化工学报 ›› 2021, Vol. 72 ›› Issue (10): 5028-5039.DOI: 10.11949/0438-1157.20210607
收稿日期:2021-04-29
修回日期:2021-06-11
出版日期:2021-10-05
发布日期:2021-10-05
通讯作者:
于建国
作者简介:曹大群(1995—),男,硕士研究生,基金资助:
Daqun CAO1( ),Yan JIN2,Hang CHEN1,Jianguo YU1(
),Yan JIN2,Hang CHEN1,Jianguo YU1( )
)
Received:2021-04-29
Revised:2021-06-11
Online:2021-10-05
Published:2021-10-05
Contact:
Jianguo YU
摘要:
采用等温溶解平衡法研究338.15 K时三元体系CaCl2-SrCl2-H2O、CaCl2-BaCl2-H2O和SrCl2-BaCl2-H2O的稳定相平衡,进一步研究四元体系CaCl2-SrCl2-BaCl2-H2O的相平衡。根据实验数据,分别绘制了三元体系的相图和密度-组成图,四元体系空间立体图、相图、水图和密度-组成图。研究结果表明:CaCl2-SrCl2-BaCl2-H2O四元体系在338.15 K时没有复盐和固溶体生成,稳定平衡相图由1个共饱点、3条单变量溶解度曲线,3个结晶区组成,分别对应BaCl2·2H2O结晶区、SrCl2·mH2O(m=2,6)结晶区和CaCl2·2H2O结晶区。采用DFT模型进行338.15 K三元体系CaCl2-SrCl2-H2O、CaCl2-BaCl2-H2O和SrCl2-BaCl2-H2O的溶解度计算,计算结果与实验结果基本吻合。针对CaCl2-SrCl2-BaCl2-H2O四元体系及三元子体系的稳定相平衡研究,可为综合开发利用油田卤水中的钙-锶-钡资源提供数据基础。
中图分类号:
曹大群,金艳,陈杭,于建国. 338.15 K时四元体系CaCl2-SrCl2-BaCl2-H2O相平衡测定及溶解度计算[J]. 化工学报, 2021, 72(10): 5028-5039.
Daqun CAO,Yan JIN,Hang CHEN,Jianguo YU. Phase equilibria determination and solubility calculation of the quaternary system CaCl2-SrCl2-BaCl2-H2O at 338.15 K[J]. CIESC Journal, 2021, 72(10): 5028-5039.
| No. | 液相组成/% | 密度/(g/cm3) | 湿渣组成/% | 平衡固相 | ||||
|---|---|---|---|---|---|---|---|---|
| CaCl2 | SrCl2 | H2O | CaCl2 | SrCl2 | H2O | |||
| 1,A | 57.83 | 0.00 | 42.17 | 1.6465 | — | — | — | C |
| 2 | 56.43 | 0.54 | 43.03 | 1.6215 | 58.19 | 0.75 | 41.06 | C |
| 3,E | 55.16 | 0.98 | 43.83 | 1.6192 | 56.44 | 2.85 | 40.71 | C+S |
| 4 | 53.61 | 1.07 | 45.32 | 1.5908 | 43.68 | 15.98 | 40.34 | S |
| 5 | 50.54 | 1.25 | 48.21 | 1.5750 | 25.76 | 40.35 | 33.89 | S |
| 6 | 48.12 | 1.32 | 50.56 | 1.5675 | 26.61 | 36.98 | 36.41 | S |
| 7 | 47.32 | 1.66 | 51.02 | 1.5418 | 22.02 | 44.61 | 33.37 | S |
| 8 | 42.61 | 3.63 | 53.76 | 1.5027 | 21.67 | 41.90 | 36.43 | S |
| 9 | 40.6 | 4.87 | 54.53 | 1.4785 | 19.80 | 43.78 | 36.42 | S |
| 10 | 33.15 | 10.51 | 56.34 | 1.4644 | 16.56 | 46.83 | 36.61 | S |
| 11 | 26.79 | 15.91 | 57.3 | 1.4559 | 10.47 | 55.76 | 33.77 | S |
| 12 | 19.85 | 22.42 | 57.73 | 1.4345 | 5.21 | 65.35 | 29.44 | S |
| 13 | 11.03 | 30.99 | 57.98 | 1.4493 | 5.78 | 55.26 | 38.95 | S |
| 14 | 9.05 | 33.38 | 57.57 | 1.4506 | 3.12 | 63.96 | 32.92 | S |
| 15,C | 8.57 | 34.07 | 57.36 | 1.4564 | 1.89 | 58.87 | 39.24 | S+SH |
| 16 | 1.11 | 43.77 | 55.12 | 1.4963 | 0.71 | 51.31 | 47.98 | S+SH |
