化工学报 ›› 2025, Vol. 76 ›› Issue (5): 2230-2240.DOI: 10.11949/0438-1157.20241125
收稿日期:2024-10-11
修回日期:2024-12-13
出版日期:2025-05-25
发布日期:2025-06-13
通讯作者:
孙林兵
作者简介:谈朋(1988—),男,博士,副教授,ptan@njtech.edu.cn
基金资助:
Peng TAN( ), Xuemei LI, Xiaoqin LIU, Linbing SUN(
), Xuemei LI, Xiaoqin LIU, Linbing SUN( )
)
Received:2024-10-11
Revised:2024-12-13
Online:2025-05-25
Published:2025-06-13
Contact:
Linbing SUN
摘要:
磁诱导的变温吸附因其操作便捷、产热速率快以及传热距离短而备受关注,其能源利用效率由吸附材料在磁场中的性能决定。目前的吸附材料性能难以调变,无法在磁诱导变温吸附中充分发挥其分离性能。构筑了磁响应柔性吸附材料MN@CPL-1,并将其应用于丙烯捕获。通过将Fe3O4纳米颗粒与柔性MOF材料原位复合实现材料的制备。当交变磁场关闭时,MN@CPL-1具有开放的孔结构,能够有效地捕获丙烯分子。当交变磁场打开时,磁诱导的热量从Fe3O4纳米颗粒快速转移至CPL-1,使其框架结构发生局部旋转,促使丙烯分子释放。最优样品在10~30℃变温区间内的工作容量(22.5 cm3·g-1)优于很多经典的丙烯吸附剂,如MIL-101(14.45 cm3·g-1)、MAC-4(13.00 cm3·g-1)和沸石5A(4.14 cm3·g-1)。在外部磁场调控下,最优样品的吸附量变化率达到了65.9%,并且经过5次交变磁场关闭/打开吸脱附循环后,复合材料依然能保持良好的吸附性能。磁感应产热与吸附材料柔性结构的耦合提升了吸附效率。
中图分类号:
谈朋, 李雪梅, 刘晓勤, 孙林兵. 基于柔性MOFs的磁响应复合材料及其丙烯吸附性能研究[J]. 化工学报, 2025, 76(5): 2230-2240.
Peng TAN, Xuemei LI, Xiaoqin LIU, Linbing SUN. Study on magnetically responsive composite materials based on flexible MOFs and their propylene adsorption performance[J]. CIESC Journal, 2025, 76(5): 2230-2240.
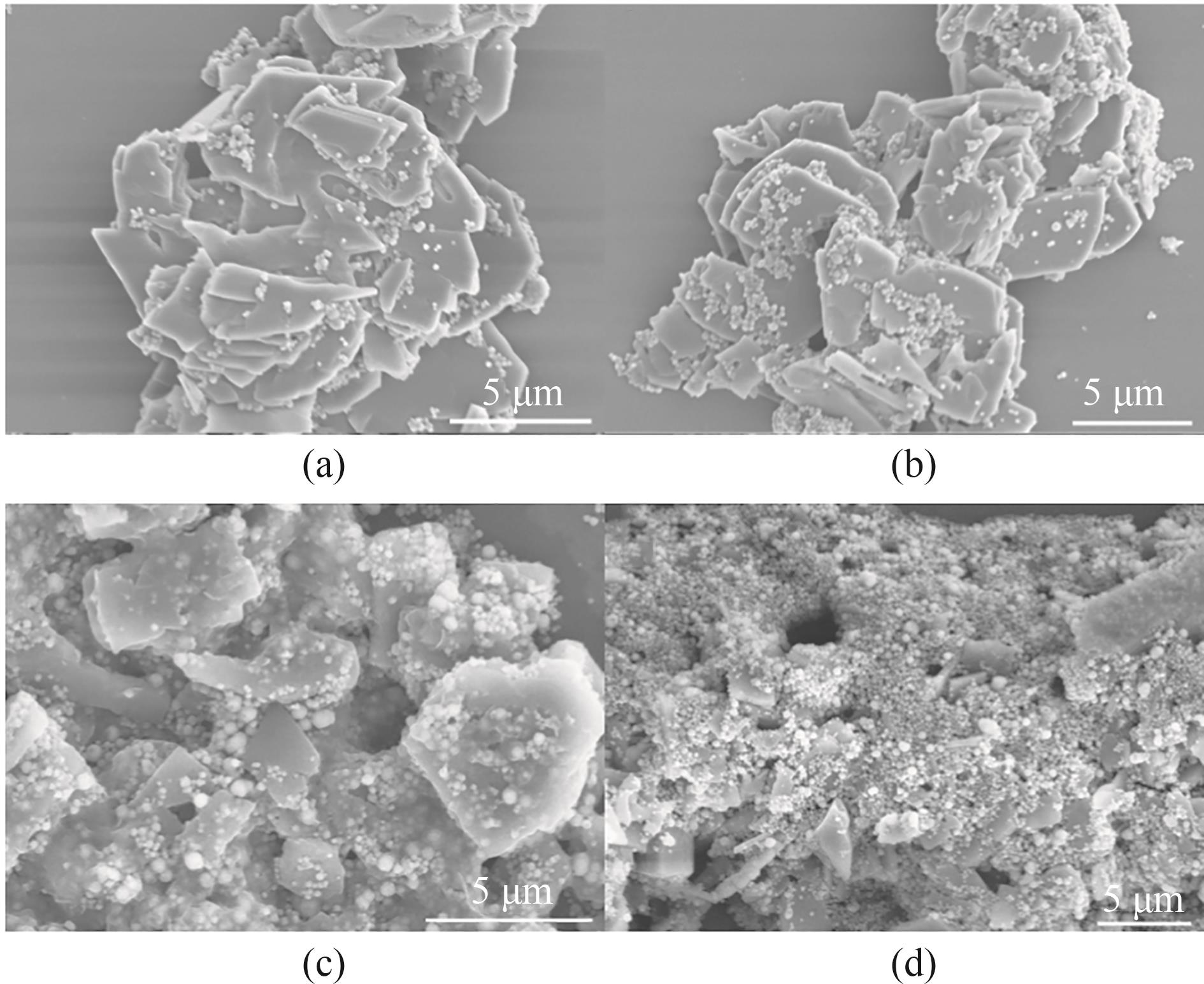
图3 MN@CPL-1-1(a)、MN@CPL-1-2(b)、MN@CPL-1-3(c)和MN@CPL-1-4(d)的SEM图像
Fig.3 SEM images of MN@CPL-1-1 (a), MN@CPL-1-2 (b), MN@CPL-1-3 (c) and MN@CPL-1-4 (d)
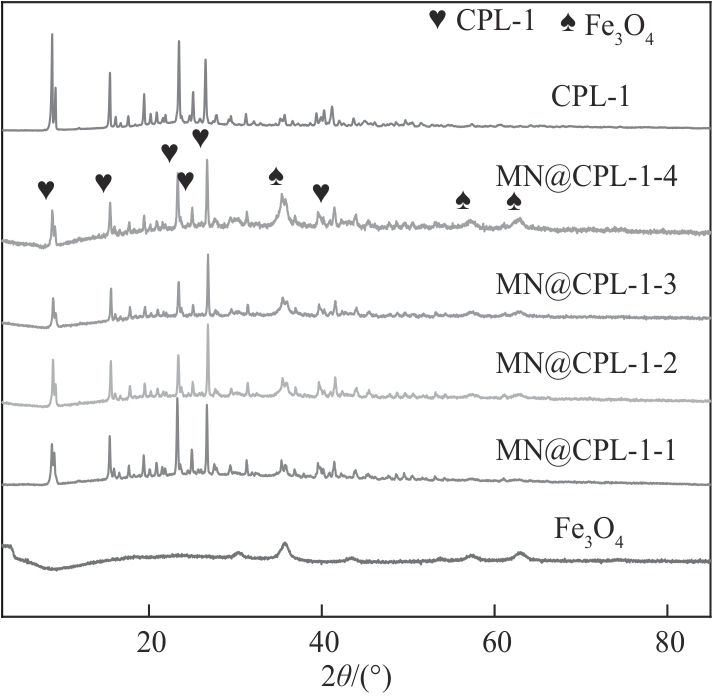
图4 Fe3O4、CPL-1、MN@CPL-1-1、MN@CPL-1-2、MN@CPL-1-3和MN@CPL-1-4的XRD谱图
Fig.4 XRD patterns of Fe3O4, CPL-1, MN@CPL-1-1, MN@CPL-1-2, MN@CPL-1-3 and MN@CPL-1-4
| Sample | SBET/(m2·g-1) | Vp/(cm3·g-1) | Vmicro/(cm3·g-1) |
|---|---|---|---|
| CPL-1 | 247 | 0.09 | 0.05 |
| MN@CPL-1-1 | 172 | 0.11 | 0.07 |
| MN@CPL-1-2 | 216 | 0.17 | 0.06 |
| MN@CPL-1-3 | 246 | 0.19 | 0.11 |
| MN@CPL-1-4 | 229 | 0.18 | 0.11 |
表1 样品的结构参数
