化工学报 ›› 2020, Vol. 71 ›› Issue (7): 3296-3303.DOI: 10.11949/0438-1157.20191579
收稿日期:2019-12-25
修回日期:2020-03-19
出版日期:2020-07-05
发布日期:2020-07-05
通讯作者:
沈意
作者简介:胡京宇(2000—),女,本科生,基金资助:
Jingyu HU( ),Rong YAO,Yuhang PAN,Chao ZHU,Shuang SONG,Yi SHEN(
),Rong YAO,Yuhang PAN,Chao ZHU,Shuang SONG,Yi SHEN( )
)
Received:2019-12-25
Revised:2020-03-19
Online:2020-07-05
Published:2020-07-05
Contact:
Yi SHEN
摘要:
利用吸附-光催化技术去除水体中有机染料,是高效节能的方法之一。然而,碳基吸附剂在光催化降解污染物过程中,由于活性氧的攻击会发生自身的逐渐氧化。本研究中,采用尿素水解水热法制备二氧化钛/层状双氢氧化物(TiO2/LDH)非碳基复合材料,不仅对水体中有机染料具有较高的吸附容量,也在光辐射后具有可重复的再生能力。对甲基橙的吸附量为527.5 mg/g,在4轮循环再生后仍具有88.6%的再生率;对亚甲基蓝的吸附量为208.3 mg/g,在4轮循环再生后仍具有94.7%的再生率。在吸附-光再生过程中,LDH基底自身具有强抗氧化能力和高吸附能力,同时提高了负载剂TiO2的光吸收效率,为水中有机污染物去除和吸附-光催化复合材料设计优化提供了可行的策略。
中图分类号:
胡京宇, 姚戎, 潘玉航, 朱超, 宋爽, 沈意. 可光助再生二氧化钛/层状双氢氧化物去除水体中有机染料[J]. 化工学报, 2020, 71(7): 3296-3303.
Jingyu HU, Rong YAO, Yuhang PAN, Chao ZHU, Shuang SONG, Yi SHEN. Photo-assisted regeneration of titanium dioxide/layered double hydroxide for removal of organic dyes in water[J]. CIESC Journal, 2020, 71(7): 3296-3303.
| 元素 | 项目 | TiO2/LDH 样品1 | TiO2/LDH 样品2 | TiO2/LDH 样品3 | 平均 |
|---|---|---|---|---|---|
| 钛 | 原样钛含量/%(质量) | 1.37 | 1.35 | 1.41 | 1.38 |
| TiO2负载量/%(质量) | 2.28 | 2.25 | 2.35 | 2.29 |
表1 TiO2/LDH中TiO2负载量实验数据
Table 1 Test on TiO2 loaded in TiO2/LDH
| 元素 | 项目 | TiO2/LDH 样品1 | TiO2/LDH 样品2 | TiO2/LDH 样品3 | 平均 |
|---|---|---|---|---|---|
| 钛 | 原样钛含量/%(质量) | 1.37 | 1.35 | 1.41 | 1.38 |
| TiO2负载量/%(质量) | 2.28 | 2.25 | 2.35 | 2.29 |
| 吸附剂 | 比表面积/(m2/g) | 平均孔径/nm | 孔体积/(cm3/g) |
|---|---|---|---|
| LDH | 59.4 | 5.66 | 0.26 |
| TiO2/LDH | 67.1 | 7.34 | 0.35 |
表2 LDH和TiO2/LDH材料的比表面积及孔隙性质
Table 2 Specific surface area and pore textural properties of LDH and TiO2/LDH
| 吸附剂 | 比表面积/(m2/g) | 平均孔径/nm | 孔体积/(cm3/g) |
|---|---|---|---|
| LDH | 59.4 | 5.66 | 0.26 |
| TiO2/LDH | 67.1 | 7.34 | 0.35 |
| 污染物 | 吸附剂 | Freundlich 模型 | Langmuir 模型 | ||||
|---|---|---|---|---|---|---|---|
| Kf | N | R2 | qm/(mg/g) | KL/(L/g) | R2 | ||
| 甲基橙 | LDH | 141.11867 | 0.29511 | 0.90351 | 462.34149 | 0.25171 | 0.96492 |
| TiO2/LDH | 163.77771 | 0.29323 | 0.90024 | 527.49174 | 0.2654 | 0.96388 | |
| 亚甲基蓝 | LDH | 75.76239 | 0.2844 | 0.89484 | 166.92703 | 0.95467 | 0.95936 |
| TiO2/LDH | 94.99944 | 0.29356 | 0.92097 | 208.32481 | 1.07183 | 0.9804 | |
