化工学报 ›› 2019, Vol. 70 ›› Issue (7): 2411-2425.DOI: 10.11949/0438-1157.20190011
收稿日期:2019-01-04
修回日期:2019-04-03
出版日期:2019-07-05
发布日期:2019-07-05
通讯作者:
成少安
作者简介:毛政中(1994—),男,博士研究生,<email>11627025@zju.edu.cn</email>
基金资助:
Zhengzhong MAO( ),Yi SUN,Zhipeng HUANG,Chaochao LI,Haobin HUANG,Shao an CHENG(
),Yi SUN,Zhipeng HUANG,Chaochao LI,Haobin HUANG,Shao an CHENG( )
)
Received:2019-01-04
Revised:2019-04-03
Online:2019-07-05
Published:2019-07-05
Contact:
Shao an CHENG
摘要:
微生物电解池(microbial electrolysis cell,MEC)产甲烷技术是以微生物为催化剂,利用外界输入的电能将CO2或有机污染物转化为甲烷的新技术。MEC在实现CO2处置与能量转化的同时,能够处理污水、污泥、沼渣等多种污染物并生产甲烷,具有能量转化率高、生产成本低、环境友好等特点,可望成为解决能源紧缺和环境破坏问题的重要途径之一。近年来,MEC在产甲烷生物阴极结构及电子传递途径、产甲烷微生物群落等方面得到了广泛关注,同时,MEC耦合厌氧消化或其他废水处理系统形成的产甲烷新技术也逐渐研发并成为研究热点。本文综述了产甲烷生物阴极、产甲烷微生物群落等方面的研究现状,介绍了MEC耦合厌氧消化或其他系统产甲烷新技术,总结并分析了MEC产甲烷技术的研究方向和实用化过程仍需解决的技术难题。
中图分类号:
毛政中, 孙怡, 黄志鹏, 李超超, 黄浩斌, 成少安. 微生物电解池产甲烷技术研究进展[J]. 化工学报, 2019, 70(7): 2411-2425.
Zhengzhong MAO, Yi SUN, Zhipeng HUANG, Chaochao LI, Haobin HUANG, Shao an CHENG. Progress of research on methanogenic microbial electrolysis cell[J]. CIESC Journal, 2019, 70(7): 2411-2425.
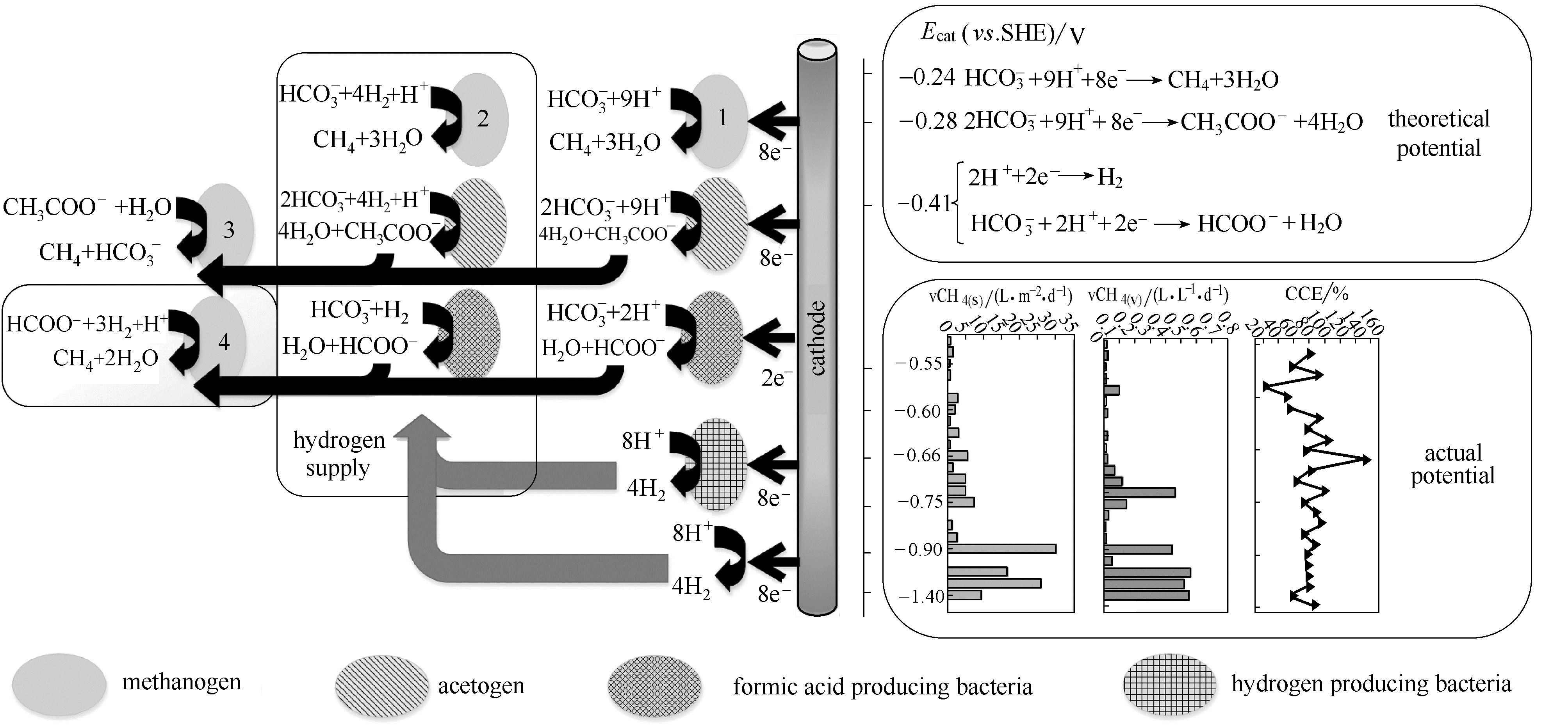
图3 产甲烷生物阴极电子传递途径和产甲烷性能总结[6,33]
Fig.3 Summary of methanogenic biocathode electron transport pathways and properties(All chemical substances involved in the calculation of the theoretical electrode potential are 1 mol·L-1 or 0.1 MPa, pH 7 and temperature 298 K) [6,33]
Exp. Num. | Type | Voltage①/V | Inoculum/substrate | vCH4(v)/(L·L-1·d-1) | Location | Dominant archaea genus | Ref. |
|---|---|---|---|---|---|---|---|
| (1) | DC②(20ml each) | 0.7 | ADS③/acetate | - | anode | Methanobacterium (65%) | [10] |
| (2) | DC(20ml each) | 0.7 | ADS/propionate | - | anode | Methanobacterium(57%) | [10] |
| (3) | SC④ | 0.6 | raw waste sludge | 0.083 | anode | Methanocorpusculum(93%) | [13] |
| (4) | SC | 0.6 | alkali pretreatment of the waste sludge | 0.1 | anode | Methanocorpusculum(85%) | [13] |
| (5) | SC(15 L) | 0.3 | food waste leachate | 0.34 L·g-1TCODremoved | bulk sludge | Methanosarcina(45%) | [53] |
| (6) | SC(20 L) | 0.3 | AD sludge FWTP | (0.34 ± 0.02) L·g-1TCODremoved | bulk sludge | Methanosarcina(24%), Methanobacterium(19%) | [54] |
| (7) | SC(0.8m3) | 4 | FTWW⑤/ADS from winery WWTP⑥ | 1.16 ± 0.06 | matured sludge | Methanomassillicoccus(22%), Methanosphaerula(14%) | [15] |
| (8) | SC(0.8m3)(anaerobic effluent recycling of 200%) | 4 | FTWW /ADS from winery WWTP | 2.01±0.13 | matured sludge | Methanothrix(37.33%), Methanosphaerula(11.17%) | [15] |
| (9) | SC (Φ80×120 mm) | 0.6 | waste activated sludge | 2.26±0.16 | suspended sludge | Methanosaeta(74%) | [55] |
| (10) | DC (300 ml each bottle) | -0.8 | CO2 | 5 L·m-2·d-1 | cathode | Methanobacterium(86.7%) | [1] |
| (11) | DC(800ml cathode working volume)continuous mode | -0.7 | AGS/ethanol and organic acids | — | cathode | Methanobacterium(77%) | [56] |
| (12) | DC(800ml cathode working volume)batch mode | -0.7 | AGS/ethanol and organic acids | — | cathode | Methanobacterium(84%) | [56] |
| (13) | SC | 0.6 | raw waste sludge | 0.083 | cathode | Methanocorpusculum(77%) | [13] |
| (14) | SC | 0.6 | alkali pretreatment of the waste sludge | 0.1 | cathode | Methanobacterium(98%) | [13] |
| (15) | SC(open circuit) | 0 | raw waste sludge | 0.064 | cathode | Methanosaeta(48.2%) | [13] |
表1 产甲烷MEC系统中古细菌组成
Table 1 Main composition of archaea in methanogenic microbial electrolysis cell
Exp. Num. | Type | Voltage①/V | Inoculum/substrate | vCH4(v)/(L·L-1·d-1) | Location | Dominant archaea genus | Ref. |
|---|---|---|---|---|---|---|---|
