化工学报 ›› 2023, Vol. 74 ›› Issue (6): 2580-2588.DOI: 10.11949/0438-1157.20230201
王新悦1( ), 王俊杰1, 曹思贤1, 王翠1, 李灵坤1, 吴宏宇1, 韩静2, 吴昊1(
), 王俊杰1, 曹思贤1, 王翠1, 李灵坤1, 吴宏宇1, 韩静2, 吴昊1( )
)
收稿日期:2023-03-07
修回日期:2023-05-08
出版日期:2023-06-05
发布日期:2023-07-27
通讯作者:
吴昊
作者简介:王新悦(1997—),女,硕士研究生,15755238685@163.com
基金资助:
Xinyue WANG1( ), Junjie WANG1, Sixian CAO1, Cui WANG1, Lingkun LI1, Hongyu WU1, Jing HAN2, Hao WU1(
), Junjie WANG1, Sixian CAO1, Cui WANG1, Lingkun LI1, Hongyu WU1, Jing HAN2, Hao WU1( )
)
Received:2023-03-07
Revised:2023-05-08
Online:2023-06-05
Published:2023-07-27
Contact:
Hao WU
摘要:
利用十八烷基三氯硅烷(octadecyltrichlorosilane,OTS)对中硼硅玻璃管制注射剂瓶内表面进行化学改性,使用接触角仪(水接触角从50°±1°增加至90°±2°)、原子力显微镜(表面粗糙度从0.448±0.086增加至1.282±0.117)、红外(出现—CH2—和—CH3特征峰)对表面进行验证,成功制备了具有疏水性能的玻璃表面。给玻璃瓶中的单克隆抗体施加机械应力,使用微流成像技术、尺寸排阻-高效液相色谱法(size exclusion-high performance liquid chromatography,SE-HPLC)、外源荧光对单抗制剂的稳定性进行全面表征。结果表明,OTS处理的疏水性界面可以减少机械应力诱导的蛋白质聚集体的产生。由于疏水表面与蛋白质强的疏水相互作用,OTS处理的疏水表面可有效抑制机械应力诱导的抗体分子的聚集。
中图分类号:
王新悦, 王俊杰, 曹思贤, 王翠, 李灵坤, 吴宏宇, 韩静, 吴昊. 玻璃内包材界面修饰对机械应力诱导的单克隆抗体聚集体形成的影响[J]. 化工学报, 2023, 74(6): 2580-2588.
Xinyue WANG, Junjie WANG, Sixian CAO, Cui WANG, Lingkun LI, Hongyu WU, Jing HAN, Hao WU. Effect of glass primary container surface modification on monoclonal antibody aggregates induced by mechanical stress[J]. CIESC Journal, 2023, 74(6): 2580-2588.
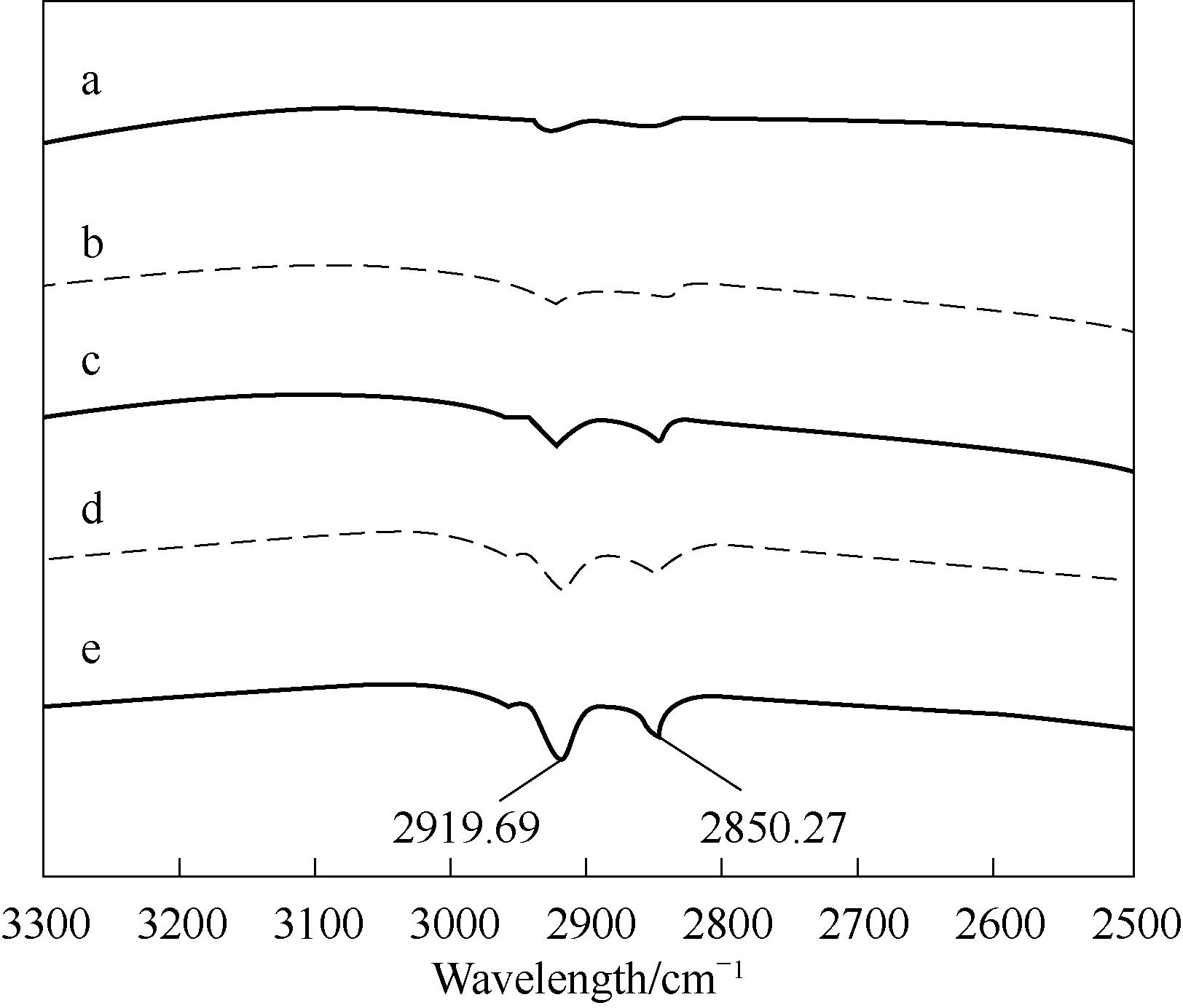
图3 玻璃表面的FTIR谱图a—未处理的玻璃表面;b—0.17 μmol/L OTS处理的玻璃表面;c—0.85 μmol/L OTS处理的玻璃表面;d—3.4 μmol/L OTS处理的玻璃表面;e—17 μmol/L OTS处理的玻璃表面
Fig.3 FTIR spectrum of glass surfaces

图5 IgG2在玻璃表面的Langmuir吸附模型a—未处理的玻璃表面;b—0.17 μmol/L OTS处理的玻璃表面;c—0.85 μmol/L OTS处理的玻璃表面;d—3.4 μmol/L OTS处理的玻璃表面;e—17 μmol/L OTS处理的玻璃表面
Fig.5 Langmuir adsorption model of IgG2 on glass surfaces

图6 在未处理、0.17 μmol/L OTS和17 μmol/L OTS处理的玻璃瓶中,经过机械应力处理的 IgG2产生的蛋白质聚集体
Fig.6 IgG2 protein aggregates produced by mechanically stressed in untreated and OTS glass vials
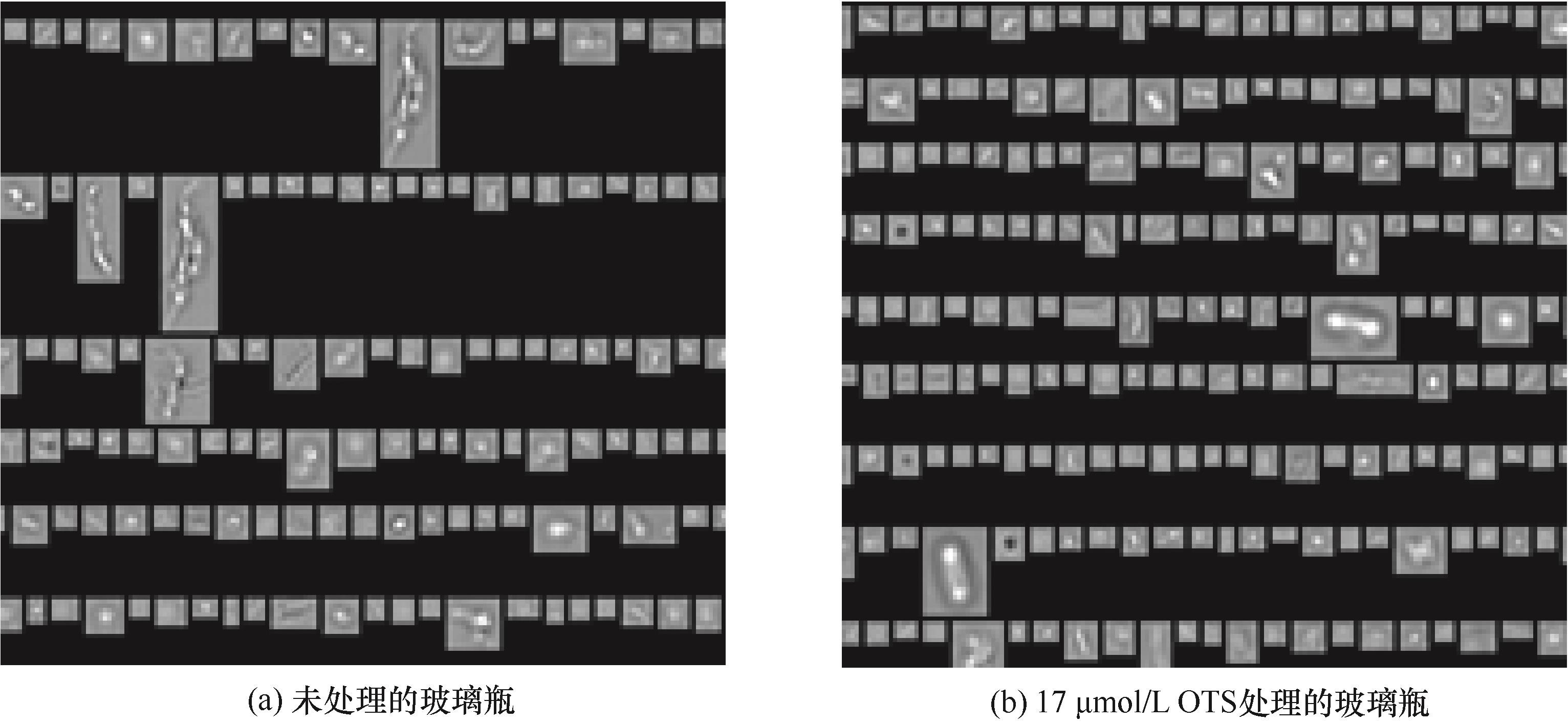
图8 在未处理和OTS处理的小瓶中经过机械应力处理后的单抗溶液产生的亚可见颗粒的形态
Fig.8 Morphology of subvisible particles in monoclonal antibody solution after mechanical stress treatment in untreated glass vials and glass vials coated by 17 μmol/L OTS

