化工学报 ›› 2020, Vol. 71 ›› Issue (12): 5443-5451.DOI: 10.11949/0438-1157.20200420
郑秋风1( ),罗军1,陈帅1,陈念粗1,于旭东1,2(
),罗军1,陈帅1,陈念粗1,于旭东1,2( ),曾英1,2
),曾英1,2
收稿日期:2020-04-22
修回日期:2020-06-30
出版日期:2020-12-05
发布日期:2020-12-05
通讯作者:
于旭东
作者简介:郑秋风(1996—),男,硕士研究生,基金资助:
ZHENG Qiufeng1( ),LUO Jun1,CHEN Shuai1,CHEN Niancu1,YU Xudong1,2(
),LUO Jun1,CHEN Shuai1,CHEN Niancu1,YU Xudong1,2( ),ZENG Ying1,2
),ZENG Ying1,2
Received:2020-04-22
Revised:2020-06-30
Online:2020-12-05
Published:2020-12-05
Contact:
YU Xudong
摘要:
采用等温溶解平衡法研究了298.2 K四元体系MgCl2-SrCl2-AlCl3-H2O的相平衡关系,测定了该体系的溶解度和平衡溶液的密度、折射率。根据实验数据,分别绘制了298.2 K该四元体系的空间立体图、相图、水图、密度-组成图和折射率-组成图。研究发现:298.2 K下,四元体系MgCl2-SrCl2-AlCl3-H2O无复盐或固溶体生成,稳定相图由2个共饱点、5条单变量曲线和4个结晶区组成。四个结晶区分别为MgCl2·6H2O、SrCl2·6H2O、SrCl2·2H2O和AlCl3·6H2O,其中SrCl2·6H2O结晶区最大,SrCl2·2H2O结晶区最小,说明SrCl2·6H2O更容易结晶析出。平衡液相的密度和折射率随着J(MgCl2)的变化呈规律性变化。采用Pitzer模型进行了298.2 K四元体系MgCl2-SrCl2-AlCl3-H2O溶解度计算,对比发现,计算结果与实验结果基本吻合。
中图分类号:
郑秋风,罗军,陈帅,陈念粗,于旭东,曾英. 298.2 K四元体系MgCl2-SrCl2-AlCl3-H2O相平衡实验及溶解度计算[J]. 化工学报, 2020, 71(12): 5443-5451.
ZHENG Qiufeng,LUO Jun,CHEN Shuai,CHEN Niancu,YU Xudong,ZENG Ying. Measurements and simulation for aqueous quaternary system MgCl2-SrCl2-AlCl3-H2O at 298.2 K[J]. CIESC Journal, 2020, 71(12): 5443-5451.
| No. | Composition of equilibrium solution, w(B)/% | J?necke index of dry salt, J(B)/(g/100 g salt) | Equilibrated solid phase | ||||||
|---|---|---|---|---|---|---|---|---|---|
| J(MgCl2)+J(SrCl2)+J(AlCl3)=100 | |||||||||
| w(MgCl2) | w(SrCl2) | w(AlCl3) | w(H2O) | J(MgCl2) | J(SrCl2) | J(AlCl3) | J(H2O) | ||
| 1, A | 34.75 | 1.42 | 0.00 | 63.83 | 96.07 | 3.93 | 0.00 | 176.47 | Bis + S2 |
| 2 | 33.98 | 1.33 | 0.83 | 63.86 | 94.02 | 3.68 | 2.30 | 176.70 | Bis + S2 |
| 3 | 32.94 | 1.27 | 1.75 | 64.04 | 91.60 | 3.53 | 4.87 | 178.09 | Bis + S2 |
| 4 | 33.01 | 1.31 | 2.53 | 63.15 | 89.58 | 3.55 | 6.87 | 171.37 | Bis + S2 |
| 5 | 31.87 | 1.33 | 3.50 | 63.30 | 86.84 | 3.62 | 9.54 | 172.48 | Bis + S2 |
| 6 | 30.37 | 1.39 | 4.57 | 63.67 | 83.59 | 3.83 | 12.58 | 175.25 | Bis + S2 |
| 7, E1 | 29.49 | 1.32 | 5.47 | 63.72 | 81.28 | 3.64 | 15.08 | 175.63 | Bis + S2 + AC |
| 8 | 28.80 | 1.23 | 6.19 | 63.78 | 79.51 | 3.40 | 17.09 | 176.09 | S2 + AC |
| 9, E2 | 27.36 | 1.39 | 7.28 | 63.97 | 75.93 | 3.86 | 20.21 | 177.55 | S6 + S2 + AC |
| 10, B | 33.16 | 2.22 | 0.00 | 64.62 | 93.73 | 6.27 | 0.00 | 182.65 | S6 + S2 |
| 11 | 32.01 | 1.98 | 1.84 | 64.17 | 89.33 | 5.53 | 5.14 | 179.10 | S6 + S2 |
| 12 | 30.74 | 2.10 | 2.89 | 64.27 | 86.03 | 5.88 | 8.09 | 179.88 | S6 + S2 |
| 13 | 29.27 | 2.12 | 4.04 | 64.57 | 82.62 | 5.98 | 11.40 | 182.25 | S6 + S2 |
| 14 | 28.45 | 2.11 | 4.90 | 64.54 | 80.23 | 5.95 | 13.82 | 182.01 | S6 + S2 |
| 15 | 28.23 | 1.96 | 5.52 | 64.29 | 79.05 | 5.49 | 15.46 | 180.03 | S6 + S2 |
| 16 | 27.73 | 1.53 | 6.55 | 64.19 | 77.44 | 4.27 | 18.29 | 179.25 | S6 + S2 |
| 17, C | 30.45 | 0.00 | 5.65 | 63.90 | 84.35 | 0.00 | 15.65 | 177.01 | Bis + AC |