| (1) | DC②(20ml each) | 0.7 | ADS③/acetate | - | anode | Methanobacterium (65%) | [10] |
| (2) | DC(20ml each) | 0.7 | ADS/propionate | - | anode | Methanobacterium(57%) | [10] |
| (3) | SC④ | 0.6 | raw waste sludge | 0.083 | anode | Methanocorpusculum(93%) | [13] |
| (4) | SC | 0.6 | alkali pretreatment of the waste sludge | 0.1 | anode | Methanocorpusculum(85%) | [13] |
| (5) | SC(15 L) | 0.3 | food waste leachate | 0.34 L·g-1TCODremoved | bulk sludge | Methanosarcina(45%) | [53] |
| (6) | SC(20 L) | 0.3 | AD sludge FWTP | (0.34 ± 0.02) L·g-1TCODremoved | bulk sludge | Methanosarcina(24%), Methanobacterium(19%) | [54] |
| (7) | SC(0.8m3) | 4 | FTWW⑤/ADS from winery WWTP⑥ | 1.16 ± 0.06 | matured sludge | Methanomassillicoccus(22%), Methanosphaerula(14%) | [15] |
| (8) | SC(0.8m3)(anaerobic effluent recycling of 200%) | 4 | FTWW /ADS from winery WWTP | 2.01±0.13 | matured sludge | Methanothrix(37.33%), Methanosphaerula(11.17%) | [15] |
| (9) | SC (Φ80×120 mm) | 0.6 | waste activated sludge | 2.26±0.16 | suspended sludge | Methanosaeta(74%) | [55] |
| (10) | DC (300 ml each bottle) | -0.8 | CO2 | 5 L·m-2·d-1 | cathode | Methanobacterium(86.7%) | [1] |
| (11) | DC(800ml cathode working volume)continuous mode | -0.7 | AGS/ethanol and organic acids | — | cathode | Methanobacterium(77%) | [56] |
| (12) | DC(800ml cathode working volume)batch mode | -0.7 | AGS/ethanol and organic acids | — | cathode | Methanobacterium(84%) | [56] |
| (13) | SC | 0.6 | raw waste sludge | 0.083 | cathode | Methanocorpusculum(77%) | [13] |
| (14) | SC | 0.6 | alkali pretreatment of the waste sludge | 0.1 | cathode | Methanobacterium(98%) | [13] |
| (15) | SC(open circuit) | 0 | raw waste sludge | 0.064 | cathode | Methanosaeta(48.2%) | [13] |
| Coupling system | Effective volume/L | OLR①/(g TCOD·L-1·d-1) | HRT/d | Applied voltage/V | Current density/ (A·m-2) | TCOD removal/% | Other contaminants removal indicators/% | Ce②/% | CCE③/% | vCH4(v)④/ (L·L-1·d-1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
AD-MEC DW⑤ treatment | 3 | 0.08 | 1 | 1 | — | 50 | — | 95 | 80 | 0.012 | [58] |
| AD-MEC | 0.5 | 2.02 | 24 | 0.8 | 0.0501±0.0028 | 87.5±2.2 | 36.9±1.7(VSS) | 56.37±3.31 | >100 | 0.073±0.001 | [65] |
| AD-MEC FWTP⑥ wastewater treatment | 20 | 3.0 | 20 | 0.3 | — | 76.1±3.3 | 73.2±2.1%(TVS⑦) | — | — | (0.34±0.02) L·(g COD)-1 | [54] |
| AD-MEC SEOR⑧ wastewater treatment | 0.022 | 0.21 | 20 | 1.2 | 80 A·m-3 | 95.8 | — | — | — | 0.133±0.0045 | [61] |
| TP-AD-MEC(fermentate-digestate mixture, 55℃) | anode:0.86; cathode:0.86 | 1.5 | 20 | anode: +0.2 V vs SHE | 0.723±0.048 (830 cm2) | 28±3 | — | 119±28(particulate COD) | 51±1 | 0.111±0.010 | [12] |
| UAR⑨-MEC | 0.6 | 1.5—2 | 1 | 0.8±0.01 | 8.6 mA | 83 | 97%(carbohydrate) 62%(protein) 83%(TOC) | 15 | — | 142.8 ml·(g COD)-1 | [23] |
| MEC-AnMBR? | 12000 | 5.88—7.85 | 10 | 0.6 | — | 88.8 | 72.1%(TN⑩) 87.4%( 86.9%(BOD5) | — | — | 0.123 | [75] |
AD-MEC (PS?treatment) | anode:0.5; cathode:0.1 | 0.89 | 9 | anode:-0.03 V vs SHE | 2 | 70±4 | 61±9 (VSS) | 63 | — | — | [79] |
ABR?-MFC-MEC fecal wastewater treatment | ABR:28;MFC:9.6; MEC:9.6 | 0.75 | 48 | MFC output voltage:(452.5±10.5)mV | — | 95.9 | 95%( | — | — | biogas components: CH4:55%–65% | [77] |
| PEC?-MEC | anode:0.08; cathode:0.15 | — | 3 | galvanostatic electrolysis at 2.5 mA | 3.33(7.5 cm2) | — | — | — | 82±10 | 0.0391 | [80] |
| PEC-MEC | anode:0.45;cathode:0.45 | — | — | — | 0.275(40 cm2) | — | — | — | 96 | (192.0 ± 3.6) μl·d-1·cm-2 | [81] |
