化工学报 ›› 2023, Vol. 74 ›› Issue (3): 1161-1174.DOI: 10.11949/0438-1157.20221479
收稿日期:2022-11-14
修回日期:2023-02-11
出版日期:2023-03-05
发布日期:2023-04-19
通讯作者:
全学军
作者简介:陈号(1996—),男,硕士研究生,309471372@qq.com
基金资助:
Hao CHEN( ), Yijuan TIAN, Xuejun QUAN(
), Yijuan TIAN, Xuejun QUAN( ), Ziwen JIANG, Gang LI
), Ziwen JIANG, Gang LI
Received:2022-11-14
Revised:2023-02-11
Online:2023-03-05
Published:2023-04-19
Contact:
Xuejun QUAN
摘要:
铬铁矿酸浸分解制铬盐因其过程可以避免危险性物质六价铬[Cr(Ⅵ)]的产生而显示出较大应用前景,以盐酸为酸浸剂可以利用金属离子在盐酸中溶解度的差异,使工艺具有易于回收剩余酸和铬盐的特点。但是,也因为溶解平衡,使盐酸浸出铬铁矿的浸出效率较低,为了提高铬铁矿盐酸浸出过程的效率,提出了氢氟酸(HF)强化铬铁矿盐酸浸出的新工艺。在前期工作基础上,研究了HF强化铬铁矿盐酸浸出的规律、动力学及过程机理。结果表明,HF可以大大强化铬铁矿中Cr、Fe的浸出速率,且随HF用量增加而增大;而其中Al、Mg的浸出速率随HF用量的增加呈现先增加后减小的趋势,其主要原因是HF用量大时可以促进体系中含Al、Mg、Si物质形成Mg2SiO4、(Mg, Al)SiO3、Al2SiO5等结晶性硅酸盐,进入渣相中。这一发现为选择适当的浸出条件,实现浸出液中Cr与Al、Mg等杂质的更好分离提供了依据。在较高HF用量下,Cr、Fe、Al、Mg的浸出率可分别达到约92%、94%、17%、14%。HF的用量不仅影响铬铁矿盐酸浸出过程动力学的控制步骤,在促进浸出液中含Al、Mg、Si物质转化生成结晶性硅酸盐方面也起着重要的作用。
中图分类号:
陈号, 田仪娟, 全学军, 蒋子文, 李纲. 铬铁矿在HCl-HF体系中的分解行为[J]. 化工学报, 2023, 74(3): 1161-1174.
Hao CHEN, Yijuan TIAN, Xuejun QUAN, Ziwen JIANG, Gang LI. Decomposition behaviour of chromite in the HCl-HF system[J]. CIESC Journal, 2023, 74(3): 1161-1174.
| 元素 | 含量/(mg/g) |
|---|---|
| Cr | 293.79 |
| Fe | 181.96 |
| Al | 76.20 |
| Mg | 52.36 |
| Si | 16.40 |
表1 南非铬铁矿主要化学成分
Table 1 Main chemical composition of chromite from South Africa
| 元素 | 含量/(mg/g) |
|---|---|
| Cr | 293.79 |
| Fe | 181.96 |
| Al | 76.20 |
| Mg | 52.36 |
| Si | 16.40 |
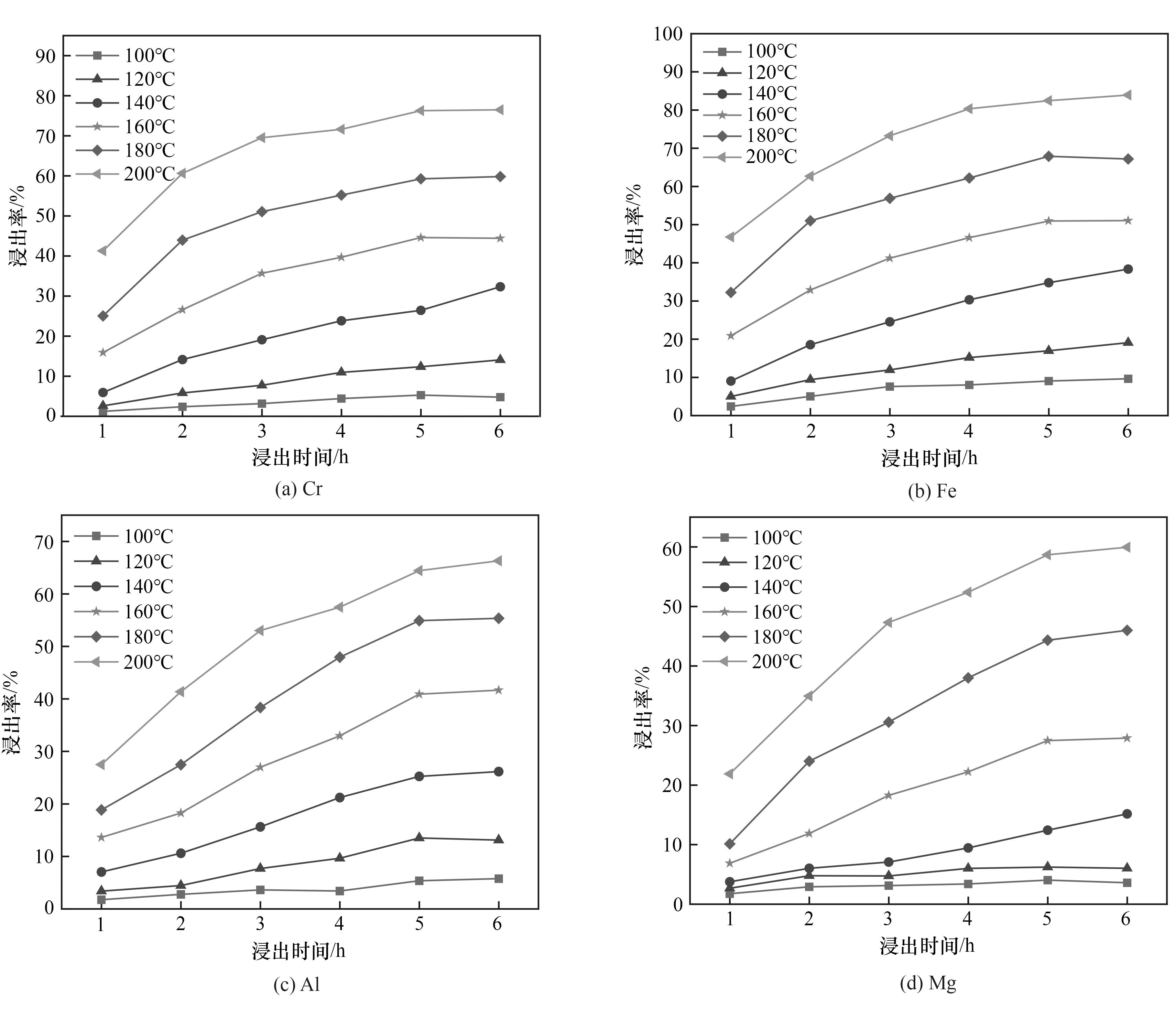
图7 HF用量为3倍理论量时各主要金属离子在不同温度下的浸出率随时间的变化