| 17,B | 0.00 | 45.30 | 54.70 | 1.5226 | — | — | — | S |
表1 CaCl2-SrCl2-H2O三元体系在338.15 K时的溶解度数据
Table 1 Solubility data of the ternary system CaCl2-SrCl2-H2O at 338.15 K
| No. | 液相组成/% | 密度/(g/cm3) | 湿渣组成/% | 平衡固相 | ||||
|---|---|---|---|---|---|---|---|---|
| CaCl2 | SrCl2 | H2O | CaCl2 | SrCl2 | H2O | |||
| 1,A | 57.83 | 0.00 | 42.17 | 1.6465 | — | — | — | C |
| 2 | 56.43 | 0.54 | 43.03 | 1.6215 | 58.19 | 0.75 | 41.06 | C |
| 3,E | 55.16 | 0.98 | 43.83 | 1.6192 | 56.44 | 2.85 | 40.71 | C+S |
| 4 | 53.61 | 1.07 | 45.32 | 1.5908 | 43.68 | 15.98 | 40.34 | S |
| 5 | 50.54 | 1.25 | 48.21 | 1.5750 | 25.76 | 40.35 | 33.89 | S |
| 6 | 48.12 | 1.32 | 50.56 | 1.5675 | 26.61 | 36.98 | 36.41 | S |
| 7 | 47.32 | 1.66 | 51.02 | 1.5418 | 22.02 | 44.61 | 33.37 | S |
| 8 | 42.61 | 3.63 | 53.76 | 1.5027 | 21.67 | 41.90 | 36.43 | S |
| 9 | 40.6 | 4.87 | 54.53 | 1.4785 | 19.80 | 43.78 | 36.42 | S |
| 10 | 33.15 | 10.51 | 56.34 | 1.4644 | 16.56 | 46.83 | 36.61 | S |
| 11 | 26.79 | 15.91 | 57.3 | 1.4559 | 10.47 | 55.76 | 33.77 | S |
| 12 | 19.85 | 22.42 | 57.73 | 1.4345 | 5.21 | 65.35 | 29.44 | S |
| 13 | 11.03 | 30.99 | 57.98 | 1.4493 | 5.78 | 55.26 | 38.95 | S |
| 14 | 9.05 | 33.38 | 57.57 | 1.4506 | 3.12 | 63.96 | 32.92 | S |
| 15,C | 8.57 | 34.07 | 57.36 | 1.4564 | 1.89 | 58.87 | 39.24 | S+SH |
| 16 | 1.11 | 43.77 | 55.12 | 1.4963 | 0.71 | 51.31 | 47.98 | S+SH |
| 17,B | 0.00 | 45.30 | 54.70 | 1.5226 | — | — | — | S |
| No. | 液相组成/% | 密度/(g/cm3) | 湿渣组成/% | 平衡固相 | ||||
|---|---|---|---|---|---|---|---|---|
| CaCl2 | BaCl2 | H2O | CaCl2 | BaCl2 | H2O | |||
| 1 | 57.83 | 0.00 | 42.17 | 1.6465 | — | — | — | C |
| 2,E | 54.95 | 0.16 | 44.89 | 1.6122 | 45.46 | 21.85 | 32.69 | C+B |
| 3 | 48.12 | 0.46 | 51.42 | 1.5403 | 31.19 | 30.47 | 38.34 | B |
| 4 | 31.15 | 3.37 | 65.48 | 1.3872 | 19.51 | 33.89 | 46.6 | B |
| 5 | 18.96 | 9.37 | 71.67 | 1.2995 | 8.72 | 50.16 | 41.12 | B |
| 6 | 7.84 | 21.09 | 71.07 | 1.3051 | 3.79 | 53.65 | 42.56 | B |
| 7 | 0.00 | 32.19 | 67.81 | 1.3095 | — | — | — | B |
表2 CaCl2-BaCl2-H2O三元体系在338.15 K时的溶解度数据
Table 2 Solubility data of the ternary system CaCl2-BaCl2-H2O at 338.15 K
| No. | 液相组成/% | 密度/(g/cm3) | 湿渣组成/% | 平衡固相 | ||||
|---|---|---|---|---|---|---|---|---|
| CaCl2 | BaCl2 | H2O | CaCl2 | BaCl2 | H2O | |||
| 1 | 57.83 | 0.00 | 42.17 | 1.6465 | — | — | — | C |