| MEC-UASB?(pilot scale F-T? wastewater treatment) | 800 | 30.23±1.07 | MEC:0.11 UASB:1.67 | 4.0 | — | 93.5±1.6 | — | — | — | 2.01±0.13 | [15] |
N-MEC? (3 groups: M/C/N) | 1 | M:1.6 C/N:0.9 | M:0.58 C/N:1 | M:1.3 C/N:1 | — | M:80.9±3 C/N:99 | C: 65%±2.4% N: 83%±3% ( | — | — | M: 0.451 | [74] |
| UT?-UASB-MEC | UASB-MEC:35; UT:10 | 0.92±0.02 | 7 | 0.5 | 45 mA | 71.4 | 37.86%(VSS/SS) | — | — | — | [82] |
表2 MEC耦合其他系统产甲烷实验汇总
Table 2 Summary of MEC coupled other systems for methane production
| Coupling system | Effective volume/L | OLR①/(g TCOD·L-1·d-1) | HRT/d | Applied voltage/V | Current density/ (A·m-2) | TCOD removal/% | Other contaminants removal indicators/% | Ce②/% | CCE③/% | vCH4(v)④/ (L·L-1·d-1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
AD-MEC DW⑤ treatment | 3 | 0.08 | 1 | 1 | — | 50 | — | 95 | 80 | 0.012 | [58] |
| AD-MEC | 0.5 | 2.02 | 24 | 0.8 | 0.0501±0.0028 | 87.5±2.2 | 36.9±1.7(VSS) | 56.37±3.31 | >100 | 0.073±0.001 | [65] |
| AD-MEC FWTP⑥ wastewater treatment | 20 | 3.0 | 20 | 0.3 | — | 76.1±3.3 | 73.2±2.1%(TVS⑦) | — | — | (0.34±0.02) L·(g COD)-1 | [54] |
| AD-MEC SEOR⑧ wastewater treatment | 0.022 | 0.21 | 20 | 1.2 | 80 A·m-3 | 95.8 | — | — | — | 0.133±0.0045 | [61] |
| TP-AD-MEC(fermentate-digestate mixture, 55℃) | anode:0.86; cathode:0.86 | 1.5 | 20 | anode: +0.2 V vs SHE | 0.723±0.048 (830 cm2) | 28±3 | — | 119±28(particulate COD) | 51±1 | 0.111±0.010 | [12] |
| UAR⑨-MEC | 0.6 | 1.5—2 | 1 | 0.8±0.01 | 8.6 mA | 83 | 97%(carbohydrate) 62%(protein) 83%(TOC) | 15 | — | 142.8 ml·(g COD)-1 | [23] |
| MEC-AnMBR? | 12000 | 5.88—7.85 | 10 | 0.6 | — | 88.8 | 72.1%(TN⑩) 87.4%( 86.9%(BOD5) | — | — | 0.123 | [75] |
AD-MEC (PS?treatment) | anode:0.5; cathode:0.1 | 0.89 | 9 | anode:-0.03 V vs SHE | 2 | 70±4 | 61±9 (VSS) | 63 | — | — | [79] |
ABR?-MFC-MEC fecal wastewater treatment | ABR:28;MFC:9.6; MEC:9.6 | 0.75 | 48 | MFC output voltage:(452.5±10.5)mV | — | 95.9 | 95%( | — | — | biogas components: CH4:55%–65% | [77] |
| PEC?-MEC | anode:0.08; cathode:0.15 | — | 3 | galvanostatic electrolysis at 2.5 mA | 3.33(7.5 cm2) | — | — | — | 82±10 | 0.0391 | [80] |
| PEC-MEC | anode:0.45;cathode:0.45 | — | — | — | 0.275(40 cm2) | — | — | — | 96 | (192.0 ± 3.6) μl·d-1·cm-2 | [81] |
| MEC-UASB?(pilot scale F-T? wastewater treatment) | 800 | 30.23±1.07 | MEC:0.11 UASB:1.67 | 4.0 | — | 93.5±1.6 | — | — | — | 2.01±0.13 | [15] |
N-MEC? (3 groups: M/C/N) | 1 | M:1.6 C/N:0.9 | M:0.58 C/N:1 | M:1.3 C/N:1 | — | M:80.9±3 C/N:99 | C: 65%±2.4% N: 83%±3% ( | — | — | M: 0.451 | [74] |
| UT?-UASB-MEC | UASB-MEC:35; UT:10 | 0.92±0.02 | 7 | 0.5 | 45 mA | 71.4 | 37.86%(VSS/SS) | — | — | — | [82] |
| 1 | ChengS A, XingD F, CallD F, et al. Direct biological conversion of electrical current into methane by electromethanogenesis[J]. Environmental Science & Technology, 2009, 43(10): 3953-3958. |
| 2 | AryalN, TremblayP, LizakD M, et al. Performance of different Sporomusa species for the microbial electrosynthesis of acetate from carbon dioxide[J]. Bioresource Technology, 2017, 233: 184-190. |
| 3 | BajracharyaS, YuliasniR, VanbroekhovenK, et al. Long-term operation of microbial electrosynthesis cell reducing CO2 to multi-carbon chemicals with a mixed culture avoiding methanogenesis[J]. Bioelectrochemistry, 2017, 113: 26-34. |
| 4 | SchlagerS, HaberbauerM, FuchsbauerA, et al. Bio-electrocatalytic application of microorganisms for carbon dioxide reduction to methane[J]. Chemsuschem, 2017, 10(1SI): 226-233. |
| 5 | RabaeyK, RozendalR A. Microbial electrosynthesis - revisiting the electrical route for microbial production[J]. Nature Reviews Microbiology, 2010, 8(10): 706. |