Fig.7 Variation of leaching rate of major metal ions with time at different temperatures when HF dosage is 3 times the theoretical amount
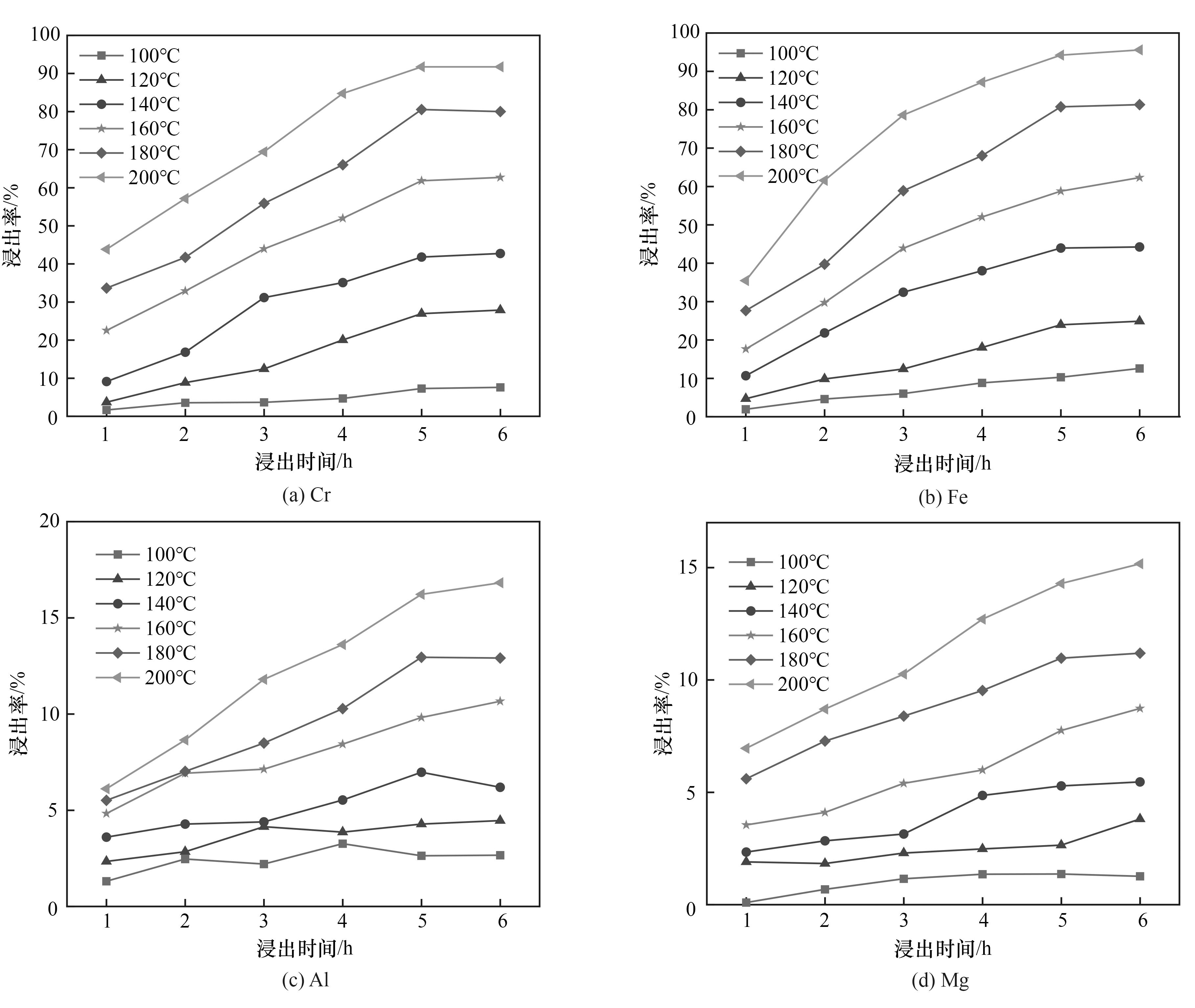
图8 HF用量为10倍理论量时各主要金属离子在不同温度下的浸出率随时间的变化
Fig.8 Variation of leaching rate of major metal ions with time at different temperatures when HF dosage is 10 times the theoretical amount
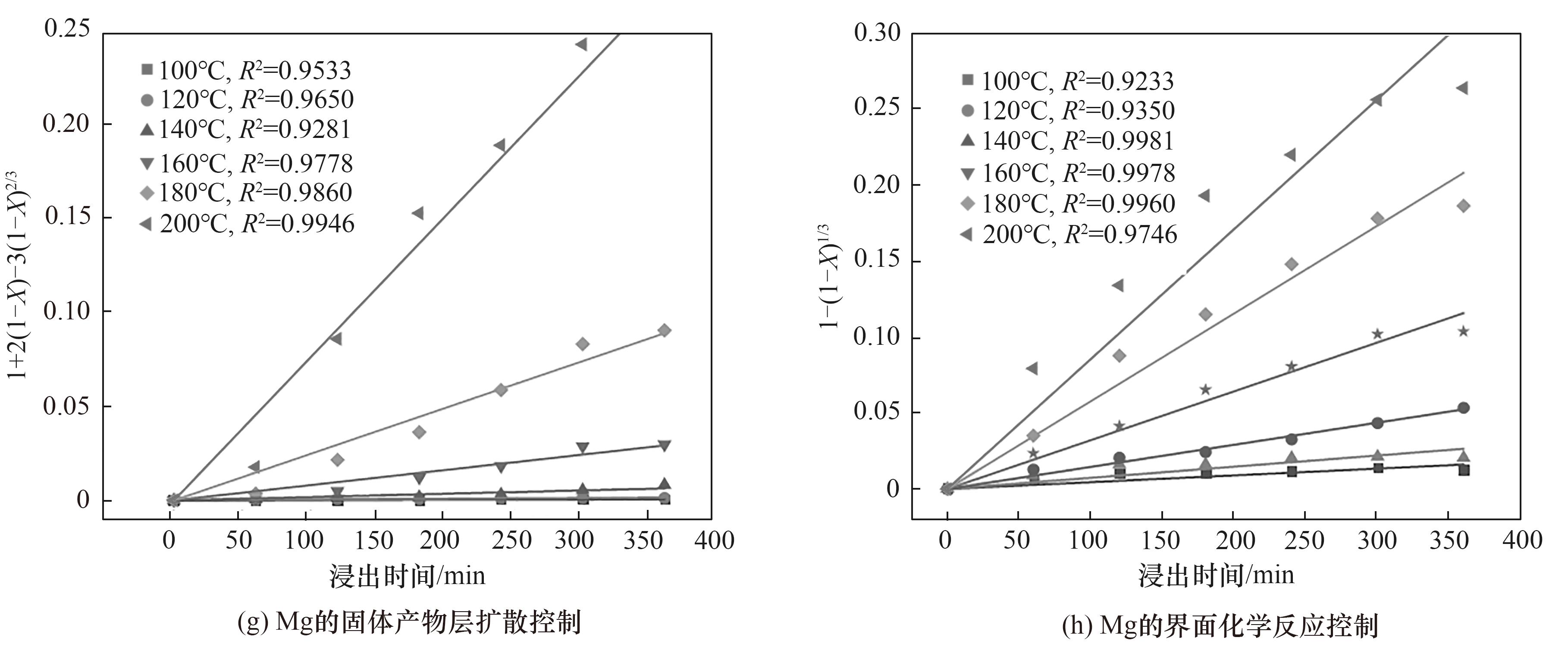
图13 HF用量为理论量3倍时不同温度下各金属浸出动力学拟合曲线
Fig.13 Fitted curves for the leaching kinetic of the metals at different temperatures when HF dosage is 3 times theoretical amout
| Temperature/℃ | 固体产物层扩散控制 | 界面化学反应控制 | ||||||
|---|---|---|---|---|---|---|---|---|
| ki(Cr)/min-1 | ki(Fe)/min-1 | ki(Al)/min-1 | ki(Mg)/min-1 | kr(Cr)/min-1 | kr(Fe)/min-1 | kr(Al)/min-1 | kr(Mg)/min-1 | |
| 100 | 2.46051×10-6 | 9.19429×10-6 | 5.27136×10-6 | 1.56694×10-6 | 5.44832×10-5 | 1.06872×10-4 | 5.76641×10-5 | 4.38630×10-5 |
| 120 | 1.73265×10-5 | 3.41549×10-5 | 3.57455×10-5 | 4.22081×10-6 | 1.44503×10-4 | 2.06567×10-4 | 1.39782×10-4 | 7.21222×10-5 |
| 140 | 9.50439×10-5 | 1.53615×10-4 | 1.65633×10-4 | 1.77324×10-5 | 3.45840×10-4 | 4.47295×10-4 | 2.94154×10-4 | 1.42844×10-4 |
| 160 | 2.55933×10-4 | 3.57935×10-4 | 4.91442×10-4 | 8.11095×10-5 | 5.93726×10-4 | 5.55457×10-4 | 5.06541×10-4 | 3.15485×10-4 |
| 180 | 5.38569×10-4 | 7.36588×10-4 | 9.23902×10-4 | 2.46691×10-4 | 8.93989×10-4 | 1.06979×10-3 | 7.47020×10-4 | 5.66920×10-4 |
| 200 | 1.06968×10-3 | 1.37972×10-3 | 1.4200×10-3 | 7.58054×10-4 | 1.32973×10-3 | 1.54000×10-3 | 9.83350×10-4 | 8.39131×10-4 |