Table 1 Textural properties of different samples
| Sample | SBET/(m2·g-1) | Vp/(cm3·g-1) | Vmicro/(cm3·g-1) |
|---|---|---|---|
| CPL-1 | 247 | 0.09 | 0.05 |
| MN@CPL-1-1 | 172 | 0.11 | 0.07 |
| MN@CPL-1-2 | 216 | 0.17 | 0.06 |
| MN@CPL-1-3 | 246 | 0.19 | 0.11 |
| MN@CPL-1-4 | 229 | 0.18 | 0.11 |
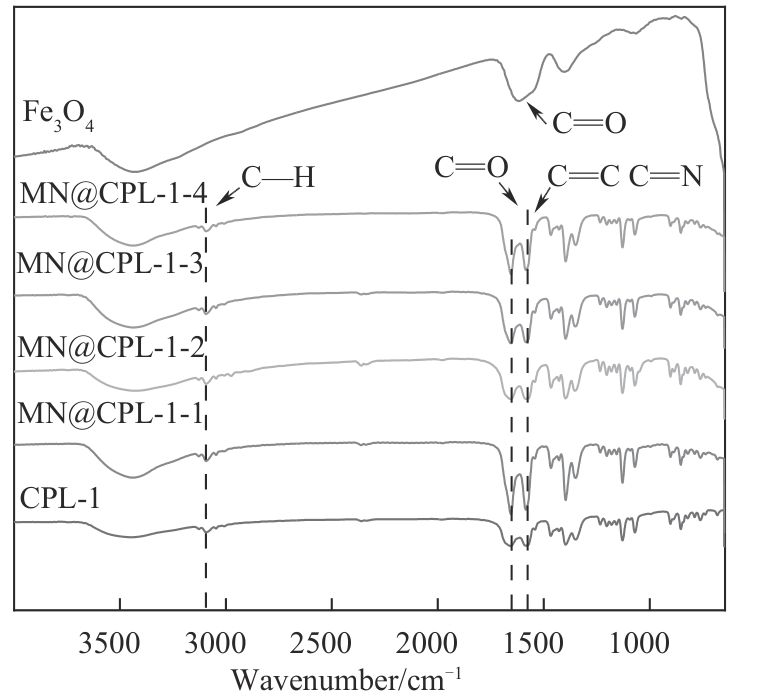
图6 Fe3O4、CPL-1、MN@CPL-1-1、MN@CPL-1-2、MN@CPL-1-3和MN@CPL-1-4的FTIR谱图
Fig.6 FTIR spectra of Fe3O4, CPL-1, MN@CPL-1-1, MN@CPL-1-2, MN@CPL-1-3 and MN@CPL-1-4
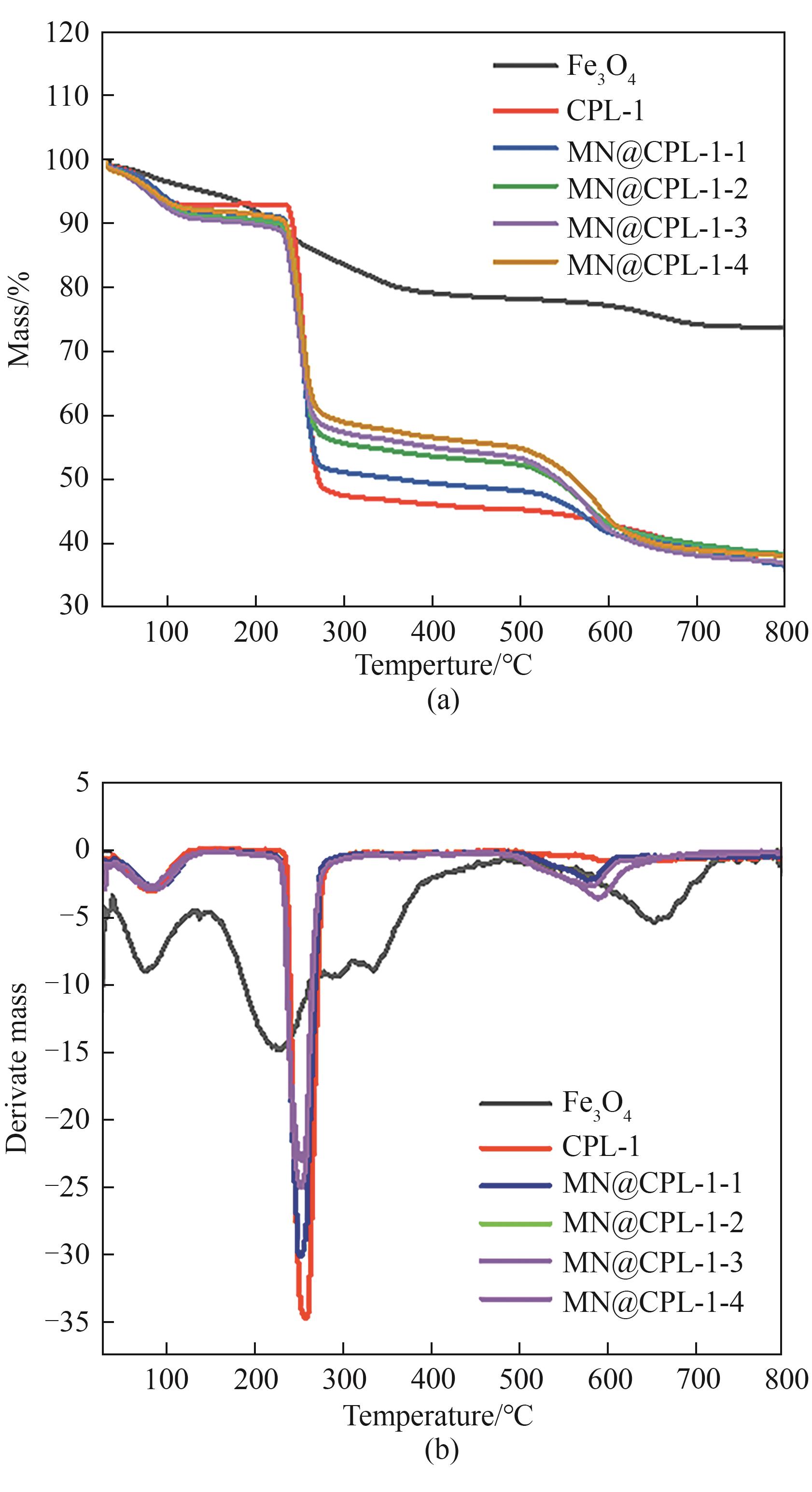
图7 Fe3O4、CPL-1、MN@CPL-1-1、MN@CPL-1-2、MN@CPL-1-3和MN@CPL-1-4的TG(a)和DTG曲线(b)
Fig.7 TG (a) and DTG (b) curves of Fe3O4, CPL-1, MN@CPL-1-1, MN@CPL-1-2, MN@CPL-1-3 and MN@CPL-1-4
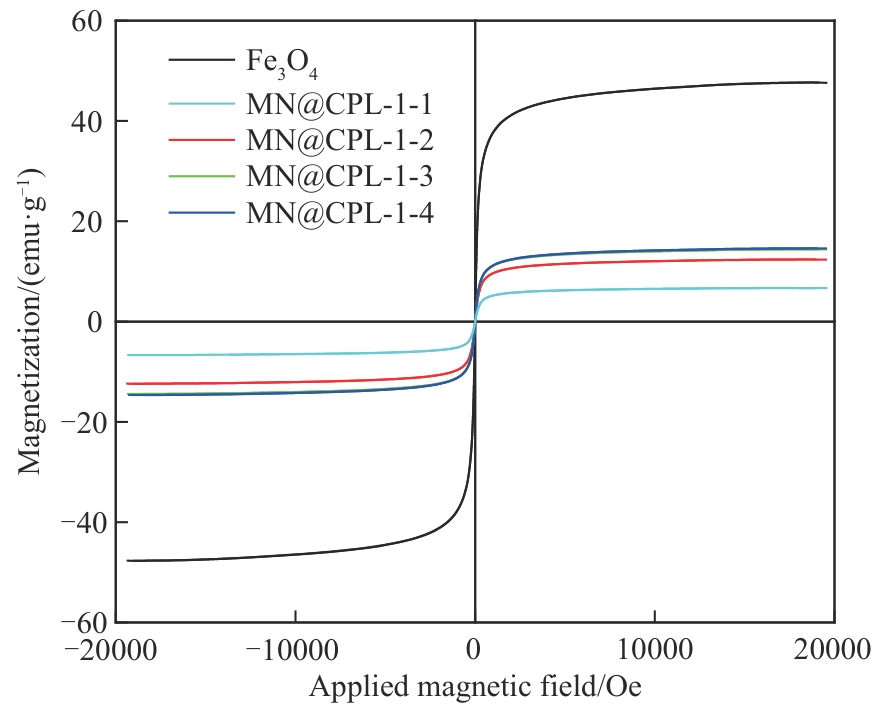
图8 Fe3O4、MN@CPL-1-1、MN@CPL-1-2、MN@CPL-1-3和MN@CPL-1-4的磁滞回线(1 Oe=79.5774715 A·m-1)
Fig.8 Magnetic hysteretic loops of Fe3O4, MN@CPL-1-1, MN@CPL-1-2, MN@CPL-1-3 and MN@CPL-1-4(1 Oe=79.5774715 A·m-1)
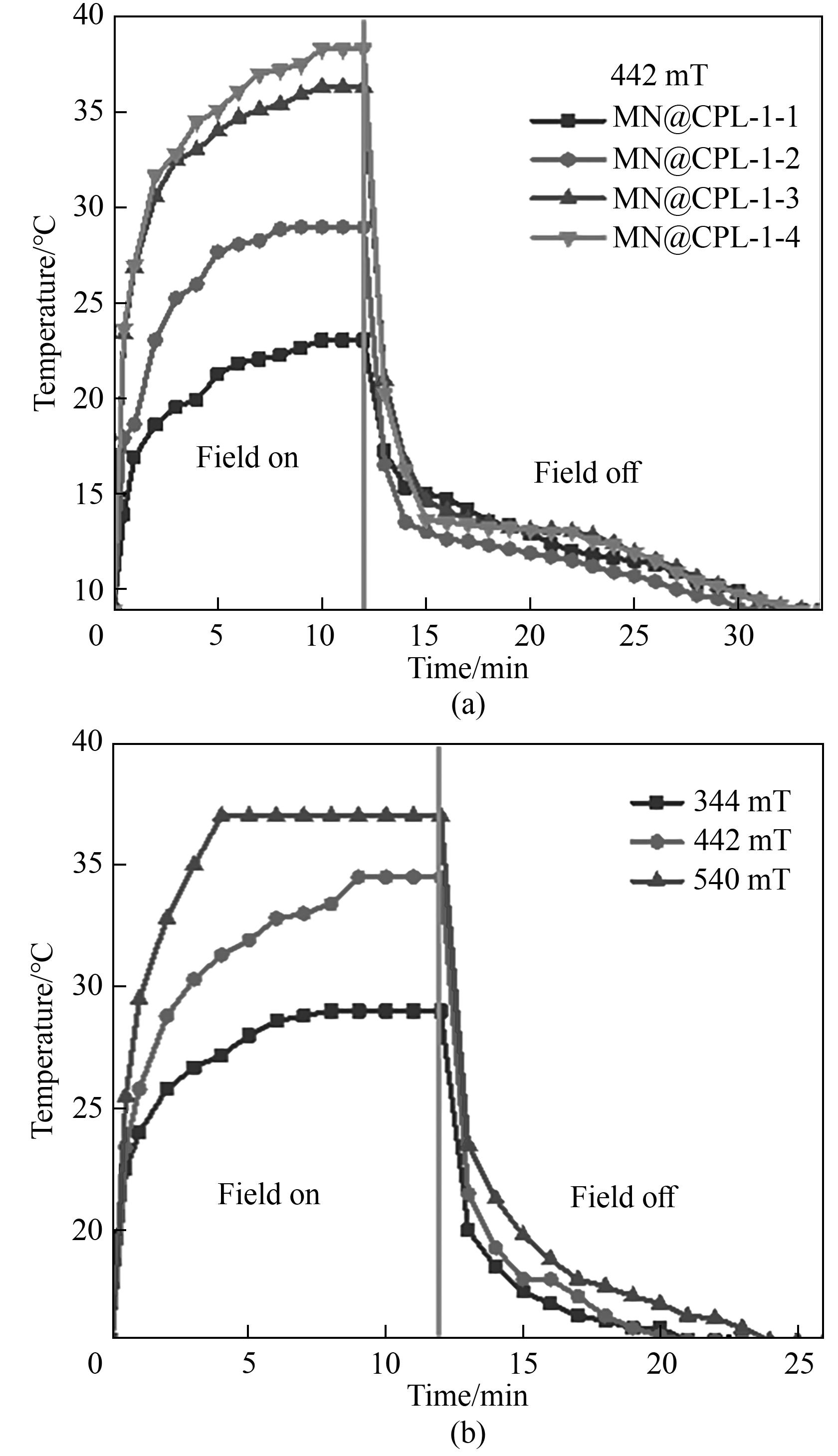
图10 (a)MN@CPL-1-1、MN@CPL-1-2、MN@CPL-1-3和MN@CPL-1-4暴露在442 mT交变磁场和(b)MN@CPL-1-3暴露在不同强度的交变磁场中的温度变化
Fig.10 Temperature changes of (a) MN@CPL-1-1, MN@CPL-1-2, MN@CPL-1-3 and MN@CPL-1-4 exposed to the alternating magnetic field of 442 mT and (b) MN@CPL-1-3 exposed to alternating magnetic fields of different intensities
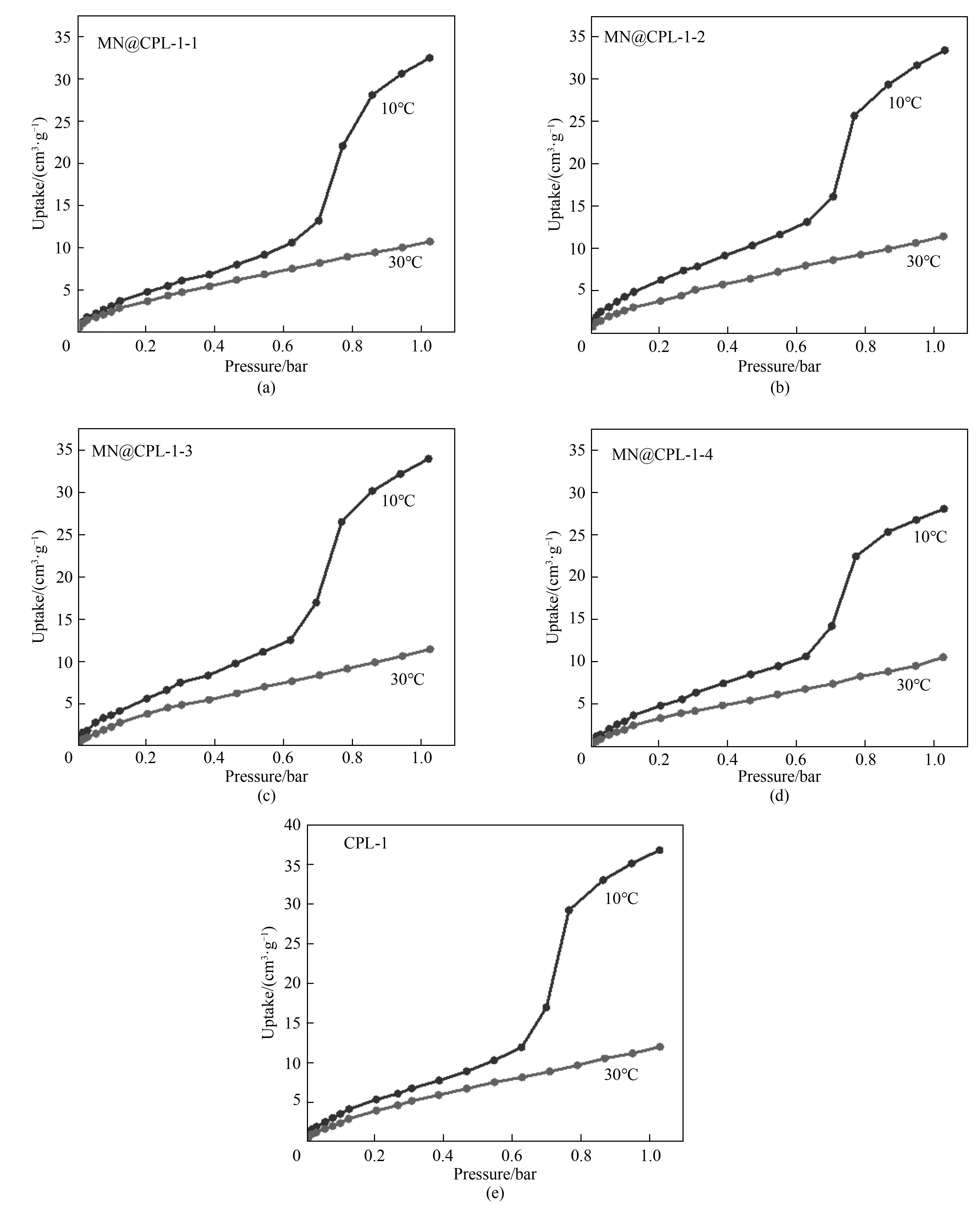
图12 MN@CPL-1-1(a),MN@CPL-1-2(b),MN@CPL-1-3(c),MN@CPL-1-4(d)和CPL-1(e)在10℃和30℃下的C3H6吸附等温线
Fig.12 C2H6 adsorption isotherms of MN@CPL-1-1 (a), MN@CPL-1-2 (b), MN@CPL-1-3 (c), MN@CPL-1-4 (d) and CPL-1 (e) at 10℃ and 30℃ (1 bar=105 Pa)
| Sample | Adsorption capacity at 10℃/ (cm3·g-1) | Adsorption capacity at 30℃/ (cm3·g-1) | Working capacity/ (cm3·g-1) |
|---|---|---|---|
| CPL-1 | 36.8 | 12.0 | 24.8 |
| MN@CPL-1-1 | 32.5 | 10.8 | 21.7 |
| MN@CPL-1-2 | 33.4 | 11.4 | 22.0 |
