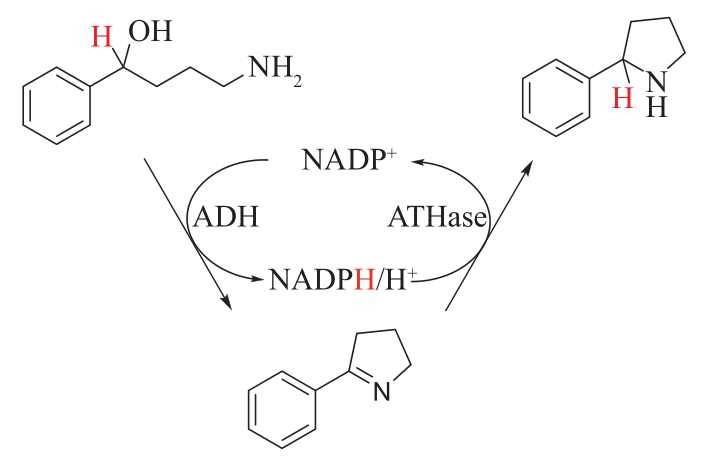
图14 醇脱氢酶(ADH)偶联人工转移氢化酶(ATHase)制备吡咯烷
Fig.14 Synthesis of pyrrolidine by alcohol dehydrogenase (ADH) coupled with artificial transfer hydrogenase (ATHase)

图15 烯还原酶(ER)偶联醛脱氢酶(ALDH)催化α, β-不饱和醛转化为饱和羧酸
Fig.15 Conversion of saturated carboxylic acids from α, β-unsaturated aldehydes catalyzed by (ER) coupled with aldehyde dehydrogenase (ALDH)
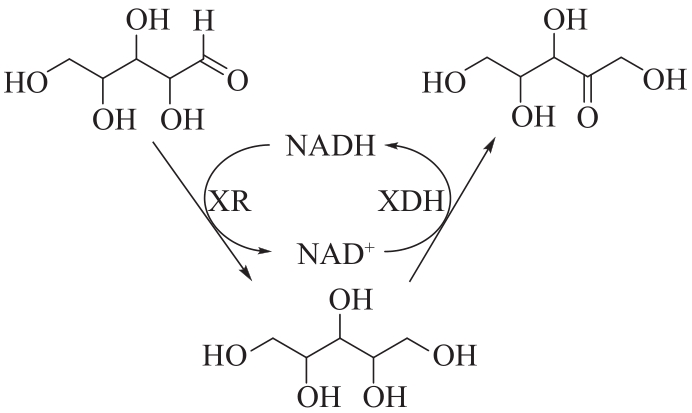
图16 将木糖还原酶(XR)与木糖醇脱氢酶(XDH)共固定于DNA支架上催化木糖生成木酮糖
Fig.16 Co-immobilization of xylose reductase (XR) and xylitol dehydrogenase (XDH) on DNA scaffolds to catalyze xylose to xylulose

图19 5-外-羟基樟脑脱氢酶(FdeH)偶联P450单加氧酶合成2,5-二酮硼烷
Fig.19 The synthesis of 2,5-diketoborane by 5-exo-hydroxycamphor dehydrogenase (FdeH) coupled with P450 monooxygenase

图21 以环酮和二醇为底物经过单加氧酶和醇脱氢酶组成的级联体系合成内酯
Fig.21 Synthesis of lactones by a convergent cascade system consisting of monooxygenase and alcohol dehydrogenase using cyclic ketones and diols as substrates
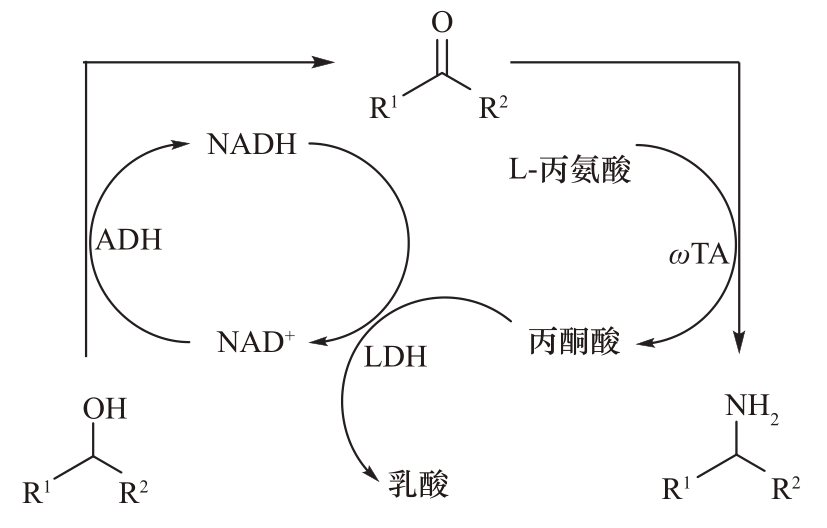
图25 醇脱氢酶(ADH)偶联转氨酶(ωTA)和乳酸脱氢酶(LDH)转化仲醇生成手性胺
Fig.25 Conversion of sencondary alcohols to chiral amines by alcohol dehydrogenase (ADH) coupled with transaminase (ωTA) and lactate dehydrogenase (LDH)

图26 醇脱氢酶(ADH)偶联脱羧酶(PyDC)和乳酸脱氢酶(LDH)催化乙醇生成L-乳酸
Fig.26 The production of L-lactic acid from ethanol catalyzed by alcohol dehydrogenase (ADH) coupled decarboxylase (PyDC) and lactate dehydrogenase (LDH)
| 1 | 伍开琳, 陈永正. 氧化还原酶介导生物级联催化研究进展[J]. 合成化学, 2018, 26(4): 292-299. |
| Wu K L, Chen Y Z. Research progress on oxidoreductase mediated biocatalytic cascades[J]. Chinese Journal of Synthetic Chemistry, 2018, 26(4): 292-299. | |
| 2 | Paul C E, Arends I W C E, Hollmann F. Is simpler better? Synthetic nicotinamide cofactor analogues for redox chemistry[J]. ACS Catalysis, 2014, 4(3): 788-797. |
| 3 | 朱晨杰, 付静雯, 谭卓涛, 等. 天然烟酰胺辅因子再生体系及其人工类似物研究进展[J]. 化工学报, 2018, 69(1): 259-271. |
| Zhu C J, Fu J W, Tan Z T, et al. Advances in regeneration system of natural nicotinamide cofactor and its artificial analogues[J]. CIESC Journal, 2018, 69(1): 259-271. | |
| 4 | Guarneri A, van Berkel W J H, Paul C E. Alternative coenzymes for biocatalysis[J]. Current Opinion in Biotechnology, 2019, 60: 63-71. |
| 5 | Wu H, Tian C Y, Song X K, et al. Methods for the regeneration of nicotinamide coenzymes[J]. Green Chemistry, 2013, 15(7): 1773-1789. |
| 6 | Mordhorst S, Andexer J N. Round, round we go—strategies for enzymatic cofactor regeneration[J]. Natural Product Reports, 2020, 37(10): 1316-1333. |
| 7 | Seelbach K, Riebel B, Hummel W, et al. A novel, efficient regenerating method of NADPH using a new formate dehydrogenase[J]. Tetrahedron Letters, 1996, 37(9): 1377-1380. |
| 8 | Pire C, Esclapez J, Díaz S, et al. Alteration of coenzyme specificity in halophilic NAD(P)+ glucose dehydrogenase by site-directed mutagenesis[J]. Journal of Molecular Catalysis B: Enzymatic, 2009, 59(4): 261-265. |