表3 LDH和TiO2/LDH对有机染料的Freundlich和Langmuir等温吸附参数
Table 3 Regression parameters of adsorption isotherms of organic dyes onto LDH and TiO2/LDH fitted by Freundlich and Langmuir models
| 污染物 | 吸附剂 | Freundlich 模型 | Langmuir 模型 | ||||
|---|---|---|---|---|---|---|---|
| Kf | N | R2 | qm/(mg/g) | KL/(L/g) | R2 | ||
| 甲基橙 | LDH | 141.11867 | 0.29511 | 0.90351 | 462.34149 | 0.25171 | 0.96492 |
| TiO2/LDH | 163.77771 | 0.29323 | 0.90024 | 527.49174 | 0.2654 | 0.96388 | |
| 亚甲基蓝 | LDH | 75.76239 | 0.2844 | 0.89484 | 166.92703 | 0.95467 | 0.95936 |
| TiO2/LDH | 94.99944 | 0.29356 | 0.92097 | 208.32481 | 1.07183 | 0.9804 | |
| 污染物 | 吸附剂 | 拟一级动力学 | 拟二级动力学 | ||||
|---|---|---|---|---|---|---|---|
| qe/(mg/g) | k1/(1/s) | R2 | qe/(mg/g) | k2/(g/(mg·s)) | R2 | ||
| 甲基橙 | LDH | 178.46944 | 0.01407 | 0.97968 | 198.49873 | 0.01815 | 0.92925 |
| TiO2/LDH | 206.09581 | 0.02074 | 0.98208 | 225.49671 | 0.02831 | 0.92758 | |
| 亚甲基蓝 | LDH | 69.2104 | 0.01416 | 0.98638 | 76.08457 | 0.01969 | 0.95594 |
| TiO2/LDH | 81.4685 | 0.01977 | 0.99344 | 89.16015 | 0.02729 | 0.95272 | |
表4 LDH和TiO2/LDH对有机染料的吸附动力学参数
Table 4 Adsorption kinetic parameters of organic dyes onto LDH and TiO2/LDH
| 污染物 | 吸附剂 | 拟一级动力学 | 拟二级动力学 | ||||
|---|---|---|---|---|---|---|---|
| qe/(mg/g) | k1/(1/s) | R2 | qe/(mg/g) | k2/(g/(mg·s)) | R2 | ||
| 甲基橙 | LDH | 178.46944 | 0.01407 | 0.97968 | 198.49873 | 0.01815 | 0.92925 |
| TiO2/LDH | 206.09581 | 0.02074 | 0.98208 | 225.49671 | 0.02831 | 0.92758 | |
| 亚甲基蓝 | LDH | 69.2104 | 0.01416 | 0.98638 | 76.08457 | 0.01969 | 0.95594 |
| TiO2/LDH | 81.4685 | 0.01977 | 0.99344 | 89.16015 | 0.02729 | 0.95272 | |
| 污染物 | 吸附剂 | Recovery/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 h | 1 h | 1.5 h | 2 h | 3 h | 4 h | 5 h | 7 h | 10 h | ||
| 甲基橙 | LDH | 0 | 0 | 0 | 0 | 0.4 | 1.0 | 1.3 | 1.9 | 2.2 |
| TiO2/LDH | 22.52 | 42.36 | 59.95 | 73.60 | 84.89 | 93.05 | 93.95 | 93.95 | 93.94 | |
| 亚甲基 | LDH | 0 | 0 | 0 | 0 | 0.3 | 0.8 | 1.0 | 1.6 | 3.0 |
| TiO2/LDH | 29.48 | 49.55 | 68.41 | 81.26 | 90.29 | 95.69 | 96.87 | 96.85 | 96.85 | |
表5 LDH和TiO2/LDH对有机染料的再生动力学参数
Table 5 Regenerated kinetic parameters of LDH and TiO2/LDH for organic dyes in timeline of photocatalytic regeneration