表2 HF用量为理论量的3倍时铬铁矿中各金属浸出反应速率常数随温度的变化
Table 2 Variation of the reaction rate constant with temperature for each metal in the leaching process when HF dosage is 3 times theoretical amount
| Temperature/℃ | 固体产物层扩散控制 | 界面化学反应控制 | ||||||
|---|---|---|---|---|---|---|---|---|
| ki(Cr)/min-1 | ki(Fe)/min-1 | ki(Al)/min-1 | ki(Mg)/min-1 | kr(Cr)/min-1 | kr(Fe)/min-1 | kr(Al)/min-1 | kr(Mg)/min-1 | |
| 100 | 2.46051×10-6 | 9.19429×10-6 | 5.27136×10-6 | 1.56694×10-6 | 5.44832×10-5 | 1.06872×10-4 | 5.76641×10-5 | 4.38630×10-5 |
| 120 | 1.73265×10-5 | 3.41549×10-5 | 3.57455×10-5 | 4.22081×10-6 | 1.44503×10-4 | 2.06567×10-4 | 1.39782×10-4 | 7.21222×10-5 |
| 140 | 9.50439×10-5 | 1.53615×10-4 | 1.65633×10-4 | 1.77324×10-5 | 3.45840×10-4 | 4.47295×10-4 | 2.94154×10-4 | 1.42844×10-4 |
| 160 | 2.55933×10-4 | 3.57935×10-4 | 4.91442×10-4 | 8.11095×10-5 | 5.93726×10-4 | 5.55457×10-4 | 5.06541×10-4 | 3.15485×10-4 |
| 180 | 5.38569×10-4 | 7.36588×10-4 | 9.23902×10-4 | 2.46691×10-4 | 8.93989×10-4 | 1.06979×10-3 | 7.47020×10-4 | 5.66920×10-4 |
| 200 | 1.06968×10-3 | 1.37972×10-3 | 1.4200×10-3 | 7.58054×10-4 | 1.32973×10-3 | 1.54000×10-3 | 9.83350×10-4 | 8.39131×10-4 |
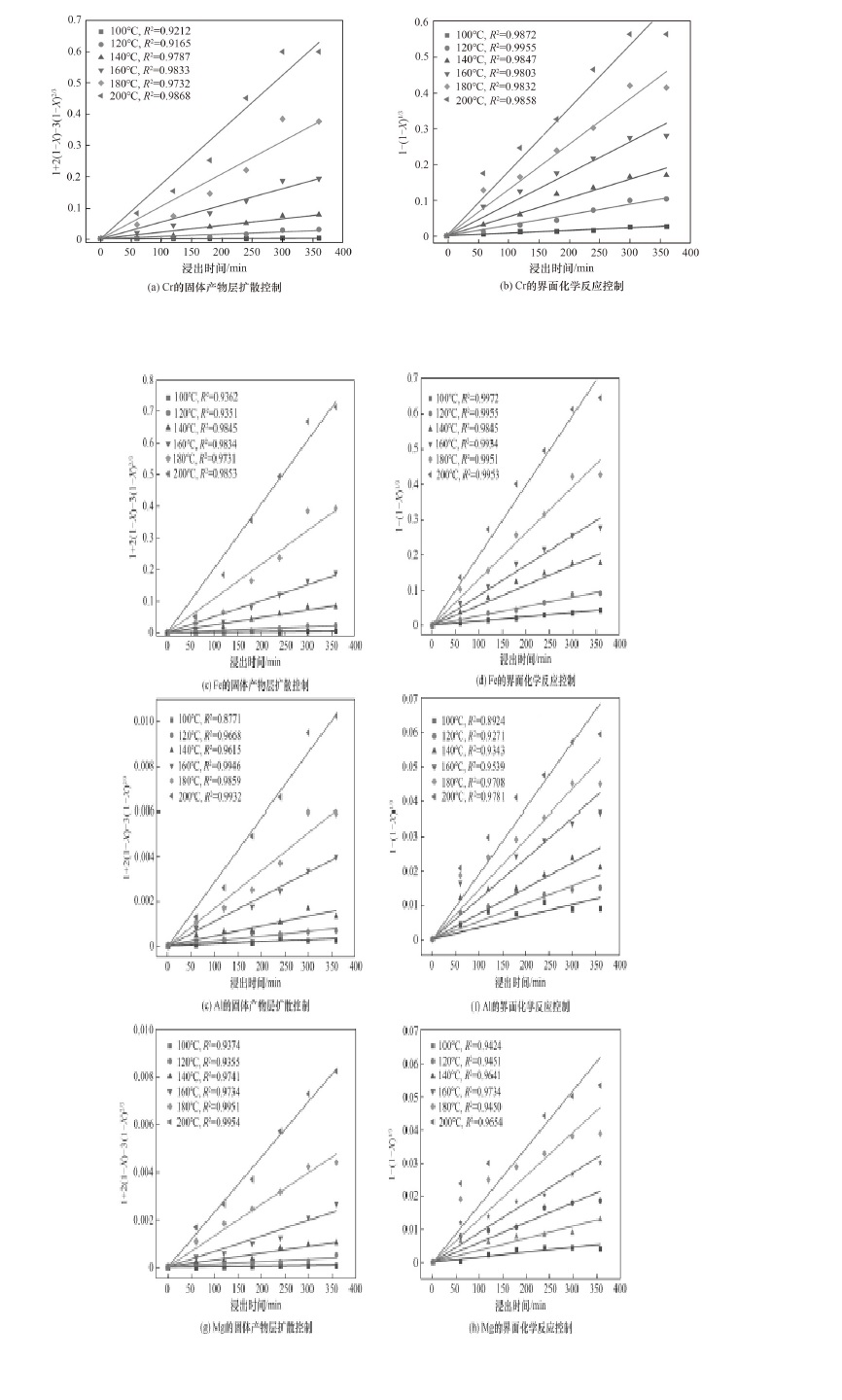
图15 HF用量为10倍时不同温度下各金属浸出动力学拟合曲线
Fig.15 Fitted curves for the leaching kinetic of the metals at different temperatures when HF dosage is 10 times theoretical amount
