化工学报 ›› 2021, Vol. 72 ›› Issue (4): 1885-1894.DOI: 10.11949/0438-1157.20201162
王东博1( ),张蕾蕾1,翟晶焕1,张丽娟1,刘锡建1,朱雪焱2,陆杰1(
),张蕾蕾1,翟晶焕1,张丽娟1,刘锡建1,朱雪焱2,陆杰1( )
)
收稿日期:2020-08-17
修回日期:2020-12-18
出版日期:2021-04-05
发布日期:2021-04-05
通讯作者:
陆杰
作者简介:王东博(1995—),男,硕士研究生,基金资助:
WANG Dongbo1( ),ZHANG Leilei1,ZHAI Jinghuan1,ZHANG Lijuan1,LIU Xijian1,ZHU Xueyan2,LU Jie1(
),ZHANG Leilei1,ZHAI Jinghuan1,ZHANG Lijuan1,LIU Xijian1,ZHU Xueyan2,LU Jie1( )
)
Received:2020-08-17
Revised:2020-12-18
Online:2021-04-05
Published:2021-04-05
Contact:
LU Jie
摘要:
实验测定了DL-及D-青霉胺在273.15~333.15 K、不同质量比乙醇-水混合溶剂中的溶解度,以此确定了合适的结晶溶剂比例。绘制了273.15、283.15、293.15、303.15、313.15和323.15 K下青霉胺溶解度曲线。测定了D-和DL-青霉胺以及ee值为87%溶液的介稳区宽度。研究了不同ee值饱和溶液(饱和温度为313.15 K)在不同初始过冷度下起始成核情况及ee值为87%的饱和溶液在不同初始过冷度和晶种量下的等温结晶过程。最后考察了不同降温速率、晶种量对ee值为87%原料的结晶拆分情况。结果表明,对于等温结晶过程,随着结晶进行,产品ee值下降,且初始过冷度越大、晶种加入量越多,ee>99%的产品收率越高;对于降温结晶过程,产品ee值随收率增大而减小,且降温速率越慢、晶种加入量越多,ee>99%的产品收率越高。
中图分类号:
王东博, 张蕾蕾, 翟晶焕, 张丽娟, 刘锡建, 朱雪焱, 陆杰. 青霉胺结晶热力学及拆分工艺研究[J]. 化工学报, 2021, 72(4): 1885-1894.
WANG Dongbo, ZHANG Leilei, ZHAI Jinghuan, ZHANG Lijuan, LIU Xijian, ZHU Xueyan, LU Jie. Study on the crystallization thermodynamics and chiral resolution of penicillamine[J]. CIESC Journal, 2021, 72(4): 1885-1894.

图3 D-和DL-青霉胺原料与固液平衡后残余固体的X射线衍射谱图a—DL-残余固体;b—DL-原料;c—D-残余固体;d—D-原料
Fig.3 XRD patterns of the raw materials and the residual solids after solid-liquid equilibrium of D- and DL-penicillamine
| 晶种量/% | 产品收率 | ||
|---|---|---|---|
| ΔT=6 K | ΔT=10 K | ΔT=15 K | |
| 1 | 13.47% | 19.67% | 21.03% |
| 2 | 14.27% | 21.65% | 22.31% |
| 4 | 15.14% | 23.86% | 24.74% |
| 8 | 16.21% | 24.62% | 25.35% |
表1 青霉胺恒温结晶收率
Table 1 Yield of penicillamine of thermostatic crystallization
| 晶种量/% | 产品收率 | ||
|---|---|---|---|
| ΔT=6 K | ΔT=10 K | ΔT=15 K | |
| 1 | 13.47% | 19.67% | 21.03% |
| 2 | 14.27% | 21.65% | 22.31% |
| 4 | 15.14% | 23.86% | 24.74% |
| 8 | 16.21% | 24.62% | 25.35% |
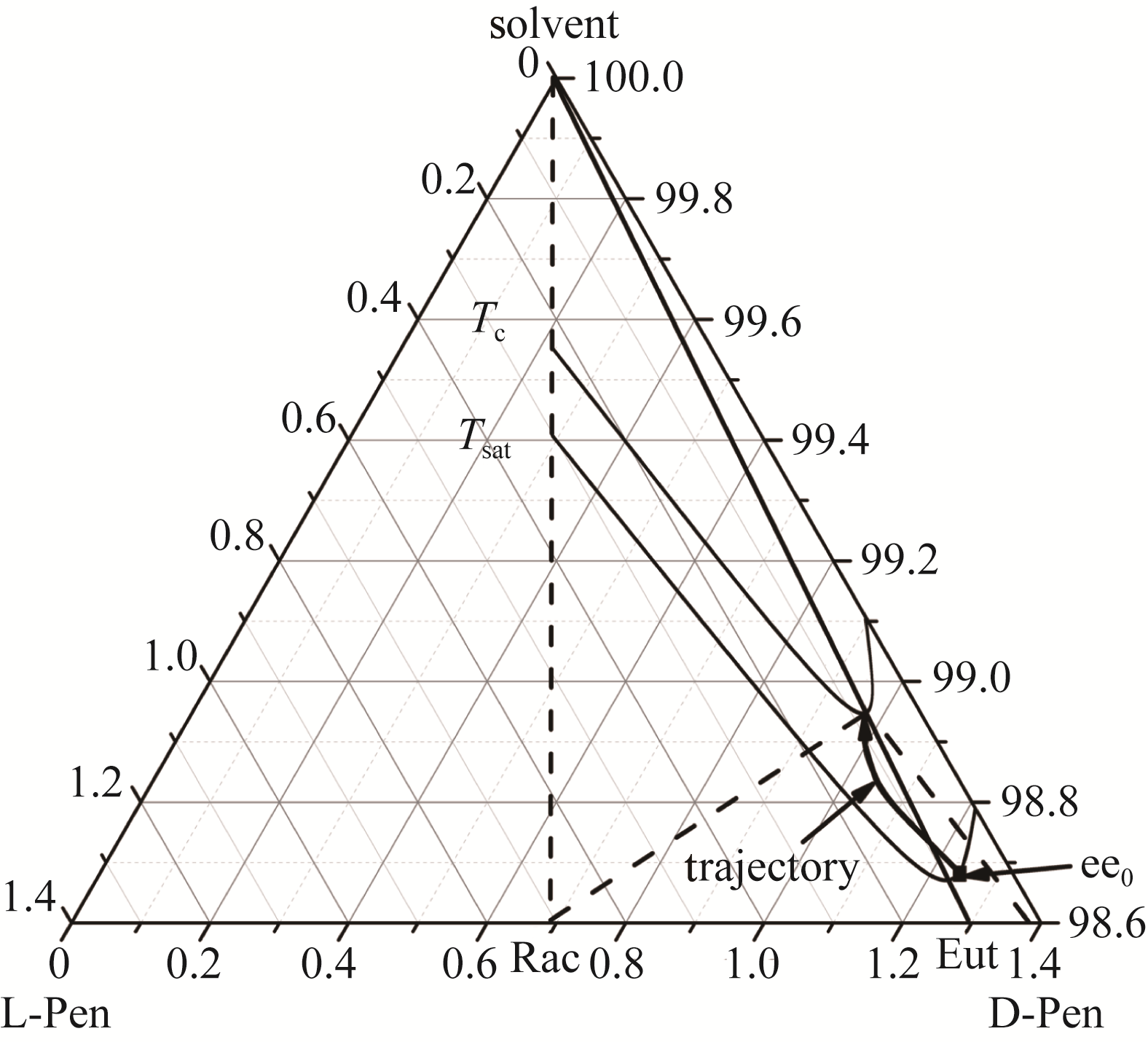
图10 青霉胺恒温结晶过程Tc—结晶温度303.15 K;Tsat—饱和溶液温度313.15 K;Eut—二元共饱和点曲线;ee0—初始饱和溶液的ee值;trajectory—结晶过程曲线
Fig.10 The thermostatic crystallization process of penicillamine
| 晶种量/% | 产品收率 | |||
|---|---|---|---|---|