| MN@CPL-1-3 | 34.0 | 11.5 | 22.5 |
| MN@CPL-1-4 | 28.1 | 10.5 | 17.6 |
表2 样品在10℃和30℃下的饱和吸附量和工作容量
Table 2 Saturated adsorption and working capacities of different samples at 10℃ and 30°C
| Sample | Adsorption capacity at 10℃/ (cm3·g-1) | Adsorption capacity at 30℃/ (cm3·g-1) | Working capacity/ (cm3·g-1) |
|---|---|---|---|
| CPL-1 | 36.8 | 12.0 | 24.8 |
| MN@CPL-1-1 | 32.5 | 10.8 | 21.7 |
| MN@CPL-1-2 | 33.4 | 11.4 | 22.0 |
| MN@CPL-1-3 | 34.0 | 11.5 | 22.5 |
| MN@CPL-1-4 | 28.1 | 10.5 | 17.6 |
| Sample | Tl /℃ | Th/℃ | Working capacity/(cm3·g-1) | References |
|---|---|---|---|---|
| MN@CPL-1-3 | 10 | 30 | 22.50 | This work |
| MAC-4 | 0 | 25 | 13.00 | [ |
| HP-Cu-BTC | 0 | 25 | 6.98 | [ |
| MIL-101 | 20 | 40 | 14.45 | [ |
| ZIF-8 | 0 | 20 | 8.58 | [ |
| Ni-MOF-74 | 25 | 50 | 7.84 | [ |
| 5A | 0 | 20 | 4.14 | [ |
| zeolite 4A | 150 | 185 | 10.30 | [ |
| SG-1 | 20 | 40 | 3.18 | [ |
表3 不同类型的C3H6吸附剂的工作容量
Table 3 Working capacities of different kinds of C3H6 adsorbents
| Sample | Tl /℃ | Th/℃ | Working capacity/(cm3·g-1) | References |
|---|---|---|---|---|
| MN@CPL-1-3 | 10 | 30 | 22.50 | This work |
| MAC-4 | 0 | 25 | 13.00 | [ |
| HP-Cu-BTC | 0 | 25 | 6.98 | [ |
| MIL-101 | 20 | 40 | 14.45 | [ |
| ZIF-8 | 0 | 20 | 8.58 | [ |
| Ni-MOF-74 | 25 | 50 | 7.84 | [ |
| 5A | 0 | 20 | 4.14 | [ |
| zeolite 4A | 150 | 185 | 10.30 | [ |
| SG-1 | 20 | 40 | 3.18 | [ |
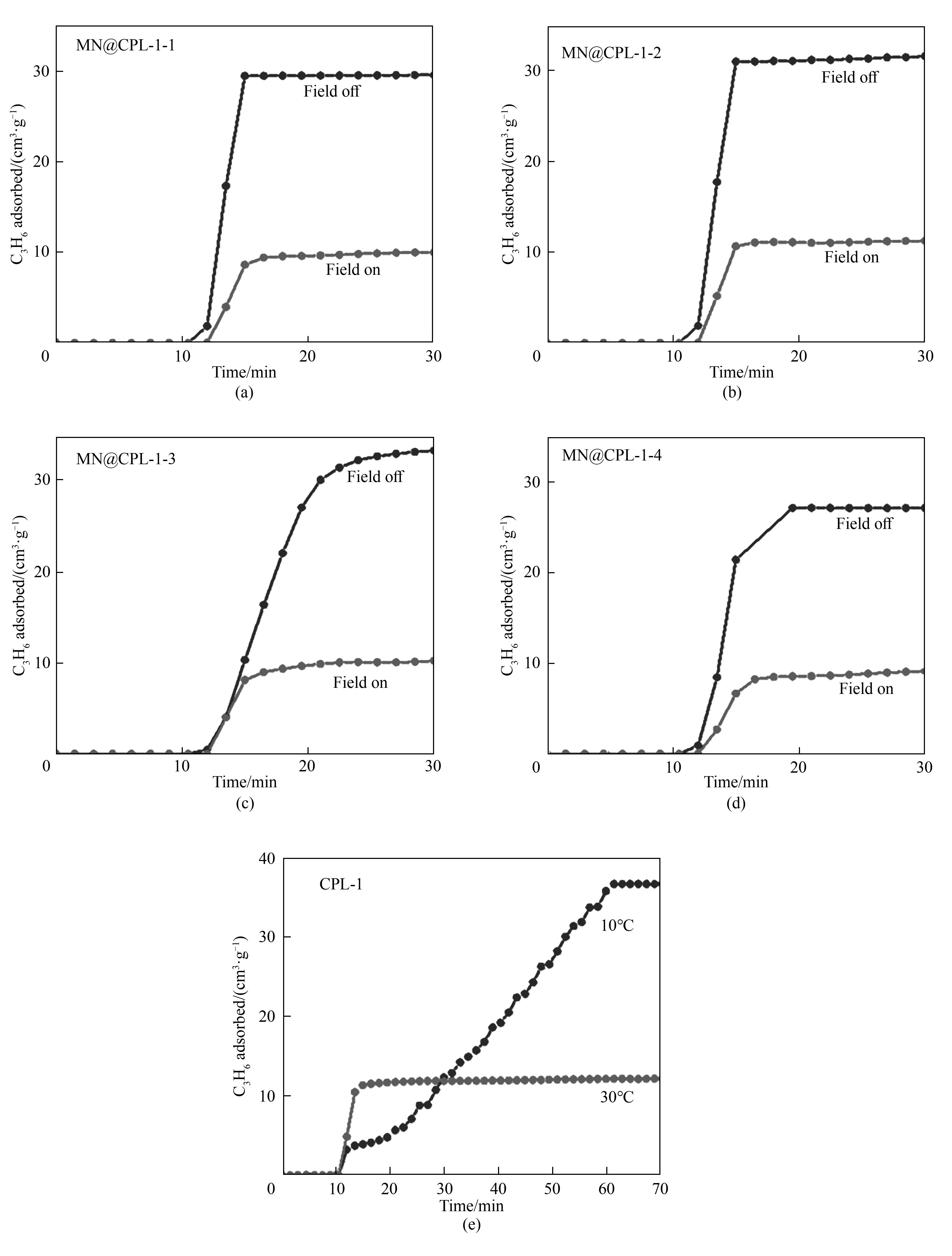
图14 MN@CPL-1-1(a),MN@CPL-1-2(b),MN@CPL-1-(c)3和MN@CPL-1-4(d)在打开和关闭交变磁场下的动态吸附曲线;(e)CPL-1在10 ℃和30 ℃下的动态吸附曲线
Fig.14 Dynamic adsorption curves of MN@CPL-1-1 (a), MN@CPL-1-2 (b), MN@CPL-1-3 (c), and MN@CPL-1-4 (d) with the alternating magnetic field on and off; (e) Dynamic adsorption curve of CPL-1 at 10 ℃ and 30 ℃
| 1 | Cui J Y, Zhang Z Q, Yang L F, et al. A molecular sieve with ultrafast adsorption kinetics for propylene separation[J]. Science, 2024, 383(6679): 179-183. |