| 9 | Johannes T W, Woodyer R D, Zhao H M. Directed evolution of a thermostable phosphite dehydrogenase for NAD(P)H regeneration[J]. Applied and Environmental Microbiology, 2005, 71(10): 5728-5734. |
| 10 | Matsuda T, Yamagishi Y, Koguchi S, et al. An effective method to use ionic liquids as reaction media for asymmetric reduction by Geotrichum candidum [J]. Tetrahedron Letters, 2006, 47(27): 4619-4622. |
| 11 | Tassano E, Hall M. Enzymatic self-sufficient hydride transfer processes[J]. Chemical Society Reviews, 2019, 48(23): 5596-5615. |
| 12 | Koesoema A A, Standley D M, Senda T, et al. Impact and relevance of alcohol dehydrogenase enantioselectivities on biotechnological applications[J]. Applied Microbiology and Biotechnology, 2020, 104(7): 2897-2909. |
| 13 | Musa M M, Phillips R S, Laivenieks M, et al. Racemization of enantiopure secondary alcohols by Thermoanaerobacter ethanolicus secondary alcohol dehydrogenase[J]. Organic & Biomolecular Chemistry, 2013, 11(17): 2911-2915. |
| 14 | Musa M M, Patel J M, Nealon C M, et al. Thermoanaerobacter ethanolicus secondary alcohol dehydrogenase mutants with improved racemization activity[J]. Journal of Molecular Catalysis B: Enzymatic, 2015, 115: 155-159. |
| 15 | Musa M M. Racemization of enantiopure alcohols using two mutants of Thermoanaerobacter pseudoethanolicus secondary alcohol dehydrogenase[J]. ChemistrySelect, 2021, 6(46): 13261-13264. |
| 16 | Liu Y C, Merten C, Deska J. Enantioconvergent biocatalytic redox isomerization[J]. Angewandte Chemie International Edition, 2018, 57(37): 12151-12156. |
| 17 | Gruber C, Nestl B, Gross J, et al. Emulation of racemase activity by employing a pair of stereocomplementary biocatalysts[J]. Chemistry-A European Journal, 2007, 13(29): 8271-8276. |
| 18 | Bodlenner A, Glueck S M, Nestl B M, et al. Biocatalytic racemization of α-hydroxycarboxylic acids using a stereo-complementary pair of α-hydroxycarboxylic acid dehydrogenases[J]. Tetrahedron, 2009, 65(36): 7752-7755. |
| 19 | Zhang R Z, Xu Y, Xiao R, et al. Efficient one-step production of (S)-1-phenyl-1,2-ethanediol from (R)-enantiomer plus NAD(+)-NADPH in situ regeneration using engineered Escherichia coli [J]. Microbial Cell Factories, 2012, 11: 167. |
| 20 | Kostyanovsky R G, Kadorkina G K, Lyssenko K A, et al. Chiral drugs via the spontaneous resolution[J]. Mendeleev Communications, 2002, 12(1): 6-8. |
| 21 | Perdih A, Sollner Dolenc M. Recent advances in the synthesis of unnatural α-amino acids—an updated version[J]. Current Organic Chemistry, 2011, 15(22): 3750-3799. |
| 22 | Wandrey C, Fiolitakis E, Wichmann U, et al. L-amino acids from a racemic mixture of α-hydroxy acids[J]. Annals of the New York Academy of Sciences, 1984, 434(1): 91-94. |
