化工学报 ›› 2020, Vol. 71 ›› Issue (9): 4282-4291.DOI: 10.11949/0438-1157.20200468
收稿日期:2020-05-05
修回日期:2020-07-08
出版日期:2020-09-05
发布日期:2020-09-05
通讯作者:
王振华
作者简介:白哲(1996—),男,硕士研究生,基金资助:
Zhe BAI1( ),Ruijian LI1,Wenshuo HOU1,Haijun LI2,Zhenhua WANG1(
),Ruijian LI1,Wenshuo HOU1,Haijun LI2,Zhenhua WANG1( )
)
Received:2020-05-05
Revised:2020-07-08
Online:2020-09-05
Published:2020-09-05
Contact:
Zhenhua WANG
摘要:
锂硫电池因较高的比能量近年来得到了广泛的关注,然而其发展需要克服中间产物的穿梭效应、硫的绝缘性和正极体积膨胀等诸多问题。为了有效抑制穿梭效应,采用普鲁士蓝类似物衍生的方法合成了一种尖晶石结构的双金属硫化物CuCo2S4,并将其用于锂硫电池正极。利用XRD、SEM、TEM、BET、XPS等手段对合成的材料的晶体结构、形貌等性质进行分析,采用循环伏安法及恒流充放电对CuCo2S4-S复合正极的电化学性能进行测试。研究表明,CuCo2S4-S正极展现出优异的电化学性能,在0.2C倍率下首次放电容量为959 mA·h·g-1,经过100个循环后容量保持在591 mA·h·g-1。较高的放电比容量和良好的循环稳定性归因于CuCo2S4材料内部的中空结构可容纳活性物质硫,并起到物理限域作用;同时,极性CuCo2S4可有效地化学吸附多硫化物,抑制多硫化物的穿梭效应造成的容量损失。
中图分类号:
白哲, 李睿健, 侯文烁, 李海军, 王振华. 双金属硫化物CuCo2S4的合成及其在锂硫电池中的应用[J]. 化工学报, 2020, 71(9): 4282-4291.
Zhe BAI, Ruijian LI, Wenshuo HOU, Haijun LI, Zhenhua WANG. Synthesis of bimetallic sulfide CuCo2S4 and its application in lithium-sulfur batteries[J]. CIESC Journal, 2020, 71(9): 4282-4291.
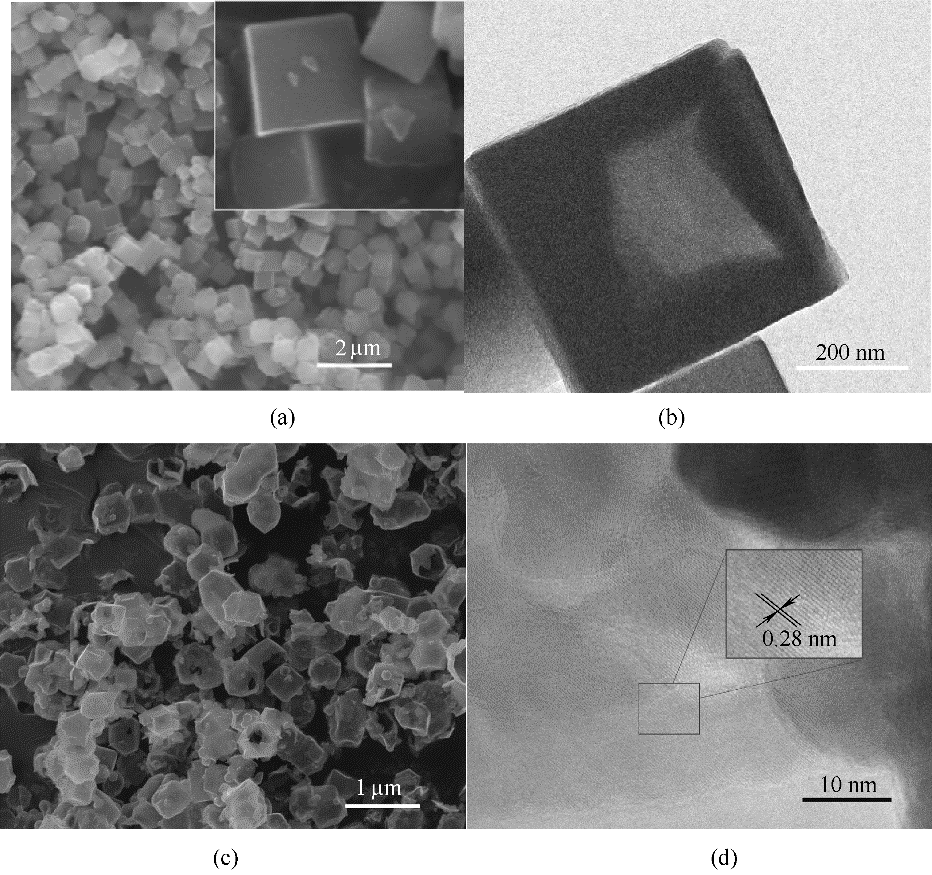
图3 Cu5/3Co4/3[Co(CN)6]2 SEM (a)和TEM (b)图; CuCo2S4的SEM(c)和HRTEM (d)图
Fig.3 SEM (a) and TEM (b) images of Cu5/3Co4/3[Co(CN)6]2; SEM (c) and HRTEM (d) images of CuCo2S4
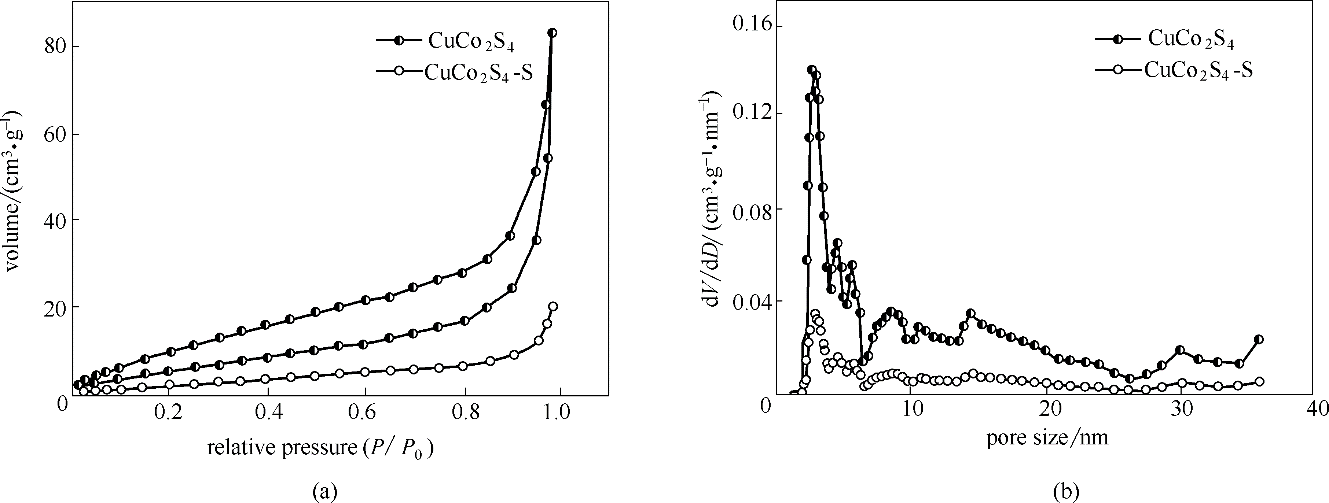
图4 CuCo2S4和CuCo2S4-S的N2吸附-脱附下的等温线图(a)和孔径分布图(b)
Fig.4 N2 adsorption-desorption isotherm curves (a) and pore size distributions (b) of CuCo2S4 and CuCo2S4-S
| Cathode material | Sulfur loading/(mg·cm-2) | Stable capacity/(mA·h·g-1) | Retention | Ref. |
|---|---|---|---|---|
| CuCo2S4-S | 1.2 | 591 at 0.2C 392 at 0.5C | 61.6% after 100 cycles 52.4% after 300 cycles | this work |
| S/a-CMs | 1.3 | 590 at 0.1C | 55.9% after 50 cycles | [ |
| MnO2@MWCNT-S | 1.2 | 560 at 0.1C | 48.7% after 100 cycles | [ |
| VS2/S | 1.16 | 467.5 at 0.2C | 54.8% after 200 cycles | [ |
| VS2@S | 1.5 | 427 at 0.2C | 33.5% after 500 cycles | [ |
| Ni3Co6S8@C | 1.5 | 340 at 0.12C | 46.8% after 200 cycles | [ |
| NiCo2S4/S | 1.0 | 421at 0.1C | 41.0% after 100 cycles | [ |
| MWCNT/Co9S8/S | 1.0 | 503 at 0.1C | 44.8% after 100 cycles | [ |
表1 本文工作与已报道正极材料性能比较