| 2 | Fu H L, Huang J Y, van der Tol J J B, et al. Supramolecular polymers form tactoids through liquid-liquid phase separation[J]. Nature, 2024, 626(8001): 1011-1018. |
| 3 | Mattocks J A, Jung J J, Lin C Y, et al. Enhanced rare-earth separation with a metal-sensitive lanmodulin dimer[J]. Nature, 2023, 618(7963): 87-93. |
| 4 | Mensah M A, Niskanen H, Magalhaes A P, et al. Aberrant phase separation and nucleolar dysfunction in rare genetic diseases[J]. Nature, 2023, 614(7948): 564-571. |
| 5 | Zhang H L, Li A, Li K, et al. Ultrafiltration separation of Am(Ⅵ)-polyoxometalate from lanthanides[J]. Nature, 2023, 616(7957): 482-487. |
| 6 | 任其龙, 杨启炜, 鲍宗必. 中国气体分离领域发展现状和未来挑战[J]. 科学观察, 2023, 18(5): 13-16. |
| Ren Q L, Yang Q W, Bao Z B. Development status and future challenges in the field of gas separation in China[J]. Science Focus, 2023, 18(5): 13-16. | |
| 7 | 张凯博, 沈佳新, 李玉霞, 等. Y沸石中Cu(Ⅰ)的可控构筑及其乙烯/乙烷吸附分离性能研究[J]. 化工学报, 2024, 75(4): 1607-1615. |
| Zhang K B, Shen J X, Li Y X, et al. Controllable construction of Cu(Ⅰ) in Y zeolite for adsorptive separation of ethylene/ethane[J]. CIESC Journal, 2024, 75(4): 1607-1615. | |
| 8 | 郭智芳, 张信伟, 王海洋, 等. 石脑油正异构烷烃吸附分离技术[J]. 当代化工, 2024, 53(7): 1703-1710. |
| Guo Z F, Zhang X W, Wang H Y, et al. Technology of adsorption and separation of n/iso-alkanes in naphtha[J]. Contemporary Chemical Industry, 2024, 53(7): 1703-1710. | |
| 9 | Dai H, Yuan X Z, Jiang L B, et al. Recent advances on ZIF-8 composites for adsorption and photocatalytic wastewater pollutant removal: fabrication, applications and perspective[J]. Coordination Chemistry Reviews, 2021, 441: 213985. |
| 10 | Deutz S, Bardow A. Life-cycle assessment of an industrial direct air capture process based on temperature-vacuum swing adsorption[J]. Nature Energy, 2021, 6: 203-213. |