| 6 | GeppertF, LiuD, van Eerten-JansenM, et al. Bioelectrochemical power-to-gas: state of the art and future perspectives[J]. Trends in Biotechnology, 2016, 34(11): 879-894. |
| 7 | VillanoM, ScardalaS, AulentaF, et al. Carbon and nitrogen removal and enhanced methane production in a microbial electrolysis cell[J]. Bioresource Technology, 2013, 130(1): 366. |
| 8 | LiF, LiY X, CaoY X, et al. Modular engineering to increase intracellular NAD(H/+) promotes rate of extracellular electron transfer of Shewanella oneidensis[J]. Nature Communications, 2018, 9(1): 3637. |
| 9 | ThauerR K, KasterA K, SeedorfH, et al. Methanogenic archaea: ecologically relevant differences in energy conservation[J]. Nature Reviews Microbiology, 2008, 6(8): 579-591. |
| 10 | HariA R, VenkidusamyK, KaturiK P, et al. Temporal microbial community dynamics in microbial electrolysis cells - influence of acetate and propionate concentration[J]. Frontiers in Microbiology, 2017, 8: 1371. |
| 11 | SteinbuschK J J, HamelersH V M, SchaapJ D, et al. Bioelectrochemical ethanol production through mediated acetate reduction by mixed cultures[J]. Environmental Science & Technology, 2010, 44(1): 513-517. |
| 12 | ZeppilliM, PavesiD, GottardoM, et al. Using effluents from two-phase anaerobic digestion to feed a methane-producing microbial electrolysis[J]. Chemical Engineering Journal, 2017, 328: 428-433. |
| 13 | LiuQ, RenZ J, HuangC, et al. Multiple syntrophic interactions drive biohythane production from waste sludge in microbial electrolysis cells[J]. Biotechnology for Biofuels, 2016, 9: 162. |
| 14 | CusickR D, KielyP D, LoganB E. A monetary comparison of energy recovered from microbial fuel cells and microbial electrolysis cells fed winery or domestic wastewaters[J]. International Journal of Hydrogen Energy, 2010, 35(17): 8855-8861. |
| 15 | WangD X, HanY X, HanH J, et al. Enhanced treatment of Fischer-Tropsch wastewater using up-flow anaerobic sludge blanket system coupled with micro-electrolysis cell: a pilot scale study[J]. Bioresource Technology, 2017, 238: 333-342. |
| 16 | YuZ S, LengX Y, ZhaoS, et al. A review on the applications of microbial electrolysis cells in anaerobic digestion[J]. Bioresource Technology, 2018, 255: 340-348. |
| 17 | ZhenG Y, LuX Q, KumarG, et al. Microbial electrolysis cell platform for simultaneous waste biorefinery and clean electrofuels generation: current, situation, challenges and future perspectives[J]. Progress in Energy and Combustion Science, 2017, 63: 119-145. |
| 18 | ZhenG Y, ZhengS J, LuX Q, et al. A comprehensive comparison of five different carbon-based cathode materials in CO2 electromethanogenesis: long-term performance, cell-electrode contact behaviors and extracellular electron transfer pathways[J]. Bioresource Technology, 2018, 266: 382-388. |
| 19 | FuQ, KuramochiY, FukushimaN, et al. Bioelectrochemical analyses of the development of a thermophilic biocathode catalyzing electromethanogenesis[J]. Environmental Science & Technology, 2015, 49(2): 1225-1232. |
| 20 | VillanoM, AulentaF, CiucciC, et al. Bioelectrochemical reduction of CO2 to CH4via direct and indirect extracellular electron transfer by a hydrogenophilic methanogenic culture[J]. Bioresource Technology, 2010, 101(9): 3085-3090. |
| 21 | van Eerten-JansenM, Ter HeijneA, BuismanC, et al. Microbial electrolysis cells for production of methane from CO2: long-term performance and perspectives[J]. International Journal of Energy Research, 2012, 36(6): 809-819. |