| 1 | Sanchez-Segado S, Jha A. Physical chemistry of roasting and leaching reactions for chromium chemical manufacturing and its impact on environment—a review[M]//Materials Processing Fundamentals. Hoboken, NJ, USA: John Wiley & Sons, Inc., 2013: 225-236. |
| 2 | Xu H B, Zheng S L, Zhang Y, et al. Oxidative leaching of a Vietnamese chromite ore in highly concentrated potassium hydroxide aqueous solution at 300℃ and atmospheric pressure[J]. Minerals Engineering, 2005, 18(5): 527-535. |
| 3 | Antony M P, Jha A, Tathavadkar V. Alkali roasting of Indian chromite ores: thermodynamic and kinetic considerations[J]. Mineral Processing and Extractive Metallurgy, 2006, 115(2): 71-79. |
| 4 | Liu Z B, Zheng J Y, Liu W Z, et al. Identification of the key host phases of Cr in fresh chromite ore processing residue (COPR)[J]. Science of the Total Environment, 2020, 703: 135075. |
| 5 | Zhang X R, Li G, Wu J, et al. Leaching of valuable elements from the waste chromite ore processing residue: a kinetic analysis[J]. ACS Omega, 2020, 5(31): 19633-19638. |
| 6 | Zhang Y, Li Z H, Qi T, et al. Green manufacturing process of chromium compounds[J]. Environmental Progress, 2005, 24(1): 44-50. |
| 7 | 陈宁, 董明甫, 黄玉西, 等. 铬盐产业绿色发展现状及展望[J]. 无机盐工业, 2018, 50(10): 10-13. |
| Chen N, Dong M F, Huang Y X, et al. Current status and prospect of green development of chromium salts industry[J]. Inorganic Chemicals Industry, 2018, 50(10): 10-13. | |
| 8 | 封承飞, 曾奎, 田仪娟, 等. 铬铁矿液相氧化浸出及动力学研究[J]. 湿法冶金, 2022, 41(3): 205-212. |
| Feng C F, Zeng K, Tian Y J, et al. Pressure oxidative leaching and leaching kinetics of chromite[J]. Hydrometallurgy of China, 2022, 41(3): 205-212. | |