| 11 | Saheed I O, Oh W D, Suah F B M. Chitosan modifications for adsorption of pollutants—a review[J]. Journal of Hazardous Materials, 2021, 408: 124889. |
| 12 | Verougstraete B, Gholami M, Gomez-Rueda Y, et al. Advancements and challenges in electric heating for enhanced temperature swing adsorption processes[J]. Separation and Purification Technology, 2025, 353: 128522. |
| 13 | Li Y C, Delmo E P, Hou G Y, et al. Enhancing local CO2 adsorption by L-histidine incorporation for selective formate production over the wide potential window[J]. Angewandte Chemie International Edition, 2023, 62(49): e202313522. |
| 14 | Zhang Z Q, Chen Y L, Chai K G, et al. Temperature-dependent rearrangement of gas molecules in ultramicroporous materials for tunable adsorption of CO2 and C2H2 [J]. Nature Communications, 2023, 14(1): 3789. |
| 15 | Du X D, Cheng Y G, Liu Z J, et al. CO2 and CH4 adsorption on different rank coals: a thermodynamics study of surface potential, Gibbs free energy change and entropy loss[J]. Fuel, 2021, 283: 118886. |
| 16 | Dang Y X, Tan P, Hu B, et al. Low-energy-consumption temperature swing system for CO2 capture by combining passive radiative cooling and solar heating[J]. Green Energy & Environment, 2024, 9(3): 507-515. |
| 17 | 王丽, 王兴杰, 李浩, 等. 葡萄糖基多孔碳材料对CO2/CH4的分离性能[J]. 化工学报, 2018, 69(2): 733-740. |
| Wang L, Wang X J, Li H, et al. Separation performance of CO2/CH4 on porous carbons derived from glucose[J]. CIESC Journal, 2018, 69(2): 733-740. | |
| 王丽, 王兴杰, 李浩, 等. 葡萄糖基多孔碳材料对CO2/CH4的分离性能[J]. 化工学报, 2018, 69(2): 733-740. | |
| Wang L, Wang X J, Li H, et al. Separation performance of CO2/CH4 on porous carbons derived from glucose[J]. CIESC Journal, 2018, 69(2): 733-740. | |