| 0.05 K/min | 0.1 K/min | 0.2 K/min | 0.4 K/min | |
| 1 | 13.04% | 12.56% | 10.31% | 8.18% |
| 2 | 20.23% | 19.13% | 17.54% | 12.53% |
| 4 | 24.91% | 24.05% | 21.43% | 14.86% |
| 8 | 25.97% | 25.28% | 22.89% | 15.94% |
表2 青霉胺降温结晶收率
Table 2 Yield of penicillamine of cooling crystallization
| 晶种量/% | 产品收率 | |||
|---|---|---|---|---|
| 0.05 K/min | 0.1 K/min | 0.2 K/min | 0.4 K/min | |
| 1 | 13.04% | 12.56% | 10.31% | 8.18% |
| 2 | 20.23% | 19.13% | 17.54% | 12.53% |
| 4 | 24.91% | 24.05% | 21.43% | 14.86% |
| 8 | 25.97% | 25.28% | 22.89% | 15.94% |
| 1 | Stinson S C. Chiral drug market shows signs of maturity[J]. Chemical & Engineering News, 1997, 75(42): 38-70. |
| 2 | 黄蓓, 杨立荣, 吴坚平. 手性拆分技术的工业应用[J]. 化工进展, 2002, 21(6): 375-380. |
| Huang B, Yang L R, Wu J P. Chiral resolution techniques in industrial practice[J]. Chemical Industry and Engineering Progress, 2002, 21(6): 375-380. | |
| 3 | 王耀国, 赵绍磊, 杨一纯, 等. 手性药物结晶拆分的研究进展[J]. 化工学报, 2019, 70(10): 3651-3662. |
| Wang Y G, Zhao S L, Yang Y C, et al. Recent progress on chiral resolution of pharmaceuticals by crystallization[J]. CIESC Journal, 2019, 70(10): 3651-3662. | |
| 4 | Anderson K C. Dark remedy: the impact of thalidomide and its revival as a vital medicine[J]. Nature Medicine, 2001, 7: 275-276. |
| 5 | He M, Zhao S L, Chen J. Chiral separation of penicillamine enantiomers by capillary electrophoresis and its application[J]. Chinese Journal of Analytical Chemistry, 2006, 34(5): 655-658. |
| 6 | Qureshi M J, Kumar M. D-Penicillamine for preventing retinopathy of prematurity in preterm infants[J]. The Cochrane Database of Systematic Reviews, 2013, (9): CD001073. |
| 7 | Kean W F, Howard-Lock H E, Lock C J L. Chirality in antirheumatic drugs[J]. The Lancet, 1991, 338(8782/8783): 1565-1568. |
| 8 | Barve I J, Chen L H, Wei P C P, et al. Enantioselective synthesis of (-)-(R) Silodosin by ultrasound-assisted diastereomeric crystallization[J]. Tetrahedron, 2013, 69(13): 2834-2843. |
| 9 | Sistla V S, Langermann J V, Lorenz H, et al. Analysis and comparison of commonly used acidic resolving agents in diastereomeric salt resolution—examples for DL-serine[J]. Crystal Growth & Design, 2011, 11(9): 3761-3768. |