| 18 | 30.08 | 0.90 | 5.29 | 63.73 | 82.93 | 2.48 | 14.59 | 175.71 | Bis + AC |
| 19 | 29.46 | 1.14 | 5.36 | 64.04 | 81.92 | 3.17 | 14.91 | 178.09 | Bis + AC |
| 20, D | 0.00 | 1.95 | 30.12 | 67.93 | 0.00 | 6.08 | 93.92 | 211.82 | S6 + AC |
| 21 | 5.34 | 1.82 | 25.76 | 67.08 | 16.22 | 5.53 | 78.25 | 203.77 | S6 + AC |
| 22 | 9.85 | 1.71 | 22.00 | 66.44 | 29.35 | 5.10 | 65.55 | 197.97 | S6 + AC |
| 23 | 13.74 | 1.59 | 18.73 | 65.94 | 40.34 | 4.67 | 54.99 | 193.60 | S6 + AC |
| 24 | 16.99 | 1.49 | 16.06 | 65.46 | 49.19 | 4.31 | 46.50 | 189.52 | S6 + AC |
| 25 | 19.76 | 1.40 | 13.67 | 65.17 | 56.73 | 4.02 | 39.25 | 187.11 | S6 + AC |
| 26 | 22.68 | 1.38 | 11.42 | 64.52 | 63.92 | 3.89 | 32.19 | 181.85 | S6 + AC |
| 27 | 25.02 | 1.39 | 9.39 | 64.20 | 69.89 | 3.88 | 26.23 | 179.33 | S6 + AC |
表1 四元体系MgCl2-SrCl2-AlCl3-H2O 298.2 K固液平衡组成
Table 1 The composition of the quaternary system MgCl2-SrCl2-AlCl3-H2O at 298.2 K
| No. | Composition of equilibrium solution, w(B)/% | J?necke index of dry salt, J(B)/(g/100 g salt) | Equilibrated solid phase | ||||||
|---|---|---|---|---|---|---|---|---|---|
| J(MgCl2)+J(SrCl2)+J(AlCl3)=100 | |||||||||
| w(MgCl2) | w(SrCl2) | w(AlCl3) | w(H2O) | J(MgCl2) | J(SrCl2) | J(AlCl3) | J(H2O) | ||
| 1, A | 34.75 | 1.42 | 0.00 | 63.83 | 96.07 | 3.93 | 0.00 | 176.47 | Bis + S2 |
| 2 | 33.98 | 1.33 | 0.83 | 63.86 | 94.02 | 3.68 | 2.30 | 176.70 | Bis + S2 |
| 3 | 32.94 | 1.27 | 1.75 | 64.04 | 91.60 | 3.53 | 4.87 | 178.09 | Bis + S2 |
| 4 | 33.01 | 1.31 | 2.53 | 63.15 | 89.58 | 3.55 | 6.87 | 171.37 | Bis + S2 |
| 5 | 31.87 | 1.33 | 3.50 | 63.30 | 86.84 | 3.62 | 9.54 | 172.48 | Bis + S2 |
| 6 | 30.37 | 1.39 | 4.57 | 63.67 | 83.59 | 3.83 | 12.58 | 175.25 | Bis + S2 |
| 7, E1 | 29.49 | 1.32 | 5.47 | 63.72 | 81.28 | 3.64 | 15.08 | 175.63 | Bis + S2 + AC |
| 8 | 28.80 | 1.23 | 6.19 | 63.78 | 79.51 | 3.40 | 17.09 | 176.09 | S2 + AC |
| 9, E2 | 27.36 | 1.39 | 7.28 | 63.97 | 75.93 | 3.86 | 20.21 | 177.55 | S6 + S2 + AC |
| 10, B | 33.16 | 2.22 | 0.00 | 64.62 | 93.73 | 6.27 | 0.00 | 182.65 | S6 + S2 |
| 11 | 32.01 | 1.98 | 1.84 | 64.17 | 89.33 | 5.53 | 5.14 | 179.10 | S6 + S2 |
| 12 | 30.74 | 2.10 | 2.89 | 64.27 | 86.03 | 5.88 | 8.09 | 179.88 | S6 + S2 |
| 13 | 29.27 | 2.12 | 4.04 | 64.57 | 82.62 | 5.98 | 11.40 | 182.25 | S6 + S2 |
| 14 | 28.45 | 2.11 | 4.90 | 64.54 | 80.23 | 5.95 | 13.82 | 182.01 | S6 + S2 |
| 15 | 28.23 | 1.96 | 5.52 | 64.29 | 79.05 | 5.49 | 15.46 | 180.03 | S6 + S2 |
| 16 | 27.73 | 1.53 | 6.55 | 64.19 | 77.44 | 4.27 | 18.29 | 179.25 | S6 + S2 |
| 17, C | 30.45 | 0.00 | 5.65 | 63.90 | 84.35 | 0.00 | 15.65 | 177.01 | Bis + AC |
| 18 | 30.08 | 0.90 | 5.29 | 63.73 | 82.93 | 2.48 | 14.59 | 175.71 | Bis + AC |
| 19 | 29.46 | 1.14 | 5.36 | 64.04 | 81.92 | 3.17 | 14.91 | 178.09 | Bis + AC |
| 20, D | 0.00 | 1.95 | 30.12 | 67.93 | 0.00 | 6.08 | 93.92 | 211.82 | S6 + AC |