| 18 | 阎海宇, 付强, 周言, 等. 真空变压吸附捕集烟道气中二氧化碳的模拟、实验及分析[J]. 化工学报, 2016, 67(6): 2371-2379. |
| Yan H Y, Fu Q, Zhou Y, et al. Simulation, experimentation and analyzation of vacuum pressure swing adsorption process for CO2 capture from dry flue gas[J]. CIESC Journal, 2016, 67(6): 2371-2379. | |
| 19 | Gu C, Tan P, Jiang T Y, et al. Solar-radiation-induced adsorption/desorption system for carbon dioxide capture[J]. Cell Reports Physical Science, 2022, 3: 101122. |
| 20 | Jacobs J H, Deering C E, Sui R H, et al. Degradation of desiccants in temperature swing adsorption processes: the temperature dependent degradation of zeolites 4A, 13X and silica gels[J]. Chemical Engineering Journal, 2023, 451: 139049. |
| 21 | Zanco S E, Ambrosetti M, Groppi G, et al. Heat transfer intensification with packed open-cell foams in TSA processes for CO2 capture[J]. Chemical Engineering Journal, 2022, 430: 131000. |
| 22 | Huang G S, Huang C, Tao Y L, et al. Localized heating driven selective growth of metal-organic frameworks (MOFs) in wood: a novel synthetic strategy for significantly enhancing MOF loadings in wood[J]. Applied Surface Science, 2021, 564: 150325. |
| 23 | Tao Y L, Li Q Q, Wu Q N, et al. Embedding metal foam into metal-organic framework monoliths for triggering a highly efficient release of adsorbed atmospheric water by localized eddy current heating[J]. Materials Horizons, 2021, 8(5): 1439-1445. |
| 24 | Li X M, Tan P, Sun Z, et al. Magnetic MOFs with flexibility for efficient magnetic-induced swing adsorption[J]. Separation and Purification Technology, 2024, 348: 127723. |
| 25 | De Belder M, Morais A F, De Vos N, et al. Performance of ferrite nanoparticles in inductive heating swing adsorption (IHSA): how tailoring material properties can circumvent the design limitations of a system[J]. Materials Horizons, 2024, 11(17): 4144-4149. |