| 23 | Bossow B, Wandrey C. Continuous enzymatically catalyzed production of L-leucine from the corresponding racemic hydroxy acid[J]. Annals of the New York Academy of Sciences, 1987, 506(1): 325-336. |
| 24 | Resch V, Fabian W M F, Kroutil W. Deracemisation of mandelic acid to optically pure non-natural L-phenylglycine via a redox-neutral biocatalytic cascade[J]. Advanced Synthesis & Catalysis, 2010, 352(6): 993-997. |
| 25 | Fan C W, Xu G C, Ma B D, et al. A novel D-mandelate dehydrogenase used in three-enzyme cascade reaction for highly efficient synthesis of non-natural chiral amino acids[J]. Journal of Biotechnology, 2015, 195: 67-71. |
| 26 | Wittcoff H A, Reuben B G, Plotkin J S. Industrial Organic Chemicals[M]. Hoboken, NJ, USA: John Wiley & Sons, Inc., 2012. |
| 27 | Nugent T C. Chiral Amine Synthesis: Methods, Developments and Applications[M]. New York: John Wiley & Sons Inc., 2010. |
| 28 | Ducrot L, Bennett M, Grogan G, et al. NAD(P)H-dependent enzymes for reductive amination: active site description and carbonyl-containing compound spectrum[J]. Advanced Synthesis & Catalysis, 2021, 363(2): 328-351. |
| 29 | Wang Z L, Sundara Sekar B, Li Z. Recent advances in artificial enzyme cascades for the production of value-added chemicals[J]. Bioresource Technology, 2021, 323: 124551. |
| 30 | Mutti F G, Knaus T, Scrutton N S, et al. Conversion of alcohols to enantiopure amines through dual-enzyme hydrogen-borrowing cascades[J]. Science, 2015, 349(6255): 1525-1529. |
| 31 | Chen F F, Liu Y Y, Zheng G W, et al. Asymmetric amination of secondary alcohols by using a redox-neutral two-enzyme cascade[J]. ChemCatChem, 2015, 7(23): 3838-3841. |
| 32 | Thompson M P, Turner N J. Two-enzyme hydrogen-borrowing amination of alcohols enabled by a cofactor-switched alcohol dehydrogenase[J]. ChemCatChem, 2017, 9(20): 3833-3836. |
| 33 | Aleku G A, France S P, Man H, et al. A reductive aminase from Aspergillus oryzae [J]. Nature Chemistry, 2017, 9(10): 961-969. |
| 34 | Montgomery S L, Mangas-Sanchez J, Thompson M P, et al. Direct alkylation of amines with primary and secondary alcohols through biocatalytic hydrogen borrowing[J]. Angewandte Chemie International Edition, 2017, 56(35): 10491-10494. |
| 35 | Lenz M, Borlinghaus N, Weinmann L, et al. Recent advances in imine reductase-catalyzed reactions[J]. World Journal of Microbiology & Biotechnology, 2017, 33(11): 199. |
| 36 | Roiban G D, Kern M, Liu Z, et al. Efficient biocatalytic reductive aminations by extending the imine reductase toolbox[J]. ChemCatChem, 2017, 9(24): 4475-4479. |
| 37 | Scholtissek A, Tischler D, Westphal A, et al. Old yellow enzyme-catalysed asymmetric hydrogenation: linking family roots with improved catalysis[J]. Catalysts, 2017, 7(12): 130. |
| 38 | Boonstra B, Rathbone D A, French C E, et al. Cofactor regeneration by a soluble pyridine nucleotide transhydrogenase for biological production of hydromorphone[J]. Applied and Environmental Microbiology, 2000, 66(12): 5161-5166. |