| 22 | van Eerten-JansenM, VeldhoenA B, PluggeC M, et al. Microbial community analysis of a methane-producing biocathode in a bioelectrochemical system[J]. Archaea-An International Microbiological Journal, 2013, 2013: 481784. |
| 23 | SangeethaT, GuoZ, LiuW, et al. Cathode material as an influencing factor on beer wastewater treatment and methane production in a novel integrated upflow microbial electrolysis cell (Upflow-MEC)[J]. International Journal of Hydrogen Energy, 2016, 41(4): 2189-2196. |
| 24 | LoganB, ChengS, WatsonV, et al. Graphite fiber brush anodes for increased power production in air-cathode microbial fuel cells[J]. Environmental Science & Technology, 2007, 41(9): 3341-3346. |
| 25 | ClauwaertP, VerstraeteW. Methanogenesis in membraneless microbial electrolysis cells[J]. Applied Microbiology and Biotechnology, 2009, 82(5): 829-836. |
| 26 | ZhenG Y, LuX Q, KobayashiT, et al. Promoted electromethanosynthesis in a two-chamber microbial electrolysis cells (MECs) containing a hybrid biocathode covered with graphite felt (GF)[J]. Chemical Engineering Journal, 2016, 284: 1146-1155. |
| 27 | JiaY, FengH, ShenD, et al. Enhanced production of methane from waste activated sludge by pretreatment using a gas-diffusion cathode[J]. Energy & Fuels, 2016, 30(12): 10511–10515. |
| 28 | ChengS A, YeY L, DingW J, et al. Enhancing power generation of scale-up microbial fuel cells by optimizing the leading-out terminal of anode[J]. Journal of Power Sources, 2014, 248: 931-938. |
| 29 | BajracharyaS, VanbroekhovenK, BuismanC J N, et al. Application of gas diffusion biocathode in microbial electrosynthesis from carbon dioxide[J]. Environmental Science and Pollution Research, 2016, 23(22): 22292-22308. |
| 30 | WangQ N, DongH, YuH B, et al. Enhanced electrochemical reduction of carbon dioxide to formic acid using a two-layer gas diffusion electrode in a microbial electrolysis cell[J]. RSC Advances, 2015, 5(14): 10346-10351. |
| 31 | AlqahtaniM F, KaturiK P, BajracharyaS, et al. Porous hollow fiber nickel electrodes for effective supply and reduction of carbon dioxide to methane through microbial electrosynthesis[J]. Advanced Functional Materials, 2018, 28(43): 1804860. |
| 32 | JourdinL, FreguiaS, DonoseB C, et al. Autotrophic hydrogen-producing biofilm growth sustained by a cathode as the sole electron and energy source[J]. Bioelectrochemistry, 2015, 102: 56-63. |
| 33 | van Eerten-JansenM, JansenN C, PluggeC M, et al. Analysis of the mechanisms of bioelectrochemical methane production by mixed cultures[J]. Journal of Chemical Technology and Biotechnology, 2015, 90(5): 963-970. |
| 34 | LienemannM, DeutzmannJ S, MiltonR D, et al. Mediator-free enzymatic electrosynthesis of formate by the Methanococcus maripaludis heterodisulfide reductase supercomplex[J]. Bioresource Technology, 2018, 254: 278-283. |
| 35 | RotaruA E, ShresthaP M, LiuF, et al. A new model for electron flow during anaerobic digestion: direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane[J]. Energy & Environmental Science, 2013, 7(1): 408-415. |
| 36 | AulentaF, CatapanoL, SnipL, et al. Linking bacterial metabolism to graphite cathodes: electrochemical insights into the H2-producing capability of Desulfovibrio sp.[J]. Chemsuschem, 2012, 5(6SI): 1080-1085. |
| 37 | FischerF, LieskeR, WinzerK. Biological gas reactions Ⅱ concerning the formation of acetic acid in the biological conversion of carbon oxide and carbonic acid with hydrogen to methane[J]. Biochemische Zeitschrift, 1932, 245: 2-12. |