Table 1 Comparison of previous reported cathode and this work
| Cathode material | Sulfur loading/(mg·cm-2) | Stable capacity/(mA·h·g-1) | Retention | Ref. |
|---|---|---|---|---|
| CuCo2S4-S | 1.2 | 591 at 0.2C 392 at 0.5C | 61.6% after 100 cycles 52.4% after 300 cycles | this work |
| S/a-CMs | 1.3 | 590 at 0.1C | 55.9% after 50 cycles | [ |
| MnO2@MWCNT-S | 1.2 | 560 at 0.1C | 48.7% after 100 cycles | [ |
| VS2/S | 1.16 | 467.5 at 0.2C | 54.8% after 200 cycles | [ |
| VS2@S | 1.5 | 427 at 0.2C | 33.5% after 500 cycles | [ |
| Ni3Co6S8@C | 1.5 | 340 at 0.12C | 46.8% after 200 cycles | [ |
| NiCo2S4/S | 1.0 | 421at 0.1C | 41.0% after 100 cycles | [ |
| MWCNT/Co9S8/S | 1.0 | 503 at 0.1C | 44.8% after 100 cycles | [ |
| 1 | 袁艳, 郑东东, 方钊, 等. 锂硫电池硫正极技术研究进展[J]. 储能科学与技术, 2018, 7(4): 618-630. |
| Yuan Y, Zheng D D, Fang Z, et al. Research progress on sulfur cathode of lithium sulfur battery[J]. Energy Storage Science and Technology, 2018, 7(4): 618-630. | |
| 2 | Xu K. A long journey of lithium: from the big bang to our smartphones[J]. Energ. Environ. Mater., 2019, 2(4): 229-233. |
| 3 | Gueon D, Hwang J T, Yang S B, et al. Spherical macroporous carbon nanotube particles with ultrahigh sulfur loading for lithium-sulfur battery cathodes[J]. ACS Nano, 2018, 12(1): 226-233. |
| 4 | Zhang L L, Wang Y J, Niu Z Q, et al. Advanced nanostructured carbon-based materials for rechargeable lithium-sulfur batteries[J]. Carbon, 2019, 141: 400-416. |
| 5 | Li Z H, He Q, Xu X, et al. A 3D nitrogen-doped graphene/tin nanowires composite as a strong polysulfide anchor for lithium-sulfur batteries with enhanced rate performance and high areal capacity[J]. Adv. Mater., 2018, 30(45): 1804089. |
| 6 | Chung S H, Manthiram A. Current status and future prospects of metal–sulfur batteries[J]. Adv. Mater., 2019, 31(27): 1901125. |
| 7 | Ren W C, Ma W, Zhang S F, et al. Recent advances in shuttle effect inhibition for lithium sulfur batteries[J]. Energy Storage Mater., 2019, 23: 23707-23732. |
| 8 | 王杰, 孙晓刚, 陈珑, 等. 多壁碳纳米管夹层抑制锂硫电池穿梭效应[J]. 化工进展, 2018, 37(3): 1070-1075. |
| Wang J, Sun X G, Chen L, et al. Multi-walled carbon nanotube interlayer for checking of the shuttle effect of lithium-sulphur battery[J]. Chemical Industry and Engineering Progress, 2018, 37(3): 1070-1075. | |