| 10 | Hein J E, Cao B H, van der Meijden M W, et al. Resolution of omeprazole using coupled preferential crystallization: efficient separation of a nonracemizable conglomerate salt under near-equilibrium conditions[J]. Organic Process Research & Development, 2013, 17(6): 946-950. |
| 11 | Ye X, Cui J, Li B, et al. Self-reporting inhibitors: a single crystallization process to obtain two optically pure enantiomers[J]. Angewandte Chemie (International Edition), 2018, 57(27): 8120-8124. |
| 12 | Ye X C, Cui J X, Li B W, et al. Enantiomer-selective magnetization of conglomerates for quantitative chiral separation[J]. Nature Communications, 2019, 10(1): 1964. |
| 13 | Takahashi H, Iwama S, Clevers S, et al. In situ observation of polymorphic transition during crystallization of organic compounds showing preferential enrichment by means of temperature-controlled video-microscopy and time-resolved X-ray powder diffraction[J]. Crystal Growth & Design, 2017, 17(2): 671-676. |
| 14 | Iwama S, Horiguchi M, Sato H, et al. Observation of the preferential enrichment phenomenon for essential α-amino acids with a racemic crystal structure[J]. Crystal Growth & Design, 2010, 10(6): 2668-2675. |
| 15 | Engwerda A H J, Koning N, Tinnemans P, et al. Deracemization of a racemic allylic sulfoxide using Viedma ripening[J]. Crystal Growth & Design, 2017, 17(8): 4454-4457. |
| 16 | Spix L, Meekes H, Blaauw R H, et al. Complete deracemization of proteinogenic glutamic acid using Viedma ripening on a metastable conglomerate[J]. Crystal Growth & Design, 2012, 12(11): 5796-5799. |
| 17 | Steendam R R, Brouwer M C, Huijs E M, et al. Enantiopure isoindolinones through Viedma ripening[J]. Chemistry, 2014, 20(42): 13527-13530. |
| 18 | Svang-Ariyaskul A, Koros W J, Rousseau R W. Chiral purification of glutamic acid enantiomers using a size-selective barrier membrane and dual-vessel crystallization[J]. Chemical Engineering Science, 2012, 77: 35-41. |