| 2,E | 54.95 | 0.16 | 44.89 | 1.6122 | 45.46 | 21.85 | 32.69 | C+B |
| 3 | 48.12 | 0.46 | 51.42 | 1.5403 | 31.19 | 30.47 | 38.34 | B |
| 4 | 31.15 | 3.37 | 65.48 | 1.3872 | 19.51 | 33.89 | 46.6 | B |
| 5 | 18.96 | 9.37 | 71.67 | 1.2995 | 8.72 | 50.16 | 41.12 | B |
| 6 | 7.84 | 21.09 | 71.07 | 1.3051 | 3.79 | 53.65 | 42.56 | B |
| 7 | 0.00 | 32.19 | 67.81 | 1.3095 | — | — | — | B |

图6 CaCl2-BaCl2-H2O三元体系在338.15 K时共饱和点的固相XRD谱图
Fig.6 XRD patterns of the solid phases of co-saturation point in the ternary system CaCl2-BaCl2-H2O at 338.15 K
| No. | 液相组成/% | 密度/(g/cm3) | 湿渣组成/% | 平衡固相 | ||||
|---|---|---|---|---|---|---|---|---|
| SrCl2 | BaCl2 | H2O | SrCl2 | BaCl2 | H2O | |||
| 1 | 45.30 | 0.00 | 54.7 | 1.5226 | — | — | — | S |
| 2 | 40.98 | 0.45 | 58.57 | 1.4953 | 53.17 | 0.58 | 46.25 | S |
| 3 | 39.11 | 0.79 | 60.10 | 1.4804 | 59.39 | 2.14 | 38.47 | S |
| 4,E | 37.86 | 1.29 | 60.85 | 1.4721 | 38.94 | 18.68 | 42.38 | B+S |
| 5 | 31.20 | 3.01 | 65.79 | 1.4182 | 10.31 | 57.94 | 31.75 | B |
| 6 | 19.06 | 11.77 | 69.17 | 1.3518 | 9.64 | 48.51 | 41.85 | B |
| 7 | 8.91 | 21.89 | 69.20 | 1.3259 | 2.48 | 67.39 | 30.13 | B |
| 8 | 0.00 | 32.19 | 67.81 | 1.3095 | — | — | — | B |
表3 SrCl2-BaCl2-H2O三元体系在338.15 K时的溶解度数据
Table 3 Solubility data of the ternary system SrCl2-BaCl2-H2O at 338.15 K
| No. | 液相组成/% | 密度/(g/cm3) | 湿渣组成/% | 平衡固相 | ||||
|---|---|---|---|---|---|---|---|---|
| SrCl2 | BaCl2 | H2O | SrCl2 | BaCl2 | H2O | |||
| 1 | 45.30 | 0.00 | 54.7 | 1.5226 | — | — | — | S |
| 2 | 40.98 | 0.45 | 58.57 | 1.4953 | 53.17 | 0.58 | 46.25 | S |
| 3 | 39.11 | 0.79 | 60.10 | 1.4804 | 59.39 | 2.14 | 38.47 | S |
| 4,E | 37.86 | 1.29 | 60.85 | 1.4721 | 38.94 | 18.68 | 42.38 | B+S |
| 5 | 31.20 | 3.01 | 65.79 | 1.4182 | 10.31 | 57.94 | 31.75 | B |
| 6 | 19.06 | 11.77 | 69.17 | 1.3518 | 9.64 | 48.51 | 41.85 | B |
| 7 | 8.91 | 21.89 | 69.20 | 1.3259 | 2.48 | 67.39 | 30.13 | B |
| 8 | 0.00 | 32.19 | 67.81 | 1.3095 | — | — | — | B |
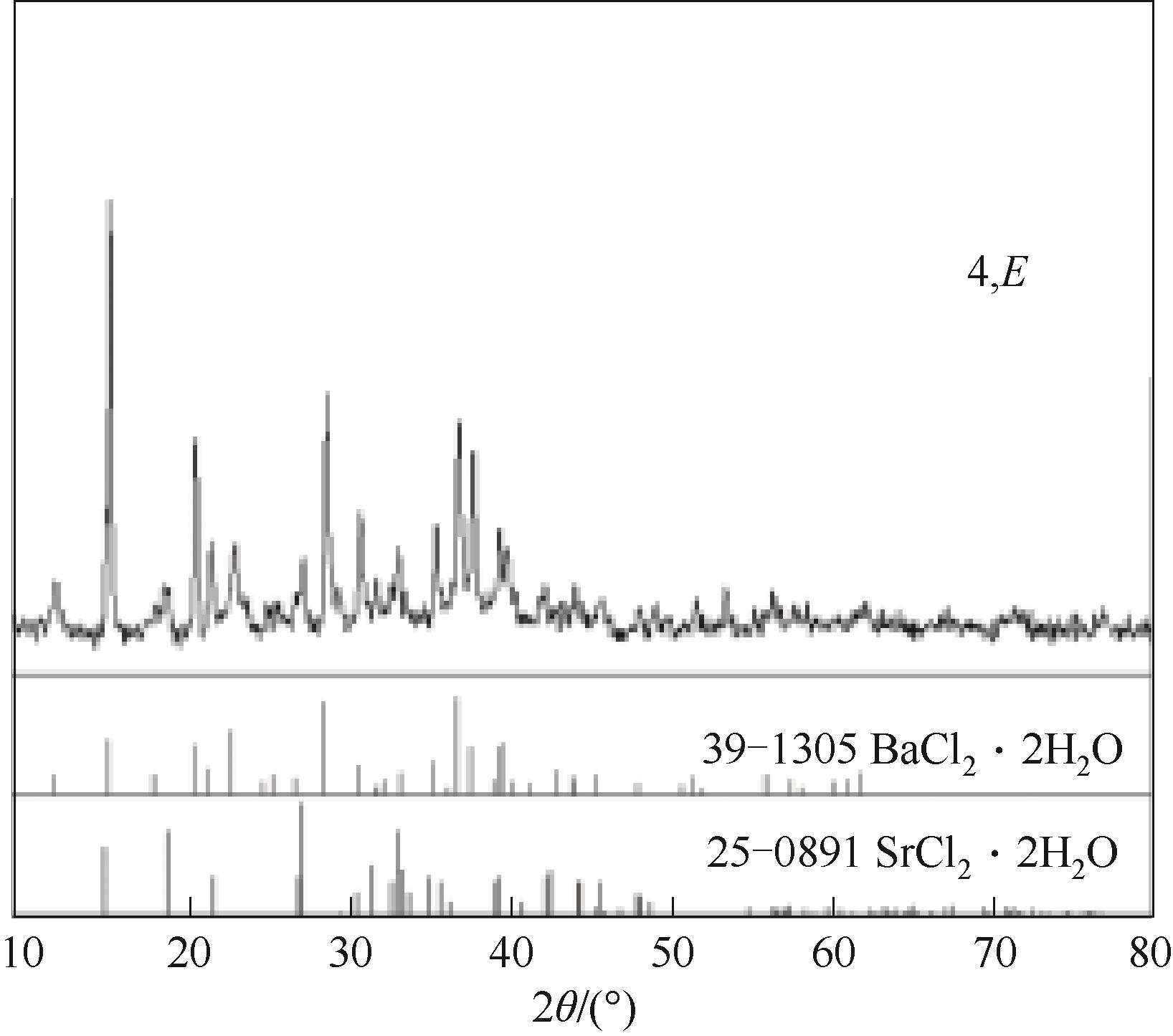
图9 SrCl2-BaCl2-H2O三元体系在338.15 K时共饱和点的固相XRD谱图
Fig.9 XRD patterns of the solid phases of co-saturation point in the ternary system SrCl2-BaCl2-H2O at 338.15K
| No. | 液相组成/% | 干盐J?necke指数 J(CaCl2)+J(SrCl2)+J(BaCl2)=100 | 平衡固相 | 密度/ (g/cm3) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CaCl2 | SrCl2 | BaCl2 | H2O | CaCl2 | SrCl2 | BaCl2 | H2O | |||