| 38 | DanielsL, SparlingR, SprottG D. The bioenergetics of methanogenesis[J]. Biochimica et Biophysica Acta, 1984, 768(2): 113-163. |
| 39 | FerryJ G. Fundamentals of methanogenic pathways that are key to the biomethanation of complex biomass[J]. Current Opinion in Biotechnology, 2011, 22(3): 351-357. |
| 40 | WelteC, DeppenmeierU. Bioenergetics and anaerobic respiratory chains of aceticlastic methanogens[J]. Biochimica et Biophysica Acta-Bioenergetics, 2014, 1837(7SI): 1130-1147. |
| 41 | NevinK P, WoodardT L, FranksA E, et al. Microbial electrosynthesis: feeding microbes electricity to convert carbon dioxide and water to multicarbon extracellular organic compounds[J]. MBIO, 2010, 1:e00103-102. |
| 42 | FaraghiparapariN, ZenglerK. Production of organics from CO2 by microbial electrosynthesis (MES) at high temperature[J]. Journal of Chemical Technology and Biotechnology, 2017, 92(2): 375-381. |
| 43 | JourdinL, FreguiaS, DonoseB C, et al. A novel carbon nanotube modified scaffold as an efficient biocathode material for improved microbial electrosynthesis[J]. Journal of Materials Chemistry A, 2014, 2(32): 13093-13102. |
| 44 | JourdinL, GriegerT, MonettiJ, et al. High acetic acid production rate obtained by microbial electrosynthesis from carbon dioxide[J]. Environmental Science & Technology, 2015, 49(22): 13566-13574. |
| 45 | ShenL, ZhaoQ C, WuX E, et al. Interspecies electron transfer in syntrophic methanogenic consortia: from cultures to bioreactors[J]. Renewable & Sustainable Energy Reviews, 2016, 54: 1358-1367. |
| 46 | RotaruA, ShresthaP M, LiuF, et al. Direct interspecies electron transfer between Geobacter metallireducens and Methanosarcina barkeri[J]. Applied and Environmental Microbiology, 2014, 80(15): 4599-4605. |
| 47 | LiuF, RotaruA, ShresthaP M, et al. Promoting direct interspecies electron transfer with activated carbon[J]. Energy & Environmental Science, 2012, 5(10): 8982-8989. |
| 48 | ChenS, RotaruA, LiuF, et al. Carbon cloth stimulates direct interspecies electron transfer in syntrophic co-cultures[J]. Bioresource Technology, 2014, 173: 82-86. |
| 49 | ChenS, RotaruA, ShresthaP M, et al. Promoting interspecies electron transfer with biochar[J]. Scientific Reports, 2014, 4(1): 5019. |
| 50 | LovleyD R. Syntrophy goes electric: direct interspecies electron transfer[J]. Annual Review of Microbiology, 2017, 71: 643-664. |
| 51 | ParkJ H, KangH J, ParkK H, et al. Direct interspecies electron transfer via conductive materials: a perspective for anaerobic digestion applications[J]. Bioresource Technology, 2018, 254: 300-311. |
| 52 | FengQ, SongY C, AhnY. Electroactive microorganisms in bulk solution contribute significantly to methane production in bioelectrochemical anaerobic reactor[J]. Bioresource Technology, 2018, 259: 119-127. |
| 53 | LeeB, ParkJ G, ShinW B, et al. Microbial communities change in an anaerobic digestion after application of microbial electrolysis cells[J]. Bioresource Technology, 2017, 234: 273-280. |
| 54 | ParkJ, LeeB, TianD, et al. Bioelectrochemical enhancement of methane production from highly concentrated food waste in a combined anaerobic digester and microbial electrolysis cell[J]. Bioresource Technology, 2018, 247: 226-233. |
| 55 | ZhaoZ S, ZhangY B, QuanX, et al. Evaluation on direct interspecies electron transfer in anaerobic sludge digestion of microbial electrolysis cell[J]. Bioresource Technology, 2016, 200: 235-244. |
| 56 | XuH, WangK, HolmesD E. Bioelectrochemical removal of carbon dioxide (CO2): an innovative method for biogas upgrading[J]. Bioresource Technology, 2014, 173: 392-398. |