| 19 | Shiraiwa T, Kubo M, Watanabe M, et al. Optical resolution by preferential crystallization of (RS)-2-amino-3-(2-carboxyethylthio) propanoic acid[J]. Bioscience, Biotechnology, and Biochemistry, 1998, 62(4): 818-820. |
| 20 | 谢志平, 孙登琼, 秦亚楠, 等. 果糖结晶过程优化[J]. 化工学报, 2014, 65(1): 251-257. |
| Xie Z P, Sun D Q, Qin Y N, et al. Optimization of crystallization process of fructose[J]. CIESC Journal, 2014, 65(1): 251-257. | |
| 21 | Nakashima K, Ishimaru T, Kuroda N, et al. High-performance liquid chromatographic separation of penicillamine enantiomers labelled with N-[4-(6-dimethylamino-2-benzofuranyl)phenyl] maleimide on a chiral stationary phase[J]. Biomedical Chromatography, 1995, 9(2): 90-93. |
| 22 | 郑大为, 王艳青, 韩永博, 等. 清洁生产在制药行业中的应用和发展[J]. 环境保护与循环经济, 2012, 32(10): 42-44, 75. |
| Zheng D W, Wang Y Q, Han Y B, et al. Status and development of clean production in the domestic and foreign pharmaceutical industry [J]. Environmental Protection and Circular Economy, 2012, 32(10): 42-44, 75. | |
| 23 | 龚俊波, 陈明洋, 黄翠, 等. 面向清洁生产的制药结晶[J]. 化工学报, 2015, 66(9): 3271-3278. |
| Gong J B, Chen M Y, Huang C, et al. Clean production of pharmaceutical crystallization[J]. CIESC Journal, 2015, 66(9): 3271-3278. | |
| 24 | Chen Z Z, Zhai J H, Liu X J, et al. Solubility measurement and correlation of the form A of ibrutinib in organic solvents from 278.15 to 323.15 K[J]. The Journal of Chemical Thermodynamics, 2016, 103: 342-348. |
| 25 | Zhang M D, Fang Z X, Zhai J H, et al. Measurement and correlation of the solubility of rivaroxaban (form I) in binary mixtures of ethyl acetate with tetrahydrofuran, N, N-dimethylformamide, and N, N-dimethylacetamide from T = (278.15 to 318.15) K[J]. The Journal of Chemical Thermodynamics, 2016, 94: 1-6. |
| 26 | Zhai J H, Chen Z Z, Liu X J, et al. Solubility measurement, model evaluation and thermodynamic analysis of rivaroxaban polymorphs in organic solvents[J]. The Journal of Chemical Thermodynamics, 2017, 104: 218-229. |
| 27 | Fan F F, Yuan H K, Feng Y H, et al. Molecular simulation approaches for the prediction of unknown crystal structures and solubilities of (R)- and (R, S)-crizotinib in organic solvents[J]. Crystal Growth & Design, 2019, 19(10): 5882-5895. |