| 39 | Gargiulo S, Opperman D J, Hanefeld U, et al. A biocatalytic redox isomerisation[J]. Chemical Communications, 2012, 48(53): 6630-6632. |
| 40 | Reich S, Nestl B M, Hauer B. Loop-grafted old yellow enzymes in the bienzymatic cascade reduction of allylic alcohols[J]. ChemBioChem, 2016, 17(7): 561-565. |
| 41 | Oberleitner N, Peters C, Muschiol J, et al. An enzymatic toolbox for cascade reactions: a showcase for an in vivo redox sequence in asymmetric synthesis[J]. ChemCatChem, 2013, 5(12): 3524-3528. |
| 42 | Okamoto Y, Köhler V, Paul C E, et al. Efficient in situ regeneration of NADH mimics by an artificial metalloenzyme[J]. ACS Catalysis, 2016, 6(6): 3553-3557. |
| 43 | Köhler V, Wilson Y M, Dürrenberger M, et al. Synthetic cascades are enabled by combining biocatalysts with artificial metalloenzymes[J]. Nature Chemistry, 2013, 5(2): 93-99. |
| 44 | Gurak J A, Yang K S, Liu Z, et al. Directed, regiocontrolled hydroamination of unactivated alkenes via protodepalladation[J]. Journal of the American Chemical Society, 2016, 138(18): 5805-5808. |
| 45 | Winkler T, Gröger H, Hummel W. Enantioselective rearrangement coupled with water addition: direct synthesis of enantiomerically pure saturated carboxylic acids from α,β-unsaturated aldehydes[J]. ChemCatChem, 2014, 6(4): 961-964. |
| 46 | Knaus T, Mutti F G, Humphreys L D, et al. Systematic methodology for the development of biocatalytic hydrogen-borrowing cascades: application to the synthesis of chiral α-substituted carboxylic acids from α-substituted α,β-unsaturated aldehydes[J]. Organic & Biomolecular Chemistry, 2015, 13(1): 223-233. |
| 47 | Ngo T A, Nakata E, Saimura M, et al. Spatially organized enzymes drive cofactor-coupled cascade reactions[J]. Journal of the American Chemical Society, 2016, 138(9): 3012-3021. |
| 48 | Staudt S, Burda E, Giese C, et al. Direct oxidation of cycloalkanes to cycloalkanones with oxygen in water[J]. Angewandte Chemie International Edition, 2013, 52(8): 2359-2363. |
| 49 | Loida P J, Sligar S G. Molecular recognition in cytochrome P-450: mechanism for the control of uncoupling reactions[J]. Biochemistry, 1993, 32(43): 11530-11538. |
| 50 | Schulz S, Girhard M, Gaßmeyer S K, et al. Selective enzymatic synthesis of the grapefruit flavor (+)-nootkatone[J]. ChemCatChem, 2015, 7(4): 601-604. |
| 51 | Hofer M, Strittmatter H, Sieber V. Biocatalytic synthesis of a diketobornane as a building block for bifunctional camphor derivatives[J]. ChemCatChem, 2013, 5(11): 3351-3357. |
| 52 | Müller C A, Dennig A, Welters T, et al. Whole-cell double oxidation of n-heptane[J]. Journal of Biotechnology, 2014, 191: 196-204. |
| 53 | O’Reilly E, Köhler V, Flitsch S L, et al. Cytochromes P450 as useful biocatalysts: addressing the limitations[J]. Chemical Communications, 2011, 47(9): 2490-2501. |