| 9 | Shen J, Xu X, Liu J, et al. Mechanistic understanding of metal phosphide host for sulfur cathode in high-energy-density lithium-sulfur batteries[J]. ACS Nano, 2019, 13(8): 8986-8996. |
| 10 | 盖丽艳, 郎笑石, 蔡克迪, 等. 锂硫电池正极材料的研究进展[J]. 电池, 2019, 49(1): 72-75. |
| Gai L Y, Lang X S, Cai K D, et al. Research progress in cathode materials for lithium-sulfur battery[J]. Battery, 2019, 49(1): 72-75. | |
| 11 | Wang T, Zhu J, Wei Z, et al. Bacteria-derived biological carbon building robust Li-S batteries[J]. Nano Lett., 2019, 19(7): 4384-4390. |
| 12 | Ji X, Lee K T, Nazar L F. A highly ordered nanostructured carbon-sulphur cathode for lithium–sulphur batteries[J]. Nat. Mater., 2009, 8(6): 500-506. |
| 13 | Zhou G M, Paek E, Hwang G S, et al. Long-life Li/polysulphide batteries with high sulphur loading enabled by lightweight three-dimensional nitrogen/sulphur-codoped graphene sponge[J]. Nat. Commun., 2015, 6(1): 7760. |
| 14 | 杨蓉, 李兰, 王黎晴, 等. 微波法制备还原氧化石墨烯及其在锂硫电池中的应用[J]. 化工学报, 2017, 68(11): 4333-4340. |
| Yang R, Li L, Wang L Q, et al. Preparation of reduced graphene oxide by microwave method and its application in lithium-sulfur batteries[J]. CIESC Journal, 2017, 68(11): 4333-4340. | |
| 15 | 李巧乐, 燕映霖, 杨蓉, 等. 锂硫电池用玉米苞叶基活性炭/硫复合正极材料的电化学性能[J]. 化工学报, 2017, 68(11): 4376-4382. |
| Li Q L, Yan Y L, Yang R, et al. Electrochemical performance of activated carbon derived from corn bracts / sulfur composite cathode material for lithium-sulfur batteries[J]. CIESC Journal, 2017, 68(11): 4376-4382. | |
| 16 | Kong L, Jin Q, Zhang X T, et al. Towards full demonstration of high areal loading sulfur cathode in lithium-sulfur batteries[J]. J. Energy Chem., 2019, 39: 17-22. |
| 17 | Li H Y, Fei L F, Zhang R, et al. FeCo alloy catalysts promoting polysulfide conversion for advanced lithium-sulfur batteries[J]. J. Energy Chem., 2020, 49: 339-347. |
| 18 | Wang J Y, Si L P, Wei Q, et al. An imine-linked covalent organic framework as the host material for sulfur loading in lithium–sulfur batteries[J]. J. Energy Chem., 2019, 28: 54-60. |