| 污染物 | 吸附剂 | Recovery/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 h | 1 h | 1.5 h | 2 h | 3 h | 4 h | 5 h | 7 h | 10 h | ||
| 甲基橙 | LDH | 0 | 0 | 0 | 0 | 0.4 | 1.0 | 1.3 | 1.9 | 2.2 |
| TiO2/LDH | 22.52 | 42.36 | 59.95 | 73.60 | 84.89 | 93.05 | 93.95 | 93.95 | 93.94 | |
| 亚甲基 | LDH | 0 | 0 | 0 | 0 | 0.3 | 0.8 | 1.0 | 1.6 | 3.0 |
| TiO2/LDH | 29.48 | 49.55 | 68.41 | 81.26 | 90.29 | 95.69 | 96.87 | 96.85 | 96.85 | |
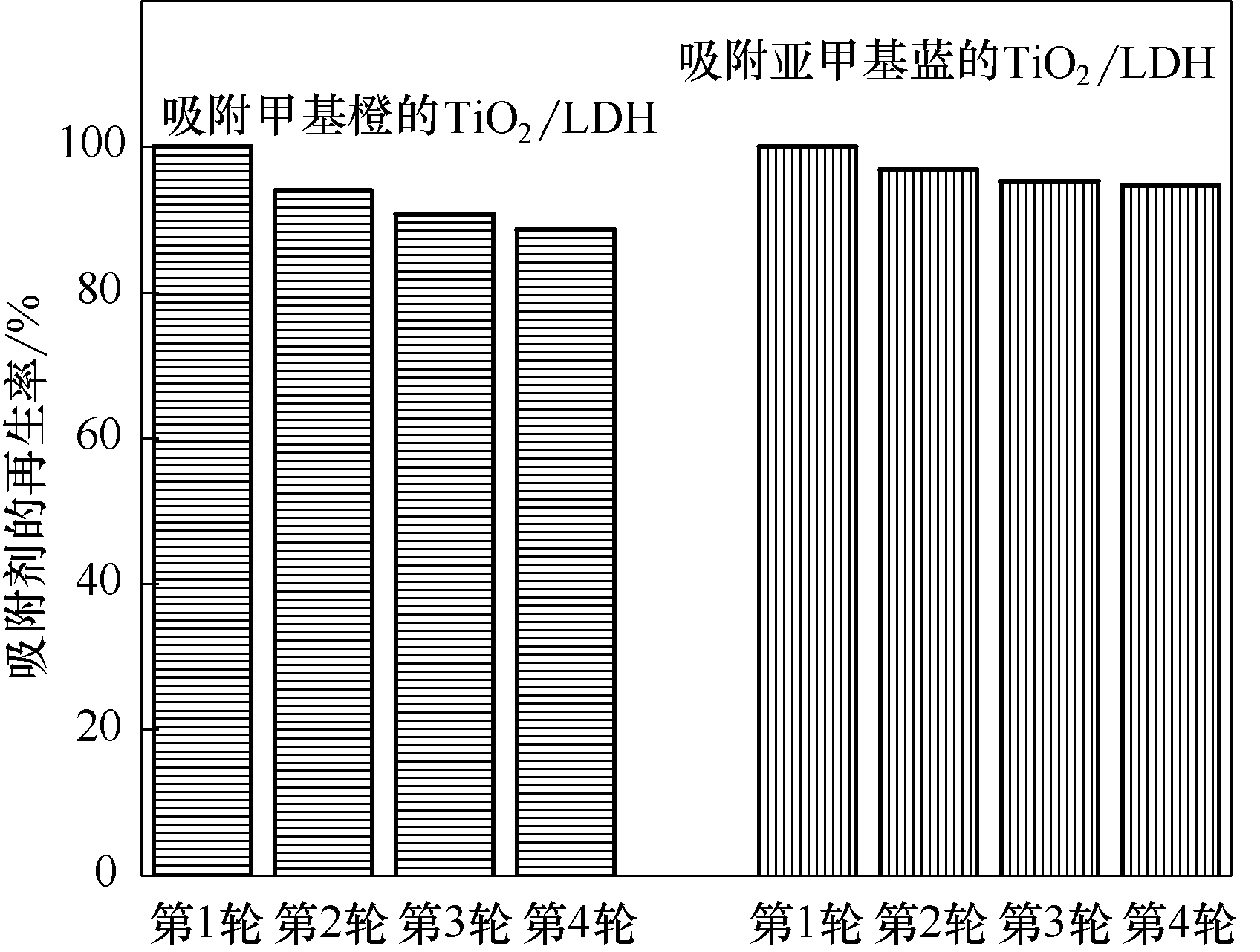
图6 TiO2/LDH吸附有机染料的多轮循环吸附再生
Fig.6 Percentage of regenerated adsorption capacity of LDH and TiO2/LDH for organic dyes in cycles of photocatalytic regeneration
| 1 | 牟铭, 顾宝珊, 王仕东, 等. 石墨烯及其复合材料在水处理中的研究进展[J]. 化工新型材料, 2019, (12): 16-21. |
| Mu M, Gu B S, Wang S D, et al. Progress of graphene and its composite in water treatment[J]. New Chemical Materials, 2019, (12): 16-21. | |
| 2 | Jiang J, Zhang X, Zhu X, et al. Removal of intermediate aromatic halogenated DBPs by activated carbon adsorption: a new approach to controlling halogenated DBPs in chlorinated drinking water[J]. Environmental Science & Technology, 2017, 51(6): 3435-3444. |
| 3 | Shen Y, Fang Q, Chen B. Environmental applications of three-dimensional graphene-based macrostructures: adsorption, transformation, and detection[J]. Environmental Science & Technology, 2015, 49(1): 67-84. |
| 4 | Shen Y, Tong Y, Xu J, et al. Ni-based layered metal-organic frameworks with palladium for electrochemical dichlorination[J]. Applied Catalysis B: Environmental, 2020, 264: 118505. |
| 5 | Shen Y, Zhu C, Song S, et al. Defect-abundant covalent triazine frameworks as sunlight-driven self-cleaning adsorbents for volatile aromatic pollutants in water[J]. Environmental Science & Technology, 2019, 53(15): 9091-9101. |
| 6 | Liu W, Cai Z Q, Zhao X, et al. High-capacity and photoregenerable composite material for efficient adsorption and degradation of phenanthrene in water[J]. Environmental Science & Technology, 2016, 50(20): 11174- 11183. |
| 7 | Li M, Lu B, Ke Q, et al. Synergetic effect between adsorption and photodegradation on nanostructured TiO2/activated carbon fiber felt porous composites for toluene removal[J]. Journal of Hazardous Materials, 2017, 333: 88-98. |
| 8 | Zaib Q, Mansoor B, Ahmad F. Photo-regenerable multi-walled carbon nanotube membranes for the removal of pharmaceutical micropollutants from water[J]. Environmental Science-Processes & Impacts, 2013, 15(8): 1582-1589. |