| 28 | 杨学智. 左氧氟沙星溶解度测定及共晶点的分析研究[D]. 天津: 天津大学, 2012. |
| Yang X Z. Measurement for solubility of levofloxacin and analysis of its eutectic point[D]. Tianjin: Tianjin University, 2012. | |
| 29 | 王波. 缬沙坦的手性拆分和稳定性的结晶工艺研究[D]. 广州: 华南理工大学, 2017. |
| Wang B. Crystallization for chiral separation and stability of valsartan[D]. Guangzhou: South China University of Technology, 2017. | |
| 30 | Polenske D, Lorenz H, Seidel-Morgenstern A. Separation of propranolol hydrochloride enantiomers by preferential crystallization: thermodynamic basis and experimental verification[J]. Crystal Growth & Design, 2007, 7(9): 1628-1634. |
| [1] | 于宏鑫, 邵双全. 水结晶过程的分子动力学模拟分析[J]. 化工学报, 2023, 74(S1): 250-258. |
| [2] | 杨欣, 王文, 徐凯, 马凡华. 高压氢气加注过程中温度特征仿真分析[J]. 化工学报, 2023, 74(S1): 280-286. |
| [3] | 常明慧, 王林, 苑佳佳, 曹艺飞. 盐溶液蓄能型热泵循环特性研究[J]. 化工学报, 2023, 74(S1): 329-337. |
| [4] | 张化福, 童莉葛, 张振涛, 杨俊玲, 王立, 张俊浩. 机械蒸汽压缩蒸发技术研究现状与发展趋势[J]. 化工学报, 2023, 74(S1): 8-24. |
| [5] | 胡建波, 刘洪超, 胡齐, 黄美英, 宋先雨, 赵双良. 有机笼跨细胞膜易位行为的分子动力学模拟研究[J]. 化工学报, 2023, 74(9): 3756-3765. |
| [6] | 张曼铮, 肖猛, 闫沛伟, 苗政, 徐进良, 纪献兵. 危废焚烧处理耦合有机朗肯循环系统工质筛选与热力学优化[J]. 化工学报, 2023, 74(8): 3502-3512. |
| [7] | 傅予, 刘兴翀, 王瀚雨, 李海敏, 倪亚飞, 邹文静, 雷月, 彭永姗. F3EACl修饰层对钙钛矿太阳能电池性能提升的研究[J]. 化工学报, 2023, 74(8): 3554-3563. |
| [8] | 卫雪岩, 钱勇. 微米级铁粉燃料中低温氧化反应特性及其动力学研究[J]. 化工学报, 2023, 74(6): 2624-2638. |
| [9] | 姚晓宇, 沈俊, 李健, 李振兴, 康慧芳, 唐博, 董学强, 公茂琼. 流体气液临界参数测量方法研究进展[J]. 化工学报, 2023, 74(5): 1847-1861. |
| [10] | 陈科, 杜理, 曾英, 任思颖, 于旭东. 四元体系LiCl+MgCl2+CaCl2+H2O 323.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(5): 1896-1903. |
| [11] | 王皓, 唐思扬, 钟山, 梁斌. MEA吸收CO2富液解吸过程中固体颗粒表面的强化作用分析[J]. 化工学报, 2023, 74(4): 1539-1548. |
| [12] | 苏晓丹, 朱干宇, 李会泉, 郑光明, 孟子衡, 李防, 杨云瑞, 习本军, 崔玉. 湿法磷酸半水工艺考察与石膏结晶过程研究[J]. 化工学报, 2023, 74(4): 1805-1817. |
| [13] | 毛元敬, 杨智, 莫松平, 郭浩, 陈颖, 罗向龙, 陈健勇, 梁颖宗. C6~C10烷醇的SAFT-VR Mie状态方程参数回归及其热物性研究[J]. 化工学报, 2023, 74(3): 1033-1041. |
| [14] | 陈毓明, 历伟, 严翔, 王靖岱, 阳永荣. 初生态聚乙烯聚集态结构调控研究进展[J]. 化工学报, 2023, 74(2): 487-499. |
| [15] | 周璇, 李孟亚, 孙杰, 岑振凯, 吕强三, 周立山, 王海涛, 韩丹丹, 龚俊波. 添加剂对氨基酸晶体生长的影响[J]. 化工学报, 2023, 74(2): 500-510. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号