| 1,E1 | 54.95 | 0 | 0.17 | 44.88 | 99.69 | 0.00 | 0.31 | 81.42 | C+B | 1.6122 |
| 2,E2 | 55.16 | 0.98 | 0 | 43.86 | 98.25 | 1.75 | 0.00 | 78.13 | C+S | 1.6192 |
| 3,E3 | 52.38 | 0.75 | 0.16 | 46.71 | 98.29 | 1.41 | 0.30 | 87.65 | C+S | 1.5933 |
| 4 | 43.61 | 1.83 | 0.15 | 54.41 | 95.66 | 4.01 | 0.33 | 119.35 | B+S | 1.5226 |
| 5 | 35.07 | 6.42 | 0.18 | 58.33 | 84.16 | 15.41 | 0.43 | 139.98 | B+S | 1.4893 |
| 6 | 24.68 | 13.91 | 0.26 | 61.15 | 63.53 | 35.80 | 0.67 | 157.40 | B+S | 1.4618 |
| 7 | 19.08 | 18.93 | 0.36 | 61.63 | 49.73 | 49.34 | 0.94 | 160.62 | B+S | 1.4705 |
| 8 | 14.82 | 22.97 | 0.48 | 61.73 | 38.72 | 60.02 | 1.25 | 161.30 | B+S | 1.4717 |
| 9 | 8.14 | 29.43 | 0.77 | 61.66 | 21.23 | 76.76 | 2.01 | 160.82 | B+S | 1.4739 |
| 10,E4 | 0 | 37.86 | 1.29 | 60.85 | 0.00 | 96.70 | 3.30 | 155.43 | B+S | 1.4721 |
表4 CaCl2-SrCl2-BaCl2-H2O四元体系在338.15 K时的溶解度数据
Table 4 Solubility data of the quaternary system CaCl2-SrCl2-BaCl2-H2O at 338.15 K
| No. | 液相组成/% | 干盐J?necke指数 J(CaCl2)+J(SrCl2)+J(BaCl2)=100 | 平衡固相 | 密度/ (g/cm3) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CaCl2 | SrCl2 | BaCl2 | H2O | CaCl2 | SrCl2 | BaCl2 | H2O | |||
| 1,E1 | 54.95 | 0 | 0.17 | 44.88 | 99.69 | 0.00 | 0.31 | 81.42 | C+B | 1.6122 |
| 2,E2 | 55.16 | 0.98 | 0 | 43.86 | 98.25 | 1.75 | 0.00 | 78.13 | C+S | 1.6192 |
| 3,E3 | 52.38 | 0.75 | 0.16 | 46.71 | 98.29 | 1.41 | 0.30 | 87.65 | C+S | 1.5933 |
| 4 | 43.61 | 1.83 | 0.15 | 54.41 | 95.66 | 4.01 | 0.33 | 119.35 | B+S | 1.5226 |
| 5 | 35.07 | 6.42 | 0.18 | 58.33 | 84.16 | 15.41 | 0.43 | 139.98 | B+S | 1.4893 |
| 6 | 24.68 | 13.91 | 0.26 | 61.15 | 63.53 | 35.80 | 0.67 | 157.40 | B+S | 1.4618 |
| 7 | 19.08 | 18.93 | 0.36 | 61.63 | 49.73 | 49.34 | 0.94 | 160.62 | B+S | 1.4705 |
| 8 | 14.82 | 22.97 | 0.48 | 61.73 | 38.72 | 60.02 | 1.25 | 161.30 | B+S | 1.4717 |
| 9 | 8.14 | 29.43 | 0.77 | 61.66 | 21.23 | 76.76 | 2.01 | 160.82 | B+S | 1.4739 |
| 10,E4 | 0 | 37.86 | 1.29 | 60.85 | 0.00 | 96.70 | 3.30 | 155.43 | B+S | 1.4721 |
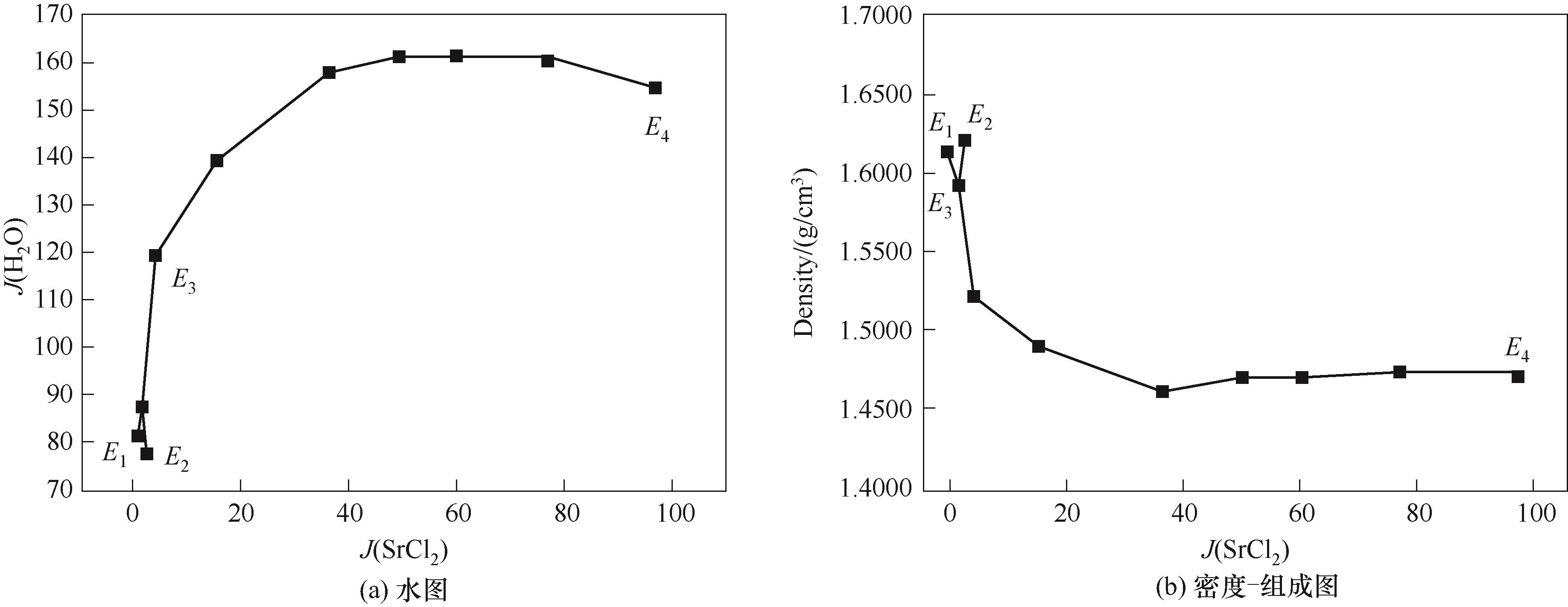
图11 CaCl2-SrCl2-BaCl2-H2O四元体系在338.15 K时的水图和密度-组成图
Fig.11 Water content diagram and density composition diagram of the quaternary system CaCl2-SrCl2-BaCl2-H2O at 338.15 K
| 离子 | σ/? | ε/(kJ/mol) | Z | 文献 |
|---|---|---|---|---|
| Cl- | 4.830 | 0.053 | -1 | [ |