图10 可溶性IgG2的SE-HPLC谱图a—未受应力的IgG2; b—未处理的玻璃瓶中经过应力处理的IgG2;c—0.17 μmol/L OTS处理的玻璃瓶中应力处理的IgG2;d—17 μmol/L OTS处理的玻璃瓶中应力处理的IgG2
Fig.10 SE-HPLC spectrum of soluble IgG2
| 1 | Nuévalos M, García-Ríos E, Mancebo F J, et al. Novel monoclonal antibody-based therapies: implications for the treatment and prevention of HCMV disease[J]. Trends in Microbiology, 2023, 31(5): 480-497. |
| 2 | Chen Y, Zhang G L, Yang Y W, et al. The treatment of inflammatory bowel disease with monoclonal antibodies in Asia[J]. Biomedicine & Pharmacotherapy, 2023, 157: 114081. |
| 3 | Chi E Y, Krishnan S, Randolph T W, et al. Physical stability of proteins in aqueous solution: mechanism and driving forces in nonnative protein aggregation[J]. Pharmaceutical Research, 2003, 20(9): 1325-1336. |
| 4 | Kosloski M P, Miclea R D, Balu-Iyer S V. Role of glycosylation in conformational stability, activity, macromolecular interaction and immunogenicity of recombinant human factor Ⅷ[J]. The AAPS, Journal, 2009, 11(3): 424-431. |
| 5 | Johann F, Wöll S, Winzer M, et al. Miniaturized forced degradation of therapeutic proteins and ADCs by agitation-induced aggregation using orbital shaking of microplates[J]. Journal of Pharmaceutical Sciences, 2022, 111(5): 1401-1413. |
| 6 | 金鹤. 静脉输液中不溶性微粒对人体的危害及控制[J]. 上海护理, 2007, 7(5): 55-57. |
| Jin H. Harm and control of insoluble particles in intravenous infusion to human body[J]. Shanghai Nursing, 2007, 7(5): 55-57. | |
| 7 | Joubert M K, Hokom M, Eakin C, et al. Highly aggregated antibody therapeutics can enhance the in vitro innate and late-stage T-cell immune responses[J]. Journal of Biological Chemistry, 2012, 287(30): 25266-25279. |
| 8 | 郭莎, 贾哲, 吴昊, 等. 单克隆抗体颗粒表征的现状与挑战[J]. 中国药事, 2022, 36(2)161-169. |
| Guo S, Jia Z, Wu H, et al. Current status and challenges of particle characterization in monoclonal antibody formulation[J]. Chinese Pharmaceutical Affairs, 2022, 36(2)161-169. | |
| 9 | Patapoff T W, Esue O. Polysorbate 20 prevents the precipitation of a monoclonal antibody during shear[J]. Pharmaceutical Development and Technology, 2009, 14(6): 659-664. |