| 9 | Zhang Y, Li Z H, Qi T, et al. Green chemistry of chromate cleaner production[J]. Chinese Journal of Chemistry, 1999, 17(3): 258-266. |
| 10 | 张懿, 李佐虎, 王志宽, 等. 铬酸钠的清洁生产方法: 1226512[P]. 1999-08-25. |
| Zhang Y, Li Z H, Wang Z K, et al. Method for cleaner production of sodium vanadate and sodium chromate by pressure leaching of vanadium slag: 1226512[P]. 1999-08-25. | |
| 11 | 中国科学院过程工程研究所. 铬盐清洁生产技术实现万吨规模生产: 万吨级铬盐清洁生产技术优化集成与标志性工程建设[J]. 中国科学院院刊, 2007, 22(5): 423-424. |
| Institute of Process Engineering, Chinese Academy of Sciences. Cleaner production technology of chromium salt realizes 10,000-ton scale production—optimization and integration of cleaner production technology of 10,000-ton chromium salt and landmark project construction[J]. Bulletin of Chinese Academy of Sciences, 2007, 22(5): 423-424. | |
| 12 | Zhang B, Shi P Y, Jiang M F. Advances towards a clean hydrometallurgical process for chromite[J]. Minerals, 2016, 6(1): 7. |
| 13 | Vardar E, Eric R H, Letowski F K. Acid leaching of chromite[J]. Minerals Engineering, 1994, 7(5/6): 605-617. |
| 14 | Zhao Q, Liu C J, Shi P Y, et al. Cleaner production of chromium oxide from low Fe(Ⅱ)-chromite[J]. Minerals, 2020, 10(5): 460. |
| 15 | Zhao Q, Liu C J, Li B K, et al. Decomposition mechanism of chromite in sulfuric acid-dichromic acid solution[J]. International Journal of Minerals, Metallurgy, and Materials, 2017, 24(12): 1361-1369. |