| 54 | Willetts A J, Knowles C J, Levitt M S, et al. Biotransformation of endo-bicyclo[2.2.1]heptan-2-ols and endo-bicyclo[3.2.0]hept-2-en-6-ol into the corresponding lactones[J]. Journal of the Chemical Society, Perkin Transactions 1, 1991(6): 1608. |
| 55 | Grogan G, Roberts S, Willetts A. Biotransformations by microbial Baeyer-Villiger monooxygenases stereoselective lactone formation in vitro by coupled enzyme systems[J]. Biotechnology Letters, 1992, 14(12): 1125-1130. |
| 56 | Gagnon R, Grogan G, Roberts S M, et al. Enzymatic Baeyer-Villiger oxidations of some bicyclo[2.2.1]heptan-2-ones using monooxygenases from Pseudomonas putida NCIMB 10007: enantioselective preparation of a precursor of azadirachtin[J]. Journal of the Chemical Society, Perkin Transactions 1, 1995(12): 1505-1511. |
| 57 | Mallin H, Wulf H, Bornscheuer U T. A self-sufficient Baeyer-Villiger biocatalysis system for the synthesis of ɛ-caprolactone from cyclohexanol[J]. Enzyme & Microbial Technology, 2013, 53(4): 283-287. |
| 58 | Staudt S, Bornscheuer U T, Menyes U, et al. Direct biocatalytic one-pot-transformation of cyclohexanol with molecular oxygen into ɛ-caprolactone[J]. Enzyme & Microbial Technology, 2013, 53(4): 288-292. |
| 59 | Kara S, Spickermann D, Schrittwieser J H, et al. More efficient redox biocatalysis by utilising 1,4-butanediol as a ‘smart cosubstrate’[J]. Green Chemistry, 2013, 15(2): 330-335. |
| 60 | Bornadel A, Hatti-Kaul R, Hollmann F, et al. A bi-enzymatic convergent cascade for ε-caprolactone synthesis employing 1, 6-hexanediol as a ‘double-smart cosubstrate’[J]. ChemCatChem, 2015, 7(16): 2442-2445. |
| 61 | Bornadel A, Hatti-Kaul R, Hollmann F, et al. Enhancing the productivity of the bi-enzymatic convergent cascade for ɛ-caprolactone synthesis through design of experiments and a biphasic system[J]. Tetrahedron, 2016, 72(46): 7222-7228. |
| 62 | Engel J, Mthethwa K S, Opperman D J, et al. Characterization of new Baeyer-Villiger monooxygenases for lactonizations in redox-neutral cascades[J]. Molecular Catalysis, 2019, 468: 44-51. |
| 63 | Huang L, Romero E, Ressmann A K, et al. Nicotinamide adenine dinucleotide-dependent redox-neutral convergent cascade for lactonizations with type Ⅱ flavin-containing monooxygenase[J]. Advanced Synthesis & Catalysis, 2017, 359(12): 2142-2148. |
| 64 | Qi S Y, Tan Z T, Na Q, et al. Constructing a multienzyme cascade redox-neutral system for the synthesis of halogenated indoles[J]. Chemical Communications, 2022, 58(40): 6016-6019. |
| 65 | Lo H C, Fish R H. Biomimetic NAD+ models for tandem cofactor regeneration, horse liver alcohol dehydrogenase recognition of 1, 4-NADH derivatives, and chiral synthesis[J]. Angewandte Chemie International Edition, 2002, 41(3): 478-481. |