| 21 | 5.34 | 1.82 | 25.76 | 67.08 | 16.22 | 5.53 | 78.25 | 203.77 | S6 + AC |
| 22 | 9.85 | 1.71 | 22.00 | 66.44 | 29.35 | 5.10 | 65.55 | 197.97 | S6 + AC |
| 23 | 13.74 | 1.59 | 18.73 | 65.94 | 40.34 | 4.67 | 54.99 | 193.60 | S6 + AC |
| 24 | 16.99 | 1.49 | 16.06 | 65.46 | 49.19 | 4.31 | 46.50 | 189.52 | S6 + AC |
| 25 | 19.76 | 1.40 | 13.67 | 65.17 | 56.73 | 4.02 | 39.25 | 187.11 | S6 + AC |
| 26 | 22.68 | 1.38 | 11.42 | 64.52 | 63.92 | 3.89 | 32.19 | 181.85 | S6 + AC |
| 27 | 25.02 | 1.39 | 9.39 | 64.20 | 69.89 | 3.88 | 26.23 | 179.33 | S6 + AC |
| No. | J(MgCl2)/ (g/100 g salt) | Density, ρ/ (g/cm3) | Refractive index, nD | No. | J(MgCl2)/ (g/100 g salt) | Density, ρ/ (g/cm3) | Refractive index, nD |
|---|---|---|---|---|---|---|---|
| 1, A | 96.07 | 1.3332 | 1.4339 | 15 | 79.05 | 1.3266 | 1.4324 |
| 2 | 94.02 | 1.3368 | 1.4337 | 16 | 77.44 | 1.3282 | 1.4341 |
| 3 | 91.60 | 1.3352 | 1.4338 | 17, C | 84.35 | 1.3403 | 1.4347 |
| 4 | 89.58 | 1.3329 | 1.4339 | 18 | 82.93 | 1.3266 | 1.4343 |
| 5 | 86.84 | 1.3299 | 1.4338 | 19 | 81.92 | 1.3265 | 1.4341 |
| 6 | 83.59 | 1.3293 | 1.4336 | 20, D | 0.00 | 1.3027 | 1.4346 |
| 7, E1 | 81.28 | 1.3261 | 1.4333 | 21 | 16.22 | 1.3100 | 1.4331 |
| 8 | 79.51 | 1.3295 | 1.4344 | 22 | 29.35 | 1.3133 | 1.4330 |
| 9, E2 | 75.93 | 1.3295 | 1.4350 | 23 | 40.34 | 1.3142 | 1.4330 |
| 10, B | 93.73 | 1.3147 | 1.4301 | 24 | 49.19 | 1.3206 | 1.4331 |
| 11 | 89.33 | 1.3235 | 1.4305 | 25 | 56.73 | 1.3259 | 1.4338 |
| 12 | 86.03 | 1.3241 | 1.4310 | 26 | 63.92 | 1.3286 | 1.4340 |
| 13 | 82.62 | 1.3245 | 1.4312 | 27 | 69.89 | 1.3290 | 1.4342 |
| 14 | 80.23 | 1.3255 | 1.4315 |
表2 四元体系MgCl2-SrCl2-AlCl3-H2O 298.2 K密度ρ、折射率nD数据
Table 2 The density (ρ) and refractive index (nD) of the quaternary system MgCl2-SrCl2-AlCl3-H2O at 298.2 K
| No. | J(MgCl2)/ (g/100 g salt) | Density, ρ/ (g/cm3) | Refractive index, nD | No. | J(MgCl2)/ (g/100 g salt) | Density, ρ/ (g/cm3) | Refractive index, nD |
|---|---|---|---|---|---|---|---|
| 1, A | 96.07 | 1.3332 | 1.4339 | 15 | 79.05 | 1.3266 | 1.4324 |
| 2 | 94.02 | 1.3368 | 1.4337 | 16 | 77.44 | 1.3282 | 1.4341 |
| 3 | 91.60 | 1.3352 | 1.4338 | 17, C | 84.35 | 1.3403 | 1.4347 |
| 4 | 89.58 | 1.3329 | 1.4339 | 18 | 82.93 | 1.3266 | 1.4343 |
| 5 | 86.84 | 1.3299 | 1.4338 | 19 | 81.92 | 1.3265 | 1.4341 |
| 6 | 83.59 | 1.3293 | 1.4336 | 20, D | 0.00 | 1.3027 | 1.4346 |
| 7, E1 | 81.28 | 1.3261 | 1.4333 | 21 | 16.22 | 1.3100 | 1.4331 |
| 8 | 79.51 | 1.3295 | 1.4344 | 22 | 29.35 | 1.3133 | 1.4330 |
| 9, E2 | 75.93 | 1.3295 | 1.4350 | 23 | 40.34 | 1.3142 | 1.4330 |
| 10, B | 93.73 | 1.3147 | 1.4301 | 24 | 49.19 | 1.3206 | 1.4331 |
| 11 | 89.33 | 1.3235 | 1.4305 | 25 | 56.73 | 1.3259 | 1.4338 |
| 12 | 86.03 | 1.3241 | 1.4310 | 26 | 63.92 | 1.3286 | 1.4340 |
| 13 | 82.62 | 1.3245 | 1.4312 | 27 | 69.89 | 1.3290 | 1.4342 |
| 14 | 80.23 | 1.3255 | 1.4315 |
| System | β(0) | β(1) | Cf | Ref. |
|---|---|---|---|---|