| 9 | 杜瑞安, 马小帅, 张萌迪, 等. 多壁碳纳米管/TiO2复合材料的合成及其光催化性能[J]. 有色金属科学与工程, 2019, (5): 75-84. |
| Du R A, Ma X S, Zhang M D, et al. Synthesis of multi-walled carbon nanotubes/TiO2 composites and their photocatalytic performance[J]. Nonferrous Metals Science and Engineering, 2019, (5): 75-84. | |
| 10 | Zhu C, Xu J, Song S, et al. TiO2 quantum dots loaded sulfonated graphene aerogel for effective adsorption-photocatalysis of PFOA[J]. Science of the Total Environment, 2020, 698: 134275. |
| 11 | 李梦悦, 房国丽, 张刚, 等. TiO2/AC复合材料的吸附性与光催化性能[J]. 河北大学学报(自然科学版), 2018, (5): 480-489. |
| Li M Y, Fang G L, Zhang G, et al. Adsorption and photocatalytic performance of TiO2/AC composites[J]. Journal of Hebei University(Natural Science Edition), 2018, (5): 480-489. | |
| 12 | 唐祝兴, 余孟. ZnO/石墨烯复合光催化材料的制备及性能研究[J]. 电镀与精饰, 2019, (7): 20-24. |
| Tang Z X, Yu M. Preparation and properties of ZnO/graphene composite photocatalyst[J]. Plating & Finishing, 2019, (7): 20-24. | |
| 13 | Mohamed M M, Ghanem M A, Khairy M, et al. Zinc oxide incorporated carbon nanotubes or graphene oxide nanohybrids for enhanced sonophotocatalytic degradation of methylene blue dye[J]. Applied Surface Science, 2019, 487: 539-549. |
| 14 | 孟亮, 孙阳, 公晗, 等. 石墨烯基材料应用于水污染物治理领域的研究进展[J]. 新型碳材料, 2019, (3): 220-237. |
| Meng L, Sun Y, Gong H, et al. Research progress of the application of graphene-based materials in the treatment of water pollutants[J]. New Carbon Materials, 2019, (3): 220-237. | |
| 15 | Cavani F, Trifiro F, Vaccari A. Hydrotalcite-type anionic clays: preparation, properties and applications[J]. Catalysis Today, 1991, 11(2): 173-301. |
| 16 | Darmograi G, Prelot B, Layrac G, et al. Study of adsorption and intercalation of orange-type dyes into Mg-Al layered double hydroxide[J]. Journal of Physical Chemistry C, 2015, 119(41): 23388-23397. |
| 17 | Valente J S, Tzompantzi F, Prince J, et al. Adsorption and photocatalytic degradation of phenol and 2,4-dichlorophenoxiacetic acid by Mg-Zn-Al layered double hydroxides[J]. Applied Catalysis B: Environmental, 2009, 90(3/4): 330-338. |
| 18 | Inacio J, Taviot-Gueho C, Forano C, et al. Adsorption of MCPA pesticide by MgAl-layered double hydroxides[J]. Applied Clay Science, 2001, 18(5/6): 255-264. |