| 10 | Bam N B, Cleland J L, Yang J, et al. Tween protects recombinant human growth hormone against agitation-induced damage via hydrophobic interactions[J]. Journal of Pharmaceutical Sciences, 1998, 87(12): 1554-1559. |
| 11 | Wang W, Wang Y J, Wang D Q. Dual effects of Tween 80 on protein stability[J]. International Journal of Pharmaceutics, 2008, 347(1/2): 31-38. |
| 12 | Perevozchikova T, Nanda H, Nesta D P, et al. Protein adsorption, desorption, and aggregation mediated by solid-liquid interfaces[J]. Journal of Pharmaceutical Sciences, 2015, 104(6): 1946-1959. |
| 13 | Kaivosoja E, Barreto G, Levón K, et al. Chemical and physical properties of regenerative medicine materials controlling stem cell fate[J]. Annals of Medicine, 2012, 44(7): 635-650. |
| 14 | Wu H, Randolph T W. Aggregation and particle formation during pumping of an antibody formulation are controlled by electrostatic interactions between pump surfaces and protein molecules[J]. Journal of Pharmaceutical Sciences, 2020, 109(4): 1473-1482. |
| 15 | Movafaghi S, Wu H, Francino Urdániz I M, et al. The effect of container surface passivation on aggregation of intravenous immunoglobulin induced by mechanical shock[J]. Biotechnology Journal, 2020, 15(9): e2000096. |
| 16 | Yoneda S, Maruno T, Mori A, et al. Influence of protein adsorption on aggregation in prefilled syringes[J]. Journal of Pharmaceutical Sciences, 2021, 110(11): 3568-3579. |
| 17 | Biddlecombe J G, Craig A V, Zhang H, et al. Determining antibody stability: creation of solid-liquid interfacial effects within a high shear environment[J]. Biotechnology Progress, 2007, 23(5): 1218-1222. |
| 18 | Oliva A, Santoveña A, Fariña J, et al. Effect of high shear rate on stability of proteins: kinetic study[J]. Journal of Pharmaceutical and Biomedical Analysis, 2003, 33(2): 145-155. |
| 19 | Sediq A S, van Duijvenvoorde R B, Jiskoot W, et al. No touching!Abrasion of adsorbed protein is the root cause of subvisible particle formation during stirring[J]. Journal of Pharmaceutical Sciences, 2016, 105(2): 519-529. |
| 20 | Gerhardt A, McGraw N R, Schwartz D K, et al. Protein aggregation and particle formation in prefilled glass syringes[J]. Journal of Pharmaceutical Sciences, 2014, 103(6): 1601-1612. |