| MgCl2-H2O | 0.3511 | 1.6512 | 0.0065 | [ |
| AlCl3-H2O | 0.7153 | 5.6509 | –0.0042 | [ |
| SrCl2-H2O | 0.2827 | 1.5625 | –0.0023 | [ |
表3 二元体系MgCl2-H2O、SrCl2-H2O和AlCl3-H2O 298.2 K单盐参数
Table 3 Pitzer single-salt parameters of the systems MgCl2-H2O, SrCl2-H2O and AlCl3-H2O at 298.2 K
| System | β(0) | β(1) | Cf | Ref. |
|---|---|---|---|---|
| MgCl2-H2O | 0.3511 | 1.6512 | 0.0065 | [ |
| AlCl3-H2O | 0.7153 | 5.6509 | –0.0042 | [ |
| SrCl2-H2O | 0.2827 | 1.5625 | –0.0023 | [ |
| C | C′ | θCC′ | ψCC′Cl | Ref. |
|---|---|---|---|---|
| Mg2+ | Sr2+ | –0.1965 | 0.0305 | [ |
| Sr2+ | Al3+ | 0.1244 | –0.0161 | [ |
| Mg2+ | Al3+ | 0.0341 | –0.0053 | this work |
表4 四元体系MgCl2-SrCl2-AlCl3-H2O 298.2 K混合离子作用参数
Table 4 Pitzer mixing ion-interaction parameters of the system MgCl2-SrCl2-AlCl3-H2O at 298.2 K
| C | C′ | θCC′ | ψCC′Cl | Ref. |
|---|---|---|---|---|
| Mg2+ | Sr2+ | –0.1965 | 0.0305 | [ |
| Sr2+ | Al3+ | 0.1244 | –0.0161 | [ |
| Mg2+ | Al3+ | 0.0341 | –0.0053 | this work |
| Species | ln Ksp | Ref. |
|---|---|---|
| MgCl2·6H2O | 10.60 | [ |
| AlCl3·6H2O | 16.05 | [ |
| SrCl2·6H2O | 4.33 | [ |
| SrCl2·2H2O | 8.46 | [ |
表5 四元体系MgCl2-SrCl2-AlCl3-H2O 298.2 K中各盐溶度积常数(lnKsp)
Table 5 Solubility product parameters (lnKsp) of the quaternary system MgCl2-SrCl2-AlCl3-H2O at 298.2 K
| Species | ln Ksp | Ref. |
|---|---|---|
| MgCl2·6H2O | 10.60 | [ |
| AlCl3·6H2O | 16.05 | [ |
| SrCl2·6H2O | 4.33 | [ |
| SrCl2·2H2O | 8.46 | [ |
| No. | J?necke index of dry salt, J(B)/(g/100 g) | No. | J?necke index of dry salt, J(B)/(g/100 g) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| J(MgCl2) | J(SrCl2) | J(AlCl3) | J(H2O) | J(MgCl2) | J(SrCl2) | J(AlCl3) | J(H2O) | ||
| 1, C | 84.56 | 0.00 | 15.44 | 177.70 | 13 | 86.50 | 6.11 | 7.39 | 184.64 |
| 2 | 83.93 | 0.84 | 15.23 | 176.91 | 14 | 79.34 | 5.80 | 14.86 | 185.78 |
| 3, E1 | 83.04 | 2.03 | 14.93 | 175.78 | 15 | 72.02 | 5.54 | 22.44 | 186.98 |
| 4 | 69.91 | 3.54 | 26.55 | 183.55 | 16 | 64.58 | 5.30 | 30.12 | 188.25 |
| 5, E2 | 55.87 | 5.07 | 39.06 | 189.78 | 17, E2 | 55.87 | 5.07 | 39.06 | 189.78 |
| 6, A | 97.13 | 2.87 | 0.00 | 176.52 | 18 | 46.27 | 5.34 | 48.39 | 194.40 |
| 7 | 93.81 | 2.66 | 3.53 | 176.43 | 19 | 37.76 | 5.50 | 56.74 | 198.29 |
| 8 | 90.49 | 2.46 | 7.05 | 176.30 | 20 | 28.88 | 5.63 | 65.49 | 202.19 |
| 9 | 87.16 | 2.27 | 10.57 | 176.10 | 21 | 19.63 | 5.71 | 74.66 | 206.14 |
| 10 | 83.85 | 2.08 | 14.07 | 175.85 | 22 | 10.01 | 5.75 | 84.24 | 210.16 |
| 11, E1 | 83.04 | 2.03 | 14.93 | 175.78 | 23, D | 0.00 | 5.76 | 94.24 | 214.27 |
| 12, B | 93.54 | 6.46 | 0.00 | 183.54 | |||||
表6 四元体系MgCl2-SrCl2-AlCl3-H2O 298.2 K溶解度计算值
Table 6 Calculated solubility of quaternary system MgCl2-SrCl2-AlCl3-H2O at 298.2 K
| No. | J?necke index of dry salt, J(B)/(g/100 g) | No. | J?necke index of dry salt, J(B)/(g/100 g) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| J(MgCl2) | J(SrCl2) | J(AlCl3) | J(H2O) | J(MgCl2) | J(SrCl2) | J(AlCl3) | J(H2O) | ||