| 19 | Kumar S, Isaacs M A, Trofimovaite R, et al. P25@CoAl layered double hydroxide heterojunction nanocomposites for CO2 photocatalytic reduction[J]. Applied Catalysis B: Environmental, 2017, 209: 394-404. |
| 20 | Liu Z P, Ma R Z, Osada M, et al. Synthesis, anion exchange, and delamination of Co-Al layered double hydroxide: assembly of the exfoliated nanosheet/polyanion composite films and magneto-optical studies[J]. Journal of the American Chemical Society, 2006, 128(14): 4872-4880. |
| 21 | 马立民, 侯凯明, 杨志刚, 等. 不同阴离子插层双金属氢氧化物的制备及摩擦学性能研究[J]. 摩擦学学报, 2017, (2): 192-198. |
| Ma L M, Hou K M, Yang Z G, et al. Synthesis of various Co-Al LDH interlayered with different anions and their tribological properties as oil additive[J]. Tribology, 2017, (2): 192-198. | |
| 22 | Khan M, Bashir J. Small angle neutron scattering and X-ray diffraction studies of nanocrystalline titanium dioxide[J]. Journal of Modern Physics, 2011, 2: 962-965. |
| 23 | Iyi N, Matsumoto T, Kaneko Y, et al. Deintercalation of carbonate ions from a hydrotalcite-like compound: enhanced decarbonation using acid-salt mixed solution[J]. Chemistry of Materials, 2004, 16(15): 2926-2932. |
| 24 | Shen Y, Chen B. Sulfonated graphene nanosheets as a superb adsorbent for various environmental pollutants in water[J]. Environmental Science & Technology, 2015, 49(12): 7364-7372. |
| 25 | 杨岳, 陈珊媛, 关成立, 等. 活性炭纤维ACF对甲基橙染料的吸附动力学研究[J]. 广州化工, 2020, (1): 66-68. |
| Yang Y, Chen S Y, Guan C L, et al. Adsorption kinetics of activated carbon fiber on methyl orange[J]. Guangzhou Chemical Industry, 2020, (1): 66-68. | |
| 26 | 时文. 兰炭的改性及其对废水中染料的吸附性能研究[D]. 合肥: 合肥工业大学, 2016. |
| Shi W. Study on modification of semi-coke and its adsorption properties of dye in waste water[D]. Hefei: Hefei University of Technology, 2016. | |
| 27 | Shen Y, Zhu C, Chen B. Immobilizing 1—3 nm Ag nanoparticles in reduced graphene oxide aerogel as a high-effective catalyst for reduction of nitroaromatic compounds[J]. Environmental Pollution, 2020, 256: 113405. |
| 28 | 刘伟, 杨旭, 王吉林, 等. N-TiO2/季铵化壳聚糖杂化膜光降解甲基橙废水[J]. 水处理技术, 2020, (3): 56-61. |
| Liu W, Yang X, Wang J L, et al. Photodegradation of methyl orange dye wastewater by N-TiO2/quaternized chitosan hybrid membrane[J]. Technology of Water Treatment, 2020, (3): 56-61. | |