| 21 | Qi L, Liu J, Ronk M, et al. A holistic approach of extractables and leachables assessment of rubber stoppered glass vial systems for biotechnology products[J]. Journal of Pharmaceutical Sciences, 2021, 110(11): 3580-3593. |
| 22 | Oom A, Poggi M, Wikström J, et al. Surface interactions of monoclonal antibodies characterized by quartz crystal microbalance with dissipation: impact of hydrophobicity and protein self-interactions[J]. Journal of Pharmaceutical Sciences, 2012, 101(2): 519-529. |
| 23 | Gao F F. Adsorption of mussel protein on polymer antifouling membranes: a molecular dynamics study[J]. Molecules (Basel, Switzerland), 2021, 26(18): 5660. |
| 24 | Marsh R J, Jones R A L, Sferrazza M. Adsorption and displacement of a globular protein on hydrophilic and hydrophobic surfaces[J]. Colloids and Surfaces B: Biointerfaces, 2002, 23(1): 31-42. |
| 25 | Lefebvre G, Maze A, Jimenez R A P, et al. Surfactant protection efficacy at surfaces varies with the nature of hydrophobic materials[J]. Pharmaceutical Research, 2021, 38(12): 2157-2166. |
| 26 | Bainor A, Chang L, McQuade T J, et al. Bicinchoninic acid (BCA) assay in low volume[J]. Analytical Biochemistry, 2011, 410(2): 310-312. |
| 27 | Gerhardt A, Bonam K, Bee J S, et al. Ionic strength affects tertiary structure and aggregation propensity of a monoclonal antibody adsorbed to silicone oil-water interfaces[J]. Journal of Pharmaceutical Sciences, 2013, 102(2): 429-440. |
| 28 | Hawe A, Sutter M, Jiskoot W. Extrinsic fluorescent dyes as tools for protein characterization[J]. Pharmaceutical Research, 2008, 25(7): 1487-1499. |
| 29 | Ying P Q, Yu Y, Jin G, et al. Competitive protein adsorption studied with atomic force microscopy and imaging ellipsometry[J]. Colloids and Surfaces B: Biointerfaces, 2003, 32(1): 1-10. |
| 30 | Rabe M, Verdes D, Seeger S. Understanding protein adsorption phenomena at solid surfaces[J]. Advances in Colloid and Interface Science, 2011, 162(1/2): 87-106. |
| 31 | Chen S F, Liu L Y, Zhou J, et al. Controlling antibody orientation on charged self-assembled monolayers[J]. Langmuir, 2003, 19: 2859-2864. |