| Ca2+ | 3.170 | 1.886 | 2 | [ |
| Sr2+ | 3.553 | 0.4948 | 2 | [ |
| Ba2+ | 3.750 | 0.1696 | 2 | [ |
表5 各离子L-J作用参数
Table 5 L-J parameters adopted in the present work
| 离子 | σ/? | ε/(kJ/mol) | Z | 文献 |
|---|---|---|---|---|
| Cl- | 4.830 | 0.053 | -1 | [ |
| Ca2+ | 3.170 | 1.886 | 2 | [ |
| Sr2+ | 3.553 | 0.4948 | 2 | [ |
| Ba2+ | 3.750 | 0.1696 | 2 | [ |
| 盐 | 溶解度/%(质量) | 数密度/(个/nm3) |
|---|---|---|
| CaCl2 | 57.83 | 7.44 |
| SrCl2 | 45.30 | 3.15 |
| BaCl2 | 32.19 | 1.37 |
表6 338.15 K时各盐的二元溶解度数据
Table 6 Solubility data of the involved pure salts in water at 338.15 K
| 盐 | 溶解度/%(质量) | 数密度/(个/nm3) |
|---|---|---|
| CaCl2 | 57.83 | 7.44 |
| SrCl2 | 45.30 | 3.15 |
| BaCl2 | 32.19 | 1.37 |
| 三元体系 | 实验共饱和点组成 | 计算共饱和点组成 |
|---|---|---|
| CaCl2-SrCl2-H2O | w(CaCl2)=55.16% w(SrCl2)=0.98% | w(CaCl2)=57.48% w(SrCl2)=0.38% |
| CaCl2-BaCl2-H2O | w(CaCl2)=54.95% w(BaCl2)=0.16% | w(CaCl2)=54.74% w(BaCl2)=0.01% |
| SrCl2-BaCl2-H2O | w(SrCl2)=1.29% w(BaCl2)=37.86% | w(SrCl2)=1.41% w(BaCl2)=39.82% |
表7 三元体系共饱和点的实验组成和计算结果
Table 7 Experimental composition and calculation results of co-saturation point of ternary system
| 三元体系 | 实验共饱和点组成 | 计算共饱和点组成 |
|---|---|---|
| CaCl2-SrCl2-H2O | w(CaCl2)=55.16% w(SrCl2)=0.98% | w(CaCl2)=57.48% w(SrCl2)=0.38% |
| CaCl2-BaCl2-H2O | w(CaCl2)=54.95% w(BaCl2)=0.16% | w(CaCl2)=54.74% w(BaCl2)=0.01% |
| SrCl2-BaCl2-H2O | w(SrCl2)=1.29% w(BaCl2)=37.86% | w(SrCl2)=1.41% w(BaCl2)=39.82% |
| 1 | 王林, 郑秋风, 刘敏, 等. 三元体系SrCl2+MgCl2+H2O 298 K相平衡测定及计算[J]. 高校化学工程学报, 2019, 33(4): 800-807. |
| Wang L, Zheng Q F, Liu M, et al. Phase equilibrium measurements and simulation of the SrCl2 + MgCl2 + H2O ternary system at 298 K[J]. Journal of Chemical Engineering of Chinese Universities, 2019, 33(4): 800-807. | |
| 2 | 胡韵. 涪陵页岩气开发污水水质分析与利用[J]. 江汉石油职工大学学报, 2017, 30(3): 75-77, 88. |
| Hu Y. Wastewater quality analysis and usage for shale gas exploitation in Fuling[J]. Journal of Jianghan Petroleum University of Staff and Workers, 2017, 30(3): 75-77, 88. | |
| 3 | 金艳, 黄伙, 张建海. 页岩气采出水处理技术进展[J]. 广东化工, 2017, 44(16): 149-151. |
| Jin Y, Huang H, Zhang J H. Technical progress of shale gas produced water treatment[J]. Guangdong Chemical Industry, 2017, 44(16): 149-151. | |
| 4 | Clynne M A, Potter R W. Solubility of some alkali and alkaline earth chlorides in water at moderate temperatures[J]. Journal of Chemical & Engineering Data, 1979, 24(4): 338-340. |