| 29 | 胡伟. 石墨烯负载TiO2复合材料的制备及光催化降解印染废水的研究[J]. 印染助剂, 2019, (8): 32-36. |
| Hu W. Preparation of graphene-loaded TiO2 composite and photocatalytic degradation of printing and dyeing wastewater[J]. Textile Auxiliaries, 2019, (8): 32-36. | |
| 30 | 殷榕灿, 崔玉民, 苗慧, 等. TiO2光催化降解有机染料反应机理[J]. 水处理技术, 2020, (3): 11-15. |
| Yin R C, Cui Y M, Miao H, et al. Reaction mechanisms of photocatalytic degradation of organic dyes with TiO2[J]. Technology of Water Treatment, 2020, (3): 11-15. | |
| 31 | Schneider J, Matsuoka M, Takeuchi M, et al. Understanding TiO2 photocatalysis: mechanisms and materials[J]. Chemical Reviews, 2014, 114(19): 9919-9986. |
| 32 | Akpan U G, Hameed B H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: a review[J]. Journal of Hazardous Materials, 2009, 170(2/3): 520-529. |
| [1] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [2] | 胡兴枝, 张皓焱, 庄境坤, 范雨晴, 张开银, 向军. 嵌有超小CeO2纳米粒子的碳纳米纤维的制备及其吸波性能[J]. 化工学报, 2023, 74(8): 3584-3596. |
| [3] | 文兆伦, 李沛睿, 张忠林, 杜晓, 侯起旺, 刘叶刚, 郝晓刚, 官国清. 基于自热再生的隔壁塔深冷空分工艺设计及优化[J]. 化工学报, 2023, 74(7): 2988-2998. |
| [4] | 张澳, 罗英武. 低模量、高弹性、高剥离强度丙烯酸酯压敏胶[J]. 化工学报, 2023, 74(7): 3079-3092. |
| [5] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [6] | 蔡斌, 张效林, 罗倩, 党江涛, 左栗源, 刘欣梅. 导电薄膜材料的研究进展[J]. 化工学报, 2023, 74(6): 2308-2321. |
| [7] | 卫雪岩, 钱勇. 微米级铁粉燃料中低温氧化反应特性及其动力学研究[J]. 化工学报, 2023, 74(6): 2624-2638. |
| [8] | 崔张宁, 胡紫璇, 吴雷, 周军, 叶干, 刘田田, 张秋利, 宋永辉. 可降解纤维素基材料的耐水性能研究进展[J]. 化工学报, 2023, 74(6): 2296-2307. |
| [9] | 李振, 张博, 王丽伟. PEG-EG固-固相变材料的制备和性能研究[J]. 化工学报, 2023, 74(6): 2680-2688. |
| [10] | 代佳琳, 毕唯东, 雍玉梅, 陈文强, 莫晗旸, 孙兵, 杨超. 热物性对混合型CPCMs固液相变特性影响模拟研究[J]. 化工学报, 2023, 74(5): 1914-1927. |
| [11] | 陈韶云, 徐东, 陈龙, 张禹, 张远方, 尤庆亮, 胡成龙, 陈建. 单层聚苯胺微球阵列结构的制备及其吸附性能[J]. 化工学报, 2023, 74(5): 2228-2238. |
| [12] | 肖川宝, 李林洋, 刘武锋, 钟年丙, 解泉华, 钟登杰, 常海星. 光催化与离子交换吸附耦合有效去除2,4,6-三氯苯酚[J]. 化工学报, 2023, 74(4): 1587-1597. |
| [13] | 潘煜, 王子航, 王佳韵, 王如竹, 张华. 基于可得然-氯化锂复合吸附剂的除湿换热器热湿性能研究[J]. 化工学报, 2023, 74(3): 1352-1359. |
| [14] | 徐银, 蔡洁, 陈露, 彭宇, 刘夫珍, 张晖. 异相可见光催化耦合过硫酸盐活化技术在水污染控制中的研究进展[J]. 化工学报, 2023, 74(3): 995-1009. |
| [15] | 刘瑞琪, 周栖桐, 张悦, 贺莹, 高静, 马丽. 基于金纳米颗粒修饰二氧化硅纳米花的生物传感器构建及应用[J]. 化工学报, 2023, 74(3): 1247-1259. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号