| 1, C | 84.56 | 0.00 | 15.44 | 177.70 | 13 | 86.50 | 6.11 | 7.39 | 184.64 |
| 2 | 83.93 | 0.84 | 15.23 | 176.91 | 14 | 79.34 | 5.80 | 14.86 | 185.78 |
| 3, E1 | 83.04 | 2.03 | 14.93 | 175.78 | 15 | 72.02 | 5.54 | 22.44 | 186.98 |
| 4 | 69.91 | 3.54 | 26.55 | 183.55 | 16 | 64.58 | 5.30 | 30.12 | 188.25 |
| 5, E2 | 55.87 | 5.07 | 39.06 | 189.78 | 17, E2 | 55.87 | 5.07 | 39.06 | 189.78 |
| 6, A | 97.13 | 2.87 | 0.00 | 176.52 | 18 | 46.27 | 5.34 | 48.39 | 194.40 |
| 7 | 93.81 | 2.66 | 3.53 | 176.43 | 19 | 37.76 | 5.50 | 56.74 | 198.29 |
| 8 | 90.49 | 2.46 | 7.05 | 176.30 | 20 | 28.88 | 5.63 | 65.49 | 202.19 |
| 9 | 87.16 | 2.27 | 10.57 | 176.10 | 21 | 19.63 | 5.71 | 74.66 | 206.14 |
| 10 | 83.85 | 2.08 | 14.07 | 175.85 | 22 | 10.01 | 5.75 | 84.24 | 210.16 |
| 11, E1 | 83.04 | 2.03 | 14.93 | 175.78 | 23, D | 0.00 | 5.76 | 94.24 | 214.27 |
| 12, B | 93.54 | 6.46 | 0.00 | 183.54 | |||||
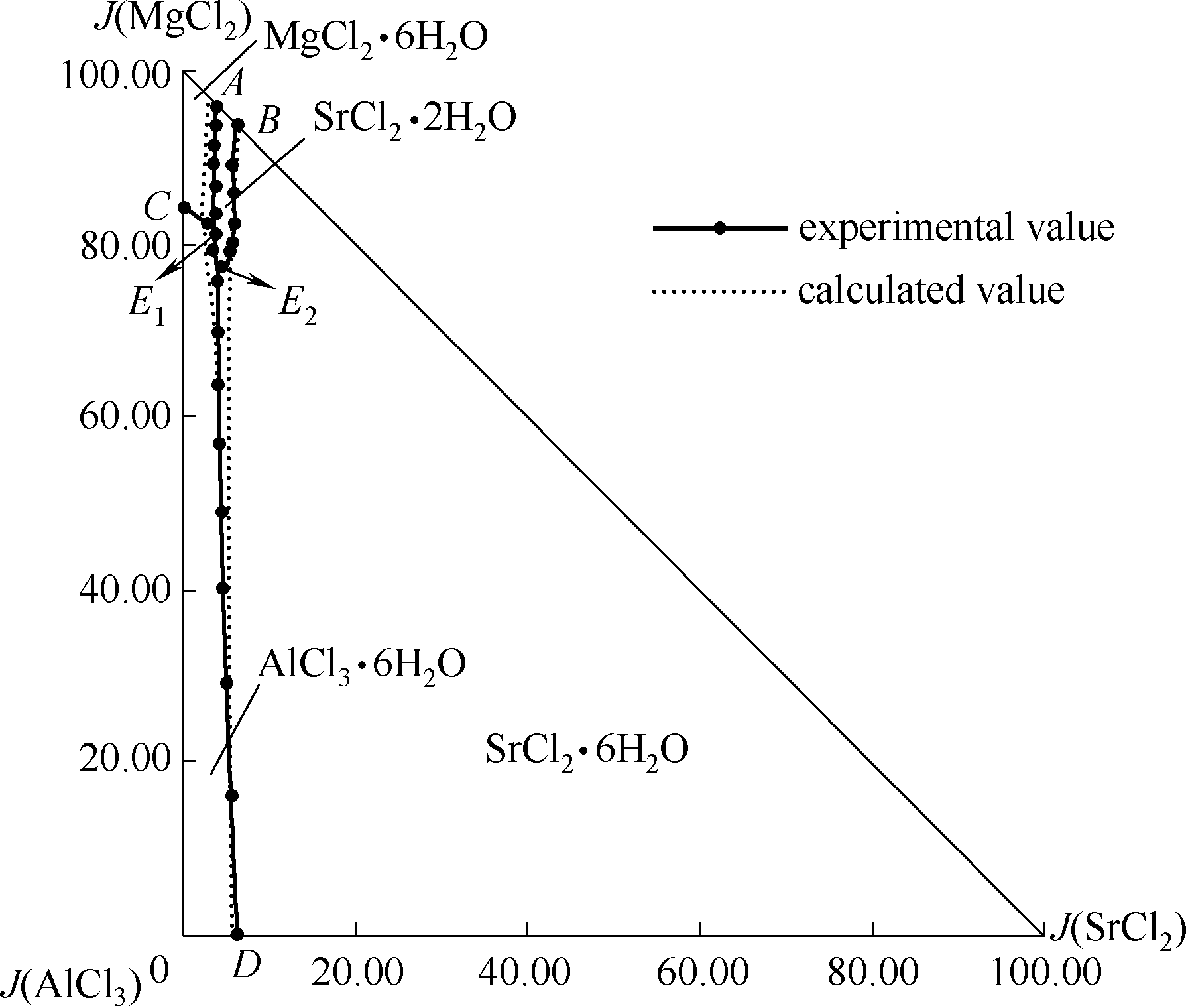
图8 四元体系MgCl2-SrCl2-AlCl3-H2O 298.2 K计算与实验相图
Fig.8 Calculated and experimental phase diagrams of the quaternary system MgCl2-SrCl2-AlCl3-H2O at 298.2 K
| 1 | 韩松昊, 税鹏, 余超, 等. 中国锶资源现状及可持续发展建议[J]. 科技通报, 2018, 34(1): 1-5. |
| Han S H, Shui P, Yu C, et al. Present situation and sustainable development suggestion of Chinese strontium resource industry[J]. Bull. Sci. Technol., 2018, 34(1): 1-5. | |
| 2 | 李波, 段东平, 王树轩, 等. 以碳酸锶废渣为原料制备高纯氯化锶[J]. 盐湖研究, 2013, 21(2): 58-61. |
| Li B, Duan D P, Wang S X, et al. The preparation of high purity strontium chloride from strontium carbonate waste[J]. J. Salt Lake Res., 2013, 21(2): 58-61. | |
| 3 | 曾英, 陈佩君, 于旭东. 四元体系Rb+, Cs+, Mg2+ // SO42- - H2O 298.2 K相平衡研究[J]. 化工学报, 2020, 71(8): 3460-3468. |
| Zeng Y, Chen P J, Yu X D. Phase equilibria of quaternary system Rb+, Cs+, Mg2+ // SO42- - H2O at T=298.2 K[J]. CIESC Journal, 2020, 71(8): 3460-3468. | |
| 4 | 聂国亮, 桑世华, 崔瑞芝. 298 K 和323 K条件下五元体系NaBr-KBr-MgBr2-CaBr2-H2O相平衡研究[J]. 化工学报, 2019, 70(9): 3267-3274. |
| Nie G L, Sang S H, Cui R Z. Phase equilibria in quinary system NaBr-KBr-MgBr2-CaBr2-H2O at 298 K and 323 K[J]. CIESC Journal, 2019, 70(9): 3267-3274. | |
| 5 | 任永胜, 曹晶, 于冰洁. 313.15 K四元体系Na+ // SO42-, CO32-, NO3- - H2O固液相平衡研究[J]. 化工学报, 2019, 70(6): 2102-2109. |
| Ren Y S, Cao J, Yu B J. Solid-liquid equilibria of quaternary system Na+ // SO42-, CO32-, NO3- - H2O at 313.15 K[J]. CIESC Journal, 2019, 70(6): 2102-2109. | |
| 6 | Yu X D, Zheng M P, Zeng Y, et al. Solid-liquid equilibrium of quinary aqueous solution composed of lithium, potassium, rubidium, magnesium, and borate at 323.15 K[J]. J. Chem. Eng. Data, 2019, 64(12): 5681-5687. |
| 7 | Wang L, Yu X D, Li M L, et al. Phase equilibrium for the aqueous ternary systems NH4+, Sr2+(Ca2+)//Cl- - H2O at T = 298 K[J]. J. Chem. Eng. Jpn., 2018, 51(7): 551-555. |
| 8 | Dong O Y, Zeng D W, Zhou H Y, et al. Phase change materials in the ternary system NH4Cl+CaCl2+H2O[J]. Calphad: Comput. Coupling Phase Diagrams Thermochem., 2011, 35(3): 269-275. |