| 19 | Li S, Cen Y, Xiang Q, et al. Vanadium dioxide- reduced graphene oxide binary host as an efficient polysulfide plague for high performance lithium-sulfur batteries[J]. J. Mater. Chem. A., 2019, 7(4): 1658-1668. |
| 20 | Song Y Z, Zhao W, Zhu X Y, et al. Vanadium dioxide-graphene composite with ultrafast anchoring behavior of polysulfides for lithium-sulfur batteries[J]. ACS Appl. Mater. Interfaces, 2018, 10(18): 15733-15741. |
| 21 | Wang S Z, Liao J X, Yang X F, et al. Designing a highly efficient polysulfide conversion catalyst with paramontroseite for high-performance and long-life lithium-sulfur batteries[J]. Nano Energy, 2019, 57: 230-240. |
| 22 | Xu J, Zhang W X, Chen Y, et al. MOF-derived porous N-Co3O4@ N-C nanododecahedra wrapped with reduced graphene oxide as a high capacity cathode for lithium–sulfur batteries[J]. J. Mater. Chem. A., 2018, 6(6): 2797-2807. |
| 23 | Wang Y K, Zhang R F, Chen J, et al. Enhancing catalytic activity of titanium oxide in lithium-sulfur batteries by band engineering[J]. Adv. Energy. Mater., 2019, 9(24): 1900953. |
| 24 | Zhang Z, Basu S, Zhu P P, et al. Highly sulfiphilic Ni-Fe bimetallic oxide nanoparticles anchored on carbon nanotubes enable effective immobilization and conversion of polysulfides for stable lithium-sulfur batteries[J]. Carbon, 2019, 142: 32-39. |
| 25 | Kong L, Chen X, Li B Q, et al. A bifunctional perovskite promoter for polysulfide regulation toward stable lithium–sulfur batteries[J]. Adv. Mater., 2018, 30(2): 1705219. |
| 26 | Yuan Z, Peng H J, Hou T Z, et al. Powering lithium-sulfur battery performance by propelling polysulfide redox at sulfiphilic hosts[J]. Nano Lett., 2016, 16(1): 519-527. |
| 27 | Chen T, Ma L B, Cheng B R, et al. Metallic and polar Co9S8 inlaid carbon hollow nanopolyhedra as efficient polysulfide mediator for lithium-sulfur batteries[J]. Nano Energy, 2017, 38: 239-248. |
| 28 | Tong W, Huang Y D, Jia W, et al. Leaf-like interconnected network structure of MWCNT/Co9S8/S for lithium-sulfur batteries[J]. J. Alloys Compd., 2018, 731: 964-970. |
| 29 | Wang H E, Li X C, Qin N, et al. Sulfur-deficient MoS2 grown inside hollow mesoporous carbon as a functional polysulfide mediator[J]. J. Mater. Chem. A., 2019, 7(19): 12068-12074. |