| 5 | Seidell A. Solubilities of inorganic and metal organic compounds. Third edition[J]. Journal of Chemical Education, 1941, 18(8): 399. |
| 6 | Sinke G C, Mossner E H, Curnutt J L. Enthalpies of solution and solubilities of calcium chloride and its lower hydrates[J]. The Journal of Chemical Thermodynamics, 1985, 17(9): 893-899. |
| 7 | Li D D, Zeng D W, Yin X, et al. Phase diagrams and thermochemical modeling of salt lake brine systems(Ⅱ): NaCl+H2O, KCl+H2O, MgCl2+H2O and CaCl2+H2O systems[J]. Calphad, 2016, 53: 78-89. |
| 8 | 袁梦霞, 乔秀臣. 三元体系AlCl3+CaCl2+H2O, AlCl3+FeCl3+H2O和CaCl2+FeCl3+H2O在35℃时的相平衡[J]. 化工学报, 2017, 68(7): 2653-2659. |
| Yuan M X, Qiao X C. Phase equilibria of AlCl3+CaCl2+H2O, AlCl3+FeCl3 +H2O and CaCl2+FeCl3+H2O ternary systems at 35℃[J]. CIESC Journal, 2017, 68(7): 2653-2659. | |
| 9 | Cao H Y, Zhou H, Bai X Q, et al. (Solid + liquid) phase equilibria of (Ca(H2PO2)2 + CaCl2 + H2O) and (Ca(H2PO2)2 + NaH2PO2 + H2O) ternary systems at T = 323.15 K[J]. The Journal of Chemical Thermodynamics, 2016, 93: 255-260. |
| 10 | Tan L N, Wang J M, Zhou H, et al. Solid-liquid phase equilibria of Ca(H2PO2)2-CaCl2-H2O and Ca(H2PO2)2-NaH2PO2-H2O ternary systems at 298.15 K[J]. Fluid Phase Equilibria, 2015, 388: 66-70. |
| 11 | Assarsson G O, Balder A. Equilibria between 18 and 114° in the aqueous ternary system containing Ca2+, Sr2+and Cl-[J]. The Journal of Physical Chemistry, 1954, 57(7): 717-720. |
| 12 | Assarsson G O. Equilibria in aqueous systems containing Sr2+, K+, Na+ and Cl-[J]. The Journal of Physical Chemistry, 1953, 57(2): 207-210. |
| 13 | Assarsson G O, Balder A. Equilibria between 18 and 100° in the aqueous ternary system containing Sr2+, Mg2+ and Cl-[J]. The Journal of Physical Chemistry, 1954, 58(5): 416. |
| 14 | Steiger M. Thermodynamic properties of SrCl2(aq) from 252 K to 524 K and phase equilibria in the SrCl2-H2O system: implications for thermochemical heat storage[J]. The Journal of Chemical Thermodynamics, 2018, 120: 106-115. |
| 15 | 毕玉敬, 孙柏, 赵静, 等. 25℃时三元体系SrCl2-CaCl2-H2O相平衡研究[J]. 无机化学学报, 2011, 27(9): 1765-1771. |
| Bi Y J, Sun B, Zhao J, et al. Phase equilibrium in ternary system SrCl2-CaCl2-H2O at 25℃[J]. Chinese Journal of Inorganic Chemistry, 2011, 27(9): 1765-1771. | |