| 9 | Zhang R Z, Yang J M, Zhang L, et al. The phase equilibriums in the NH4Cl-CaCl2-H2O system at 50 and 75℃ and their Pitzer model representations[J]. Russ. J. Phys. Chem. A, 2014, 88(13): 2325-2330. |
| 10 | Li X, Yuan J S, Ji Z Y, et al. Phase equilibrium of the ternary system of NH4Cl-CaCl2-H2O at 50℃[J]. Front. Chem. Eng. China, 2010, 4(1): 75-77. |
| 11 | Yu X D, Zeng Y, Zhang Z X. Solid-liquid metastable phase equilibria in the ternary systems KCl+NH4Cl+H2O and NH4Cl+MgCl2+H2O at 298.15 K[J]. J. Chem. Eng. Data, 2012, 57(6): 1759-1765. |
| 12 | Yu X D, Wang L, Chen J, et al. Salt-water phase equilibria in ternary systems K+(Mg2+), NH4+ // Cl- - H2O at T = 273 K[J]. J. Chem. Eng. Data, 2017, 62(4): 1427-1432. |
| 13 | Yuan M X, Qiao X C, Yu J G. Phase equilibria of AlCl3+FeCl3+H2O, AlCl3+CaCl2+H2O, and FeCl3+CaCl2+H2O at 298.15 K[J]. J. Chem. Eng. Data, 2016, 61(5): 1749-1755. |
| 14 | 袁梦霞, 乔秀臣. 三元体系AlCl3+CaCl2+H2O, AlCl3+FeCl3+H2O, CaCl2+FeCl3+H2O在35℃的相平衡[J]. 化工学报, 2017, 68(7): 2653-2659. |
| Yuan M X, Qiao X C. Phase equilibria of AlCl3+CaCl2+H2O, AlCl3+FeCl3+H2O and CaCl2+FeCl3+H2O ternary systems at 35℃[J]. CIESC Journal, 2017, 68(7): 2653-2659. | |
| 15 | Li D C, Zhang Z Y, Fan R, et al. Solid-liquid phase equilibria in the aqueous systems (CaCl2+SrCl2+H2O) and (NaCl+CaCl2+SrCl2+H2O) at 288.15 K[J]. J. Chem. Eng. Data, 2019, 64(6): 2767-2773. |
| 16 | Guo L J, Zeng D W, Yao Y, et al. Isopiestic measurement and solubility evaluation of the ternary system (CaCl2+SrCl2+H2O) at T=298.15 K[J]. J. Chem. Thermodyn., 2013, 63: 60-66. |
| 17 | Gao Y Y, Ye C, Zhang W Y, et al. Phase equilibria in the ternary system CaCl2-SrCl2-H2O and the quaternary system KCl-CaCl2-SrCl2-H2O at 373 K[J]. J. Chem. Eng. Data, 2018, 63(8): 2738-2742. |
| 18 | 毕玉敬, 孙柏, 赵静, 等. 25℃时三元体系SrCl2-CaCl2-H2O相平衡研究[J]. 无机化学学报, 2011, 27(9): 1765-1771. |
| Bi Y J, Sun B, Zhao J, et al. Phase equilibrium in ternary system SrCl2-CaCl2-H2O at 25℃[J]. Chin. J. Inorg. Chem., 2011, 27(9): 1765-1771. | |
| 19 | Zheng Q F, Wang L, Zheng H, et al. Solid-liquid equilibria and Pitzer model simulation of the SrCl2-NH4Cl-MgCl2-H2O quaternary system at T=298 K[J]. J. Chem. Eng. Data, 2018, 63(12): 4606-4613. |
| 20 | Gao W C, Li Z B. A practical approach to produce Mg-Al spinel based on the modeling of phase equilibria for NH4Cl-MgCl2-AlCl3-H2O system[J]. AIChE J., 2013, 59(6): 1855-1867. |
| 21 | Yu X D, Liu M, Zheng Q F, et al. Measurement and correlation of phase equilibria of ammonium, calcium, aluminum, and chloride in aqueous solution at 298.15 K[J]. J. Chem. Eng. Data, 2019, 64(8): 3514-3520. |
| 22 | 王林, 郑秋风, 刘敏, 等. 三元体系SrCl2 + MgCl2 + H2O 298 K相平衡测定及计算[J]. 高校化学工程学报, 2019, 33(4): 800-807. |