| 30 | 姚琳, 周玲, 李世雄, 等. 层层自组装MoS2多晶片增强锂硫电池性能(英文)[J]. 储能科学与技术, 2019, 8(3): 523-531. |
| Yao L, Zhou L, Li S X, et al. Edge-rich MoS2 nanosheets for high performance self-supporting Li-S batteries[J]. Energy Storage Science and Technology, 2019, 8(3): 523-531. | |
| 31 | You Y, Ye Y W, Wei M L, et al. Three-dimensional MoS2/rGO foams as efficient sulfur hosts for high-performance lithium-sulfur batteries[J]. Chem. Eng. J., 2019, 335: 671-678. |
| 32 | Huang X, Tang J Y, Luo B, et al. Sandwich-like ultrathin TiS2 nanosheets confined within N, S codoped porous carbon as an effective polysulfide promoter in lithium-sulfur batteries[J]. Adv. Energy. Mater., 2019, 9(32): 1901872. |
| 33 | Li S, Xu P, Aslam M K, et al. Propelling polysulfide conversion for high-loading lithium–sulfur batteries through highly sulfiphilic NiCo2S4 nanotubes[J]. Energy Storage Mater., 2020, 27: 51-60. |
| 34 | Lu X L, Zhang Q F, Wang J, et al. High performance bimetal sulfides for lithium-sulfur batteries[J]. Chem. Eng. J., 2019, 358: 955-961. |
| 35 | Wu L S, Tang S H, Qu R J. Urchin-like NiCo2S4 infused sulfur as cathode for lithium–sulfur battery[J]. J. Mater. Sci., Mater. Electron., 2019, 30(1): 189-199. |
| 36 | 彭娜, 翟鹏飞, 王景涛, 等. 二氧化锰纳米片改性隔膜在锂硫电池中的应用[J]. 化工学报, 2020, 71(5): 2389-2400. |
| Peng N, Zhai P F, Wang J T, et al. Application of manganese dioxide nanosheets modified separator for lithium-sulfur batteries[J]. CIESC Journal, 2020, 71(5): 2389-2400. | |
| 37 | Ren J, Zhou Y B, Wu H L, et al. Sulfur-encapsulated in heteroatom-doped hierarchical porous carbon derived from goat hair for high performance lithium-sulfur batteries[J]. J. Energy Chem., 2019, 30: 121-131. |
| 38 | Luo D, Li G R, Deng Y P, et al. Synergistic engineering of defects and architecture in binary metal chalcogenide toward fast and reliable lithium-sulfur batteries[J]. Adv. Energy Mater., 2019, 9(18): 1900228. |
| 39 | Song Y, Wang H, Yu W S, et al. Synergistic stabilizing lithium sulfur battery via nanocoating polypyrrole on cobalt sulfide nanobox[J]. J. Power Sources, 2018, 40: 551-560. |
| 40 | Zheng T, Li G, Meng X, et al. Porous core–shell CuCo2S4 nanospheres as anode material for enhanced lithium-ion batteries[J]. Chem. Eur. J., 2019, 25(3): 885-891. |