| 16 | 张晓. 五元体系Na+,K+,Ca2+,Sr2+//Cl--H2O及其子体系323 K时相平衡实验及不确定度研究[D]. 成都: 成都理工大学, 2018. |
| Zhang X. Studies on equilibria and uncertainty of the quinary system Na+,K+,Ca2+,Sr2+//Cl--H2O and its subsystems at 323 K[D]. Chengdu: Chengdu University of Technology, 2018. | |
| 17 | Han H J, Ji X, Ma J J, et al. Water activity, solubility determination, and model simulation of the CaCl2-SrCl2-H2O ternary system at 323.15 K[J]. Journal of Chemical & Engineering Data, 2018, 63(5): 1636-1641. |
| 18 | Li D, Meng L Z, Guo Y F, et al. Chemical engineering process simulation of brines using phase diagram and Pitzer model of the system CaCl2-SrCl2-H2O [J]. Fluid Phase Equilibria, 2019, 484: 232-238. |
| 19 | 乔占平. 三元体系BaCl2-LaCl3-H2O(30℃)的相平衡[J]. 南阳师范学院学报(自然科学版), 2004, 3(12): 46-47. |
| Qiao Z P. Study on the phase diagram of the LaCl3-BaCl2-H2O at 30℃[J]. Journal of Nanyang Normal University, 2004, 3(12): 46-47. | |
| 20 | 周久龙, 吉仁塔布, 范剑明, 等. BaCl2-NaCl-H2O三元体系溶解度分析及应用[J]. 内蒙古石油化工, 2010, 36(11): 20-22. |
| Zhou J L, Jirentabu, Fan J M, et al. Measurement and research on the solubility of BaCl2-NaCl-H2O system[J]. Inner Mongolia Petrochemical Industry, 2010, 36(11): 20-22. | |
| 21 | 朱屯. 无机盐生产中的锶钡分离[J]. 无机盐工业, 2003, 35(2): 13-15. |
| Zhu T. Separation of strontium and barium in the production of inorganic chemicals[J]. Inorganic Chemicals Industry, 2003, 35(2): 13-15. | |
| 22 | 樊秀秀, 郭旭, 孟令宗, 等. 含锶卤水体系相平衡和热力学研究进展[J]. 广东微量元素科学, 2016, 23(4): 7-11. |
| Fan X X, Guo X, Meng L Z, et al. Progresses on phase equilibria and thermodynamics of the strontium-containing brine system[J]. Guangdong Trace Elements Science, 2016, 23(4): 7-11. | |
| 23 | Chen H, Bian C, Bian J W, et al. Stable solid-liquid equilibrium of the quaternary system Na+//Cl-, NO3-, and SO42--H2O at 333.15 K[J]. Journal of Chemical & Engineering Data, 2019, 64(8): 3569-3575. |
| 24 | Qiao C Z, Zhang J, Jiang P, et al. A molecular approach for predicting phase diagrams of ternary aqueous saline solutions[J]. Chemical Engineering Science, 2020, 211: 115278. |
| 25 | Liu Y, Kermanpour F, Liu H L, et al. Molecular thermodynamic model for DNA melting in ionic and crowded solutions[J]. The Journal of Physical Chemistry B, 2010, 114(30): 9905-9911. |
| 26 | Johnson J K, Zollweg J A, Gubbins K E. The Lennard-Jones equation of state revisited[J]. Molecular Physics, 1993, 78(3): 591-618. |