| Wang L, Zheng Q F, Liu M, et al. Phase equilibrium measurements and simulation of the SrCl2 + MgCl2 + H2O ternary system at 298 K[J]. J. Chem. Eng. Chin. Univ., 2019, 33(4): 800-807. | |
| 23 | Yu X D, Zheng Q F, Wang L, et al. Solid-liquid phase equilibrium determination and correlation of ternary systems NH4Cl + AlCl3 + H2O, MgCl2 + AlCl3 + H2O and SrCl2 + AlCl3 + H2O at 298 K[J]. Fluid Phase Equilib., 2020, 507: 112426. |
| 24 | 中国科学院青海盐湖研究所. 卤水和盐的分析方法[M]. 2版. 北京: 科学出版社, 1988: 69-72. |
| Institute of Qinghai Salt-Lake of Chinese Academy of Sciences. Analytical Methods of Brines and Salts [M]. 2nd ed. Beijing: Chinese Science Press, 1988: 69-72. | |
| 25 | 袁艺, 罗维, 钟世华,等. 铝及铝合金化学分析方法第16部分: 镁含量的测定: GB/T 20975.16-2008[S]. 北京: 中国标准出版社, 2008. |
| Yuan Y, Luo W, Zhong S H, et al. Methods for chemical analysis of aluminium and aluminium alloys-part16: determination of magnesium: GB/T 20975.16—2008[S]. Beijing: China Standards Press, 2008. | |
| 26 | 冯颖新, 杨宇宏, 田光,等. 铝及铝合金化学分析方法第17部分: 锶含量的测定 火焰原子吸收光谱法: GB/T 20975.17—2008[S]. 北京: 中国标准出版社, 2008. |
| Feng Y Y, Yang Y H, Tian G, et al. Methods for chemical analysis of aluminium and aluminium alloys - part17: determination of strontium content — flame atomic absorption spectrometric method: GB/T 20975.17—2008[S]. Beijing: China Standards Press, 2008. | |
| 27 | 李跃平, 石磊, 张树朝,等. 铝及铝合金化学分析方法第25部分: 电感耦合等离子体原子发射光谱法: GB/T 20975.25—2008[S]. 北京: 中国标准出版社, 2008. |
| Li Y P, Shi L, Zhang S C, et al. Methods for chemical analysis of aluminium and aluminium alloys-part 25: inductively coupled plasma atomic emission spectrometric method: GB/T 20975.25—2008[S]. Beijing: China Standards Press, 2008. | |
| 28 | 牛自得, 程芳琴, 李宝存, 等. 水盐体系相图及其应用[M]. 天津: 天津大学出版社, 2002: 84-85. |
| Niu Z D, Cheng F Q, Li B C, et al. Salt-Water System Phase Diagrams and Applications[M]. Tianjin: Tianjin University Press, 2002: 84-85. | |
| 29 | Assarsson G O, Balder A. Equilibria between 18 and 100° in the aqueous ternary system containing Sr2+, Mg2+ and Cl-[J]. J. Phys. Chem., 1954, 58(2): 416. |
| 30 | Nie G L, Sang S H, Cui R Z, et al. Measurements and calculations of solid-liquid equilibria in two quaternary systems: LiCl-NaCl-SrCl2-H2O and LiCl-KCl-SrCl2-H2O at 298 K[J]. Fluid Phase Equilib., 2020, 509: 112458. |
| 31 | 崔瑞芝, 李武, 董亚萍, 等. 298 K四元体系LiCl-MgCl2-CaCl2-H2O相平衡实验及溶解度计算[J]. 化工学报, 2018, 69(10): 4148-4155. |
| Cui R Z, Li W, Dong Y P, et al. Measurements and calculations of solid-liquid equilibria in quaternary system LiCl-MgCl2-CaCl2-H2O at 298 K[J]. CIESC Journal, 2018, 69(10): 4148-4155. | |
| 32 | 宋彭生, 董亚萍, 李武. Li+, Na+, K+ // Cl-, SO42- - H2O五元体系25℃相图及应用[J]. 盐湖研究, 2017, 25(3): 9-17. |
| Song P S, Dong Y P, Li W. Phase diagram of Li+, Na+, K+ // Cl-, SO42- - H2O system and its utilization[J]. J. Salt Lake Res., 2017, 25(3): 9-17. | |
| 33 | Pitzer K S. Thermodynamics of electrolytes(Ⅰ): Theoretical basis and general equations[J]. J. Phys. Chem., 1973, 77(2): 268-277. |