| 16 | Shi P Y, Liu C J, Zhao Q, et al. Study on mechanisms of different sulfuric acid leaching technologies of chromite[J]. International Journal of Minerals, Metallurgy, and Materials, 2017, 24(9): 983-990. |
| 17 | Liu C J, Qi J, Jiang M F. Experimental study on sulfuric acid leaching behavior of chromite with different temperature[J]. Advanced Materials Research, 2011, 361/362/363: 628-631. |
| 18 | Geveci A, Topkaya Y, Ayhan E. Sulfuric acid leaching of Turkish chromite concentrate[J]. Minerals Engineering, 2002, 15(11): 885-888. |
| 19 | Zhao Q, Liu C J, Yang D P, et al. A cleaner method for preparation of chromium oxide from chromite[J]. Process Safety and Environmental Protection, 2017, 105: 91-100. |
| 20 | Amer M A. Processing of Ras-Shait chromite deposits[J]. Hydrometallurgy, 1992, 28(1): 29-43. |
| 21 | Biermann W J, Heinrichs M. The attack of chromite by sulphuric acid [J]. Canadian Journal of Chemistry, 1960, 38(9): 1449-1454. |
| 22 | Jiang M F, Zhao Q, Liu C J, et al. Sulfuric acid leaching of South African chromite. Part 2: Optimization of leaching conditions[J]. International Journal of Mineral Processing, 2014, 130: 102-107. |
| 23 | Zhao Q, Liu C J, Shi P Y, et al. Sulfuric acid leaching of South African chromite. Part 1: Study on leaching behavior[J]. International Journal of Mineral Processing, 2014, 130: 95-101. |
| 24 | Lai H X, Huang L Q, Gan C H, et al. Enhanced acid leaching of metallurgical grade silicon in hydrofluoric acid containing hydrogen peroxide as oxidizing agent[J]. Hydrometallurgy, 2016, 164: 103-110. |