| 66 | Paul C E, Tischler D, Riedel A, et al. Nonenzymatic regeneration of styrene monooxygenase for catalysis[J]. ACS Catalysis, 2015, 5(5): 2961-2965. |
| 67 | Simon R C, Richter N, Busto E, et al. Recent developments of cascade reactions involving ω-transaminases[J]. ACS Catalysis, 2014, 4(1): 129-143. |
| 68 | Sattler J H, Fuchs M, Tauber K, et al. Redox self-sufficient biocatalyst network for the amination of primary alcohols[J]. Angewandte Chemie, 2012, 124(36): 9290-9293. |
| 69 | Tauber K, Fuchs M, Sattler J H, et al. Artificial multi-enzyme networks for the asymmetric amination of sec-alcohols[J]. Chemistry-A European Journal, 2013, 19(12): 4030-4035. |
| 70 | Tong X D, El-Zahab B, Zhao X Y, et al. Enzymatic synthesis of L-lactic acid from carbon dioxide and ethanol with an inherent cofactor regeneration cycle[J]. Biotechnology and Bioengineering, 2011, 108(2): 465-469. |
| 71 | Sinha R, Shukla P. Current trends in protein engineering: updates and progress[J]. Current Protein & Peptide Science, 2019, 20(5): 398-407. |
| 72 | Hyster T K, Ward T R. Genetic optimization of metalloenzymes: enhancing enzymes for non-natural reactions[J]. Angewandte Chemie International Edition, 2016, 55(26): 7344-7357. |
| 73 | Turner N J, O'Reilly E. Biocatalytic retrosynthesis[J]. Nature Chemical Biology, 2013, 9(5): 285-288. |
| 74 | Schrittwieser J H, Velikogne S, Hall M, et al. Artificial biocatalytic linear cascades for preparation of organic molecules[J]. Chemical Reviews, 2018, 118(1): 270-348. |
| [1] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [2] | 孟令玎, 崇汝青, 孙菲雪, 孟子晖, 刘文芳. 改性聚乙烯膜和氧化硅固定化碳酸酐酶[J]. 化工学报, 2023, 74(8): 3472-3484. |
| [3] | 陈雅鑫, 袁航, 刘冠章, 毛磊, 杨纯, 张瑞芳, 张光亚. 蛋白质纳米笼介导的酶自固定化研究进展[J]. 化工学报, 2023, 74(7): 2773-2782. |
| [4] | 汤晓玲, 王嘉瑞, 朱玄烨, 郑仁朝. 基于Pickering乳液的卤醇脱卤酶催化合成手性环氧氯丙烷[J]. 化工学报, 2023, 74(7): 2926-2934. |
| [5] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [6] | 张兰河, 赖青燚, 王铁铮, 关潇卓, 张明爽, 程欣, 徐小惠, 贾艳萍. H2O2对SBR脱氮效率和污泥性能的影响[J]. 化工学报, 2023, 74(5): 2186-2196. |
| [7] | 刘瑞琪, 周栖桐, 张悦, 贺莹, 高静, 马丽. 基于金纳米颗粒修饰二氧化硅纳米花的生物传感器构建及应用[J]. 化工学报, 2023, 74(3): 1247-1259. |
| [8] | 贾露凡, 王艺颖, 董钰漫, 李沁园, 谢鑫, 苑昊, 孟涛. 微流控双水相贴壁液滴流动强化酶促反应研究[J]. 化工学报, 2023, 74(3): 1239-1246. |
| [9] | 苏伟怡, 丁佳慧, 李春利, 王洪海, 姜艳军. 酶促反应结晶研究进展[J]. 化工学报, 2023, 74(2): 617-629. |
| [10] | 刘昕, 戈钧, 李春. 光驱动微生物杂合系统提高生物制造水平[J]. 化工学报, 2023, 74(1): 330-341. |
| [11] | 胡阳, 孙彦. 酶分子的自驱动及其介导的微纳马达[J]. 化工学报, 2023, 74(1): 116-132. |
| [12] | 安绍杰, 许洪峰, 李思, 许远航, 李佳锡. 利用分子机器的组装与分解构建pH敏感性谷胱甘肽过氧化物人工酶[J]. 化工学报, 2022, 73(8): 3669-3678. |
| [13] | 张劢, 田瑶, 郭之旗, 王叶, 窦广进, 宋浩. 光催化-生物杂合系统设计优化用于燃料和化学品绿色合成[J]. 化工学报, 2022, 73(7): 2774-2789. |
| [14] | 孙甲琛, 孙文涛, 孙慧, 吕波, 李春. 甘草黄酮合酶Ⅱ催化甘草素特异性合成7,4′-二羟基黄酮[J]. 化工学报, 2022, 73(7): 3202-3211. |
| [15] | 张昕哲, 孙文涛, 吕波, 李春. 植物天然产物氧化与微生物制造[J]. 化工学报, 2022, 73(7): 2790-2805. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号