| 27 | Joung I S, Cheatham T E. Determination of alkali and halide monovalent ion parameters for use in explicitly solvated biomolecular simulations[J]. J. Phys. Chem. B, 2008, 112(30): 9020-9041. |
| 28 | Mountain R D. Solvation structure of ions in water[J]. International Journal of Thermophysics, 2007, 28(2): 536-543. |
| 29 | 刘燕. 应用ABEEM/MM研究Sr(Ⅱ)和Ba(Ⅱ)离子水分子体系[D]. 大连: 辽宁师范大学, 2010. |
| Liu Y. The ABEEM/MM simulations of strontium(Ⅱ) and barium(Ⅱ) cation/water systems[D]. Dalian: Liaoning Normal University, 2010. | |
| 30 | Cotterman R L, Schwarz B J, Prausnitz J M. Molecular thermodynamics for fluids at low and high densities (Part Ⅰ): Pure fluids containing small or large molecules[J]. AIChE Journal, 1986, 32(11): 1787-1798. |
| [1] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [2] | 于旭东, 李琪, 陈念粗, 杜理, 任思颖, 曾英. 三元体系KCl + CaCl2 + H2O 298.2、323.2及348.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(8): 3256-3265. |
| [3] | 姚晓宇, 沈俊, 李健, 李振兴, 康慧芳, 唐博, 董学强, 公茂琼. 流体气液临界参数测量方法研究进展[J]. 化工学报, 2023, 74(5): 1847-1861. |
| [4] | 陈科, 杜理, 曾英, 任思颖, 于旭东. 四元体系LiCl+MgCl2+CaCl2+H2O 323.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(5): 1896-1903. |
| [5] | 毛元敬, 杨智, 莫松平, 郭浩, 陈颖, 罗向龙, 陈健勇, 梁颖宗. C6~C10烷醇的SAFT-VR Mie状态方程参数回归及其热物性研究[J]. 化工学报, 2023, 74(3): 1033-1041. |
| [6] | 高靖博, 孙强, 李青, 王逸伟, 郭绪强. 考虑水合物结构转变的含氢气体水合物相平衡模型[J]. 化工学报, 2023, 74(2): 666-673. |
| [7] | 程文婷, 李杰, 徐丽, 程芳琴, 刘国际. AlCl3·6H2O在FeCl3、CaCl2、KCl及KCl–FeCl3溶液中溶解度的实验及预测[J]. 化工学报, 2023, 74(2): 642-652. |
| [8] | 蔡进, 王晓辉, 汤涵, 陈光进, 孙长宇. TBAB水溶液体系中半笼型水合物的相平衡预测模型[J]. 化工学报, 2023, 74(1): 408-415. |
| [9] | 周桓, 张梦丽, 郝晴, 吴思, 李杰, 徐存兵. 硫酸镁型光卤石转化钾盐镁矾的过程机制与动态规律[J]. 化工学报, 2022, 73(9): 3841-3850. |
| [10] | 刘潜, 张香兰, 李志平, 李玉龙, 韩梦醒. 油酚分离过程低共熔溶剂的筛选及萃取性能研究[J]. 化工学报, 2022, 73(9): 3915-3928. |
| [11] | 杨松涛, 李东洋, 牛玉清, 李鑫钢, 康绍辉, 李洪, 叶开凯, 周志全, 高鑫. 氟化物势能函数和热力学性质的分子模拟研究进展[J]. 化工学报, 2022, 73(9): 3828-3840. |
| [12] | 孙哲, 金华强, 李康, 顾江萍, 黄跃进, 沈希. 基于知识数据化表达的制冷空调系统故障诊断方法[J]. 化工学报, 2022, 73(7): 3131-3144. |
| [13] | 任玉鑫, 徐润峰, 王婉颖, 陈鹏忠, 彭孝军. 彩色光刻胶用蒽醌染料的合成及稳定性研究[J]. 化工学报, 2022, 73(5): 2251-2261. |
| [14] | 张家仁, 刘海超. 大豆油与甲醇酯交换反应体系的相平衡研究[J]. 化工学报, 2022, 73(5): 1920-1929. |
| [15] | 吴子睿, 孙瑞, 石凌峰, 田华, 王轩, 舒歌群. CO2混合工质的气液相平衡的混合规则对比与预测研究[J]. 化工学报, 2022, 73(4): 1483-1492. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号