| 34 | Pitzer K S, Mayorga G. Thermodynamics of electrolytes(Ⅱ): Activity and osmotic coefficients for strong electrolytes with one or both ions univalent[J]. J. Phys. Chem., 1973, 77(19): 2300-2308. |
| 35 | Pitzer K S. Activity Coefficients in Electrolyte Solutions[M]. 2nd ed. London: CRC Press, 1992. |
| 36 | Greenberg J P, Møller N. The prediction of mineral solubilities in natural waters: a chemical equilibrium model for the Na-K-Ca-Cl-SO4-H2O system to high concentration from 0 to 250 °C[J]. Geochim. Cosmochim. Acta, 1989, 53(10): 2503-2518. |
| 37 | Christov C. Thermodynamics of formation of double salts and mixed crystals from aqueous solutions[J]. J. Chem. Thermodyn., 2005, 37(10): 1036-1060. |
| 38 | Christov C. Thermodynamic study of the K-Mg-Al-Cl-SO4-H2O system at the temperature 298.15 K[J]. Calphad: Comput. Coupling Phase Diagrams Thermochem., 2001, 25(3): 445-454. |
| 39 | Clegg S L, Rard J A, Miller D G. Isopiestic determination of the osmotic and activity coefficients of NaCl+SrCl2+H2O at 298.15 K and representation with an extended ion-interaction model[J]. J. Chem. Eng. Data, 2005, 50(4): 1162-1170. |
| 40 | Michael S. Thermodynamic properties of SrCl2(aq) from 252 K to 524 K and phase equilibria in the SrCl2-H2O system: implications for thermochemical heat storage[J]. J. Chem. Thermodyn., 2018, 120: 106-115. |
| [1] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [2] | 于旭东, 李琪, 陈念粗, 杜理, 任思颖, 曾英. 三元体系KCl + CaCl2 + H2O 298.2、323.2及348.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(8): 3256-3265. |
| [3] | 龙臻, 王谨航, 任俊杰, 何勇, 周雪冰, 梁德青. 离子液体协同PVCap抑制天然气水合物生成实验研究[J]. 化工学报, 2023, 74(6): 2639-2646. |
| [4] | 姚晓宇, 沈俊, 李健, 李振兴, 康慧芳, 唐博, 董学强, 公茂琼. 流体气液临界参数测量方法研究进展[J]. 化工学报, 2023, 74(5): 1847-1861. |
| [5] | 徐文超, 孙志高, 李翠敏, 李娟, 黄海峰. 静态条件下表面活性剂E-1310对HCFC-141b水合物生成的影响[J]. 化工学报, 2023, 74(5): 2179-2185. |
| [6] | 陈科, 杜理, 曾英, 任思颖, 于旭东. 四元体系LiCl+MgCl2+CaCl2+H2O 323.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(5): 1896-1903. |
| [7] | 龙臻, 王谨航, 何勇, 梁德青. 离子液体与动力学抑制剂作用下混合气体水合物生成特性研究[J]. 化工学报, 2023, 74(4): 1703-1711. |
| [8] | 李明川, 樊栓狮, 徐赋海, 卢惠东, 李晓军. 水合物热分解Stefan相变模型解的存在性及Laplace变换求解[J]. 化工学报, 2023, 74(4): 1746-1754. |
| [9] | 胡晗, 杨亮, 李春晓, 刘道平. 天然烟浸滤液水合物法储甲烷动力学研究[J]. 化工学报, 2023, 74(3): 1313-1321. |
| [10] | 毛元敬, 杨智, 莫松平, 郭浩, 陈颖, 罗向龙, 陈健勇, 梁颖宗. C6~C10烷醇的SAFT-VR Mie状态方程参数回归及其热物性研究[J]. 化工学报, 2023, 74(3): 1033-1041. |
| [11] | 高靖博, 孙强, 李青, 王逸伟, 郭绪强. 考虑水合物结构转变的含氢气体水合物相平衡模型[J]. 化工学报, 2023, 74(2): 666-673. |
| [12] | 程文婷, 李杰, 徐丽, 程芳琴, 刘国际. AlCl3·6H2O在FeCl3、CaCl2、KCl及KCl–FeCl3溶液中溶解度的实验及预测[J]. 化工学报, 2023, 74(2): 642-652. |
| [13] | 蔡进, 王晓辉, 汤涵, 陈光进, 孙长宇. TBAB水溶液体系中半笼型水合物的相平衡预测模型[J]. 化工学报, 2023, 74(1): 408-415. |
| [14] | 杨松涛, 李东洋, 牛玉清, 李鑫钢, 康绍辉, 李洪, 叶开凯, 周志全, 高鑫. 氟化物势能函数和热力学性质的分子模拟研究进展[J]. 化工学报, 2022, 73(9): 3828-3840. |
| [15] | 周桓, 张梦丽, 郝晴, 吴思, 李杰, 徐存兵. 硫酸镁型光卤石转化钾盐镁矾的过程机制与动态规律[J]. 化工学报, 2022, 73(9): 3841-3850. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号