| 32 | Bee J S, Schwartz D K, Trabelsi S, et al. Production of particles of therapeutic proteins at the air-water interface during compression/dilation cycles[J]. Soft Matter, 2012, 8(40): 10329-10335. |
| 33 | Haynes Charles A, Willem N. Globular proteins at solid/liquid interfaces[J]. Colloids and Surfaces B: Biointerfaces, 1994, 2(6): 517-566. |
| 34 | Mathes J, Friess W. Influence of pH and ionic strength on IgG adsorption to vials[J]. European Journal of Pharmaceutics and Biopharmaceutics, 2011, 78(2): 239-247. |
| 35 | Quan X B, Liu J, Zhou J. Multiscale modeling and simulations of protein adsorption: progresses and perspectives[J]. Current Opinion in Colloid & Interface Science, 2019, 41: 74-85. |
| 36 | Wu H, Randolph T W. Rapid quantification of protein particles in high-concentration antibody formulations[J]. Journal of Pharmaceutical Sciences, 2019, 108(3): 1110-1116. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 赵亚欣, 张雪芹, 王荣柱, 孙国, 姚善泾, 林东强. 流穿模式离子交换层析去除单抗聚集体[J]. 化工学报, 2023, 74(9): 3879-3887. |
| [3] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [4] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [5] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [6] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [7] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [8] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [9] | 陈韶云, 徐东, 陈龙, 张禹, 张远方, 尤庆亮, 胡成龙, 陈建. 单层聚苯胺微球阵列结构的制备及其吸附性能[J]. 化工学报, 2023, 74(5): 2228-2238. |
| [10] | 侯文起, 孙彦, 董晓燕. 碱化修饰甲状腺素运载蛋白显著增强对淀粉样β蛋白聚集的抑制作用[J]. 化工学报, 2023, 74(5): 2100-2110. |
| [11] | 蔺彩虹, 王丽, 吴瑜, 刘鹏, 杨江峰, 李晋平. 沸石中碱金属阳离子对CO2/N2O吸附分离性能的影响[J]. 化工学报, 2023, 74(5): 2013-2021. |
| [12] | 李辰鑫, 潘艳秋, 何流, 牛亚宾, 俞路. 基于碳微晶结构的炭膜模型及其气体分离模拟[J]. 化工学报, 2023, 74(5): 2057-2066. |
| [13] | 王蕾, 王磊, 白云龙, 何柳柳. SA膜状锂离子筛的制备及其锂吸附性能[J]. 化工学报, 2023, 74(5): 2046-2056. |
| [14] | 肖川宝, 李林洋, 刘武锋, 钟年丙, 解泉华, 钟登杰, 常海星. 光催化与离子交换吸附耦合有效去除2,4,6-三氯苯酚[J]. 化工学报, 2023, 74(4): 1587-1597. |
| [15] | 吴选军, 王超, 曹子健, 蔡卫权. 数据与物理信息混合驱动的固定床吸附穿透深度学习模型[J]. 化工学报, 2023, 74(3): 1145-1160. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号