| 25 | Koohestani B, Darban A K, Mokhtari P, et al. Influence of hydrofluoric acid leaching and roasting on mineralogical phase transformation of pyrite in sulfidic mine tailings[J]. Minerals, 2020, 10(6): 513. |
| 26 | Li X B, Xu W B, Zhou Q S, et al. Leaching kinetics of acid-soluble Cr(Ⅵ) from chromite ore processing residue with hydrofluoric acid[J]. Journal of Central South University, 2011, 18(2): 399-405. |
| 27 | 李密, 黄婧, 戴士祥, 等. 采用HF和HClO4从铀尾矿中浸出铀的试验研究[J]. 中国矿业大学学报, 2016, 45(3): 639-645. |
| Li M, Huang J, Dai S X, et al. Extraction of uranium from uranium tailings by acid leaching with HF and HClO4 [J]. Journal of China University of Mining & Technology, 2016, 45(3): 639-645. | |
| 28 | Guo H, Yu H Z, Zhou A A, et al. Kinetics of leaching lithium from α-spodumene in enhanced acid treatment using HF/H2SO4 as medium[J]. Transactions of Nonferrous Metals Society of China, 2019, 29(2): 407-415. |
| 29 | Guo H, Kuang G, Li H, et al. Enhanced lithium leaching from lepidolite in continuous tubular reactor using H2SO4+H2SiF6 as lixiviant[J]. Transactions of Nonferrous Metals Society of China, 2021, 31(7): 2165-2173. |
| 30 | Philip K G, Charles Q J. Critical evaluation of coupling particle size distribution with the shrinking core model[J]. Chemical Engineering Science, 2004, 59(10): 1979-1987. |
| 31 | Li Y B, Kawashima N, Li J, et al. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite[J]. Advances in Colloid and Interface Science, 2013, 197/198: 1-32. |