| 26 | Bellusci M, Masi A, Albino M, et al. Fe3O4@HKUST-1 magnetic composites by mechanochemical route for induction triggered release of carbon dioxide[J]. Microporous and Mesoporous Materials, 2021, 328: 111458. |
| 27 | Maity R, Gholami M, Peter S A, et al. Strategic fast induction heating to combat hysteresis barriers in a flexible MOF for rapid CO2 desorption in biogas upgrading[J]. Small, 2023, 19(29): 2302893. |
| 28 | Schoukens M, Sharma R, Laha P, et al. Enhancing desorption performance of a compressible hybrid structured adsorbent via localized magnetic induction heating[J]. Advanced Materials Interfaces, 2024, 11(19): 2400105. |
| 29 | Zeng H, Xie M, Wang T, et al. Orthogonal-array dynamic molecular sieving of propylene/propane mixtures[J]. Nature, 2021, 595(7868): 542-548. |
| 30 | Deng Y H, Cai Y, Sun Z K, et al. Multifunctional mesoporous composite microspheres with well-designed nanostructure: a highly integrated catalyst system[J]. Journal of the American Chemical Society, 2010, 132(24): 8466-8473. |
| 31 | Wang G D, Li Y Z, Krishna R, et al. Scalable synthesis of robust MOF for challenging ethylene purification and propylene recovery with record productivity[J]. Angewandte Chemie International Edition, 2024, 63(15): e202319978. |
| 32 | Yang P, Li Y X, Liang W B, et al. Prediction of adsorption isotherms of C3H6/C3H8 on hierarchical porous HP-Cu-BTC[J]. Journal of the Indian Chemical Society, 2022, 99(9): 100657. |
| 33 | Su W, Zhang A, Sun Y, et al. Adsorption properties of C2H4 and C3H6 on 11 adsorbents[J]. Journal of Chemical & Engineering Data, 2017, 62(1): 417-421. |
| 34 | Chen D L, Shang H, Zhu W D, et al. Transient breakthroughs of CO2/CH4 and C3H6/C3H8 mixtures in fixed beds packed with Ni-MOF-74[J]. Chemical Engineering Science, 2014, 117: 407-415. |