| 57 | ZhaoZ, ZhangY, QuanX, et al. Evaluation on direct interspecies electron transfer in anaerobic sludge digestion of microbial electrolysis cell[J]. Bioresource Technology, 2016, 200: 235-244. |
| 58 | MorenoR, San-MartinM I, EscapaA, et al. Domestic wastewater treatment in parallel with methane production in a microbial electrolysis cell[J]. Renewable Energy, 2016, 93: 442-448. |
| 59 | EscapaA, GilcarreraL, GarcíaV, et al. Performance of a continuous flow microbial electrolysis cell (MEC) fed with domestic wastewater[J]. Bioresource Technology, 2012, 117(10): 55-62. |
| 60 | TencaA, CusickR D, SchievanoA, et al. Evaluation of low cost cathode materials for treatment of industrial and food processing wastewater using microbial electrolysis cells[J]. International Journal of Hydrogen Energy, 2013, 38(4): 1859-1865. |
| 61 | YuN, XingD, LiW, et al. Electricity and methane production from soybean edible oil refinery wastewater using microbial electrochemical systems[J]. International Journal of Hydrogen Energy, 2017, 42(1): 96-102. |
| 62 | KielyP D, CusickR, CallD F, et al. Anode microbial communities produced by changing from microbial fuel cell to microbial electrolysis cell operation using two different wastewaters[J]. Bioresource Technology, 2011, 102(1): 388-394. |
| 63 | WagnerR C, ReganJ M, OhS E, et al. Hydrogen and methane production from swine wastewater using microbial electrolysis cells[J]. Water Research, 2009, 43(5): 1480-1488. |
| 64 | CerrilloM, VinasM, BonmatiA. Anaerobic digestion and electromethanogenic microbial electrolysis cell integrated system: Increased stability and recovery of ammonia and methane[J]. Renewable Energy, 2018, 120: 178-189. |
| 65 | ZhaoZ, ZhangY, YuQ, et al. Enhanced decomposition of waste activated sludge via anodic oxidation for methane production and bioenergy recovery[J]. International Biodeterioration & Biodegradation, 2016, 106: 161-169. |
| 66 | ZhenG Y, LuX Q, KobayashiT, et al. Continuous micro-current stimulation to upgrade methanolic wastewater biodegradation and biomethane recovery in an upflow anaerobic sludge blanket (UASB) reactor[J]. Chemosphere, 2017, 180: 229-238. |
| 67 | DouZ, DykstraC M, PavlostathisS G. Bioelectrochemically assisted anaerobic digestion system for biogas upgrading and enhanced methane production[J]. Science of the Total Environment, 2018, 633: 1012-1021. |
| 68 | BeegleJ R, BoroleA P. Energy production from waste: evaluation of anaerobic digestion and bioelectrochemical systems based on energy efficiency and economic factors[J]. Renewable & Sustainable Energy Reviews, 2018, 96: 343-351. |
| 69 | FengQ, SongY C, BaeB U. Influence of applied voltage on the performance of bioelectrochemical anaerobic digestion of sewage sludge and planktonic microbial communities at ambient temperature[J]. Bioresource Technology, 2016, 220: 500-508. |
| 70 | PantD, SinghA, BogaertG V, et al. An introduction to the life cycle assessment (LCA) of bioelectrochemical systems (BES) for sustainable energy and product generation: relevance and key aspects[J]. Renewable & Sustainable Energy Reviews, 2011, 15(2): 1305-1313. |
| 71 | EscapaA, SanmartínM I, MoránA. Potential use of microbial electrolysis cells in domestic wastewater treatment plants for energy recovery[J]. Frontiers in Energy Research, 2014, 2(51): 17519-17527. |
| 72 | ZhenG Y, LuX Q, KatoH, et al. Overview of pretreatment strategies for enhancing sewage sludge disintegration and subsequent anaerobic digestion: current advances, full-scale application and future perspectives[J]. Renewable & Sustainable Energy Reviews, 2017, 69: 559-577. |