| 41 | Du X, Huang C, Zhang X. Surface modification of a Co9S8 nanorods with Ni(OH)2 on nickel foam for high water splitting performance[J]. Int. J. Hydrogen Energy, 2019, 44(36): 19953-19966. |
| 42 | 尚永亮, 王诚飞, 刘斌, 等. MnO2包覆的碳纳米管-硫复合正极材料的制备及性能[J]. 储能科学与技术, 2017, 6(3): 411-417. |
| Shang Y L, Wang C F, Liu B, et al. Preparation and properties of manganese dioxide coated carbon nanotubes-sulfur composite cathode material[J]. Energy Storage Science and Technology, 2017, 6(3): 411-417. | |
| 43 | Wu H L, Huan Y H, Wang D H, et al. Hierarchical VS2 nano-flowers as sulfur host for lithium sulfur battery cathodes[J]. J. Electrochem. Soc., 2019, 166(2): A188-A194. |
| 44 | Chen X J, Du G H, Zhang M, et al. Vanadium sulfide@sulfur composites as high‐performance cathode for advanced lithium–sulfur batteries[J]. Energy Technol., 2020, 8: 1901163. |
| 45 | Yan X C, Fu L, Wang X G, et al. High Performance lithium secondary batteries based on novel Ni3Co6S8@C core–shell nanoparticle[J]. J. Nanosci. Nanotechnol., 2017, 17(8): 5384-5390. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [3] | 刘远超, 关斌, 钟建斌, 徐一帆, 蒋旭浩, 李耑. 单层XSe2(X=Zr/Hf)的热电输运特性研究[J]. 化工学报, 2023, 74(9): 3968-3978. |
| [4] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [5] | 陈佳起, 赵万玉, 姚睿充, 侯道林, 董社英. 开心果壳基碳点的合成及其对Q235碳钢的缓蚀行为研究[J]. 化工学报, 2023, 74(8): 3446-3456. |
| [6] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [7] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [8] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [9] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [10] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [11] | 邢美波, 张中天, 景栋梁, 张洪发. 磁调控水基碳纳米管协同多孔材料强化相变储/释能特性[J]. 化工学报, 2023, 74(7): 3093-3102. |
| [12] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| [13] | 李彬, 徐正虎, 姜爽, 张天永. 双氧水催化氧化法清洁高效合成促进剂CBS[J]. 化工学报, 2023, 74(7): 2919-2925. |
| [14] | 涂玉明, 邵高燕, 陈健杰, 刘凤, 田世超, 周智勇, 任钟旗. 钙基催化剂的设计合成及应用研究进展[J]. 化工学报, 2023, 74(7): 2717-2734. |
| [15] | 葛加丽, 管图祥, 邱新民, 吴健, 沈丽明, 暴宁钟. 垂直多孔碳包覆的FeF3正极的构筑及储锂性能研究[J]. 化工学报, 2023, 74(7): 3058-3067. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号