| 35 | Patiño-Iglesias M E, Aguilar-Armenta G, Jiménez-López A, et al. Kinetics of the total and reversible adsorption of propylene and propane on zeolite 4A (CECA) at different temperatures[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2004, 237(1/2/3): 73-77. |
| [1] | 郭彭涛, 王婷, 薛波, 应允攀, 刘大欢. 用于CH4/N2分离的多吸附位点超微孔MOF[J]. 化工学报, 2025, 76(5): 2304-2312. |
| [2] | 唐磊, 王振菲, 李聪利, 杨佳辉, 郑浩, 石琪, 董晋湘. Co-MOF-74和Mg-MOF-74的CO工作吸附容量及操作条件[J]. 化工学报, 2025, 76(5): 2279-2293. |
| [3] | 李紫鹃, 谭晓艳, 吴永盛, 杨陈怡, 陈红, 毕小刚, 刘捷, 喻发全. 分子模拟研究三维扭曲催化芳烃-降冰片烯环化聚合物膜的CO2/N2分离机理[J]. 化工学报, 2025, 76(5): 2348-2357. |
| [4] | 花敬贤, 罗宇荣, 顾亚伟, 吴婷婷, 潘宜昌, 邢卫红. 超薄取向ZIF-8膜的制备及乙烯/乙烷高效分离[J]. 化工学报, 2025, 76(5): 2209-2218. |
| [5] | 李艳, 雷美丽, 李鑫钢. 基于分离性能的顺序式模拟移动床结构调控策略[J]. 化工学报, 2025, 76(5): 2219-2229. |
| [6] | 巴雅琪, 吴涛, 邸安頔, 陆安慧. 多孔炭材料用于低碳烃分离的研究进展[J]. 化工学报, 2025, 76(5): 2136-2157. |
| [7] | 向昕辰, 鲁丹, 赵影, 姚之侃, 寇瑞强, 郑丹军, 周志军, 张林. 聚酰胺纳滤膜表面季铵化提高荷正电性及其锂镁分离性能[J]. 化工学报, 2025, 76(5): 2377-2386. |
| [8] | 何燎, 李俊, 高梦舒, 刘东阳, 张宇豪, 赵亮, 高金森, 徐春明. 石油烃中芳烃分离技术研究进展[J]. 化工学报, 2025, 76(5): 1909-1926. |
| [9] | 徐泽海, 刘超, 张国亮. 聚合物基疏水渗透汽化膜及其溶剂回收应用[J]. 化工学报, 2025, 76(5): 2055-2069. |
| [10] | 徐智超, 俞镇东, 吴昊峰, 吴沛文, 武洪翔, 巢艳红, 朱文帅, 刘植昌, 徐春明. 富酸位13X分子筛的制备及其超深度吸附脱除生物柴油中硫醇[J]. 化工学报, 2025, 76(5): 2198-2208. |
| [11] | 茅雨洁, 路晓飞, 锁显, 杨立峰, 崔希利, 邢华斌. 工业气体中微量氧深度脱除催化剂研究进展[J]. 化工学报, 2025, 76(5): 1997-2010. |
| [12] | 时任泽, 丁秋燕, 袁振军, 那健, 刘见华, 郭树虎, 赵雄, 李洪, 高鑫. 4N电子级二乙氧基甲基硅烷的纯化技术研究[J]. 化工学报, 2025, 76(5): 2186-2197. |
| [13] | 王金月, 谢恩泽, 马翰泽, 袁晟, 何光伟, 姜忠义. 单原子层分离膜:进展与展望[J]. 化工学报, 2025, 76(5): 1943-1959. |
| [14] | 陆艳秋, 狄扬, 石文博, 殷聪聪, 汪勇. 基于新型有机多孔聚合物的智能响应膜研究进展[J]. 化工学报, 2025, 76(5): 2101-2118. |
| [15] | 齐昊, 王玉杰, 李申辉, 邹琦, 刘轶群, 赵之平. 双金属Co/Zn-ZIFs中C3H6和C3H8吸附和扩散行为分子模拟研究[J]. 化工学报, 2025, 76(5): 2313-2326. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号