| 73 | WangL, AzizT N, RdD L R F. Determining the limits of anaerobic co-digestion of thickened waste activated sludge with grease interceptor waste[J]. Water Research, 2013, 47(11): 3835. |
| 74 | HussainA, LebrunF M, TartakovskyB. Removal of organic carbon and nitrogen in a membraneless flow-through microbial electrolysis cell[J]. Enzyme and Microbial Technology, 2017, 102: 41-48. |
| 75 | 蔡文忠,张希晨,周耀辉. MEC/AnMBR反应器组合处理生活污水[J]. 南华大学学报(自然科学版), 2017, (2): 107-112. |
| CaiW Z, ZhangX C, ZhouY H. MEC/AnMBR reactor combined treatment of domestic sewage[J]. Journal of Nanhua University(Natural Science), 2017, (2): 107-112. | |
| 76 | JiangY, SuM, LiD. Removal of sulfide and production of methane from carbon dioxide in microbial fuel cells-microbial electrolysis cell (MFCs-MEC) coupled system[J]. Applied Biochemistry and Biotechnology, 2014, 172(5): 2720-2731. |
| 77 | LiuH B, LengF, GuanY L, et al. Simultaneous pollutant removal and electricity generation in a combined ABR-MFC-MEC system treating fecal wastewater[J]. Water Air and Soil Pollution, 2017,228: 179. |
| 78 | HuangL, JiangL, WangQ, et al. Cobalt recovery with simultaneous methane and acetate production in biocathode microbial electrolysis cells[J]. Chemical Engineering Journal, 2014, 253: 281-290. |
| 79 | KiD, ParameswaranP, PopatS C, et al. Maximizing Coulombic recovery and solids reduction from primary sludge by controlling retention time and pH in a flat-plate microbial electrolysis cell[J]. Environmental Science-Water Research & Technology, 2017, 3(2): 333-339. |
| 80 | NicholsE M, GallagherJ J, LiuC, et al. Hybrid bioinorganic approach to solar-to-chemical conversion[J]. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(37): 11461-11466. |
| 81 | FuQ, XiaoS, LiZ, et al. Hybrid solar-to-methane conversion system with a Faradaic efficiency of up to 96%[J]. Nano Energy, 2018, 53: 232-239. |
| 82 | LiX J, ZhuT, ZhangK, et al. Enhanced sludge degradation process using a microbial electrolysis cell in an up-flow anaerobic sludge blanket reactor with ultrasound treatment[J]. Chemical Engineering Journal, 2016, 306: 17-21. |
| 83 | BarberJ, TranP D. From natural to artificial photosynthesis[J]. Journal of the Royal Society Interface, 2013, 10(81): 20120984. |
| [1] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [2] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [3] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [4] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [5] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [6] | 洪瑞, 袁宝强, 杜文静. 垂直上升管内超临界二氧化碳传热恶化机理分析[J]. 化工学报, 2023, 74(8): 3309-3319. |
| [7] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [8] | 吕龙义, 及文博, 韩沐达, 李伟光, 高文芳, 刘晓阳, 孙丽, 王鹏飞, 任芝军, 张光明. 铁基导电材料强化厌氧去除卤代有机污染物:研究进展及未来展望[J]. 化工学报, 2023, 74(8): 3193-3202. |
| [9] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [10] | 牛超, 沈胜强, 杨艳, 潘泊年, 李熠桥. 甲烷BOG喷射器流动过程计算与性能分析[J]. 化工学报, 2023, 74(7): 2858-2868. |
| [11] | 刘晓洋, 喻健良, 侯玉洁, 闫兴清, 张振华, 吕先舒. 螺旋微通道对掺氢甲烷爆轰传播的影响[J]. 化工学报, 2023, 74(7): 3139-3148. |
| [12] | 周小文, 杜杰, 张战国, 许光文. 基于甲烷脉冲法的Fe2O3-Al2O3载氧体还原特性研究[J]. 化工学报, 2023, 74(6): 2611-2623. |
| [13] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [14] | 张艳梅, 袁涛, 李江, 刘亚洁, 孙占学. 高效SRB混合菌群构建及其在酸胁迫条件下的性能研究[J]. 化工学报, 2023, 74(6): 2599-2610. |
| [15] | 李晨曦, 刘永峰, 张璐, 刘海峰, 宋金瓯, 何旭. O2/CO2氛围下正庚烷的燃烧机理研究[J]. 化工学报, 2023, 74(5): 2157-2169. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号