| 32 | An J, Yang W Q, Yin J G, et al. Kinetics of phosphorus removal from high-phosphorus iron ores by HCl leaching[J]. Advanced Materials Research, 2013, 868: 455-458. |
| [1] | 孙永尧, 高秋英, 曾文广, 王佳铭, 陈艺飞, 周永哲, 贺高红, 阮雪华. 面向含氮油田伴生气提质利用的膜耦合分离工艺设计优化[J]. 化工学报, 2023, 74(5): 2034-2045. |
| [2] | 刘倩, 曹禹, 周琦, 穆景山, 历伟. 孔道结构修饰的Ziegler-Natta催化剂设计与高抗冲低缠结UHMWPE的制备[J]. 化工学报, 2023, 74(3): 1092-1101. |
| [3] | 侯跃辉, 刘璇, 廉应江, 韩梅, 尧超群, 陈光文. 超声微反应器内三硝基间苯三酚合成工艺研究[J]. 化工学报, 2022, 73(8): 3597-3607. |
| [4] | 周晨阳, 贾颖, 赵跃民, 张勇, 付芝杰, 冯昱清, 段晨龙. 介尺度视角下干法重介流态化分选过程强化[J]. 化工学报, 2022, 73(6): 2452-2467. |
| [5] | 刘梦溪, 范怡平, 闫子涵, 姚秀颖, 卢春喜. 提升管进料区内气体射流流动行为的调控及工业应用[J]. 化工学报, 2022, 73(6): 2496-2513. |
| [6] | 张亚爽, 李洪, 从海峰, 韩红明, 李鑫钢, 高鑫. 微波强化液桥式螺旋降膜蒸发器数值模拟[J]. 化工学报, 2021, 72(S1): 227-235. |
| [7] | 初广文,廖洪钢,王丹,李晖,李洒,姜红,金万勤,陈建峰. 微纳介尺度气液反应过程强化[J]. 化工学报, 2021, 72(7): 3435-3444. |
| [8] | 江澜, 罗勇, 邹海魁, 孙宝昌, 张亮亮, 初广文. 超重力多相催化反应器的研究进展[J]. 化工学报, 2021, 72(6): 3194-3201. |
| [9] | 蔡润夏, 李凡星. 复杂氧化物载氧体的调变策略及在过程强化中的应用[J]. 化工学报, 2021, 72(12): 6122-6130. |
| [10] | 张姬一哲, 王运东, 费维扬. 液液萃取塔研究的若干新进展及展望[J]. 化工学报, 2021, 72(12): 6016-6029. |
| [11] | 许劲, 朱杰东, 李卷利, 刘孟秋, 龚河洛. 湿化学法回收污泥水热炭中磷的潜能研究[J]. 化工学报, 2021, 72(11): 5779-5789. |
| [12] | 吴沛文, 荀苏杭, 蒋伟, 李华明, 朱文帅. 离子液体反应型萃取燃油脱硫研究进展[J]. 化工学报, 2021, 72(1): 276-291. |
| [13] | 李光晓,刘塞尔,苏远海. 微尺度内液-液传质及反应过程强化的研究进展[J]. 化工学报, 2021, 72(1): 452-467. |
| [14] | 盛磊, 李培钰, 牛宇超, 贺高红, 姜晓滨. 微尺度过程强化的结晶颗粒制备研究进展[J]. 化工学报, 2021, 72(1): 143-157. |
| [15] | 李志康, 商鲁伟, 聂苗苗, 邓文生, 谭璟. G/O/W微分散体系实现甲酸/三辛胺-正辛醇体系萃取分离[J]. 化工学报, 2020, 71(9): 4219-4227. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号