化工学报 ›› 2022, Vol. 73 ›› Issue (5): 2251-2261.DOI: 10.11949/0438-1157.20211726
任玉鑫1( ),徐润峰1,王婉颖1,陈鹏忠1,2(
),徐润峰1,王婉颖1,陈鹏忠1,2( ),彭孝军1
),彭孝军1
收稿日期:2021-12-02
修回日期:2022-03-08
出版日期:2022-05-05
发布日期:2022-05-24
通讯作者:
陈鹏忠
作者简介:任玉鑫(1997—),女,硕士研究生,基金资助:
Yuxin REN1( ),Runfeng XU1,Wanying WANG1,Pengzhong CHEN1,2(
),Runfeng XU1,Wanying WANG1,Pengzhong CHEN1,2( ),Xiaojun PENG1
),Xiaojun PENG1
Received:2021-12-02
Revised:2022-03-08
Online:2022-05-05
Published:2022-05-24
Contact:
Pengzhong CHEN
摘要:
为实现彩色滤光片更高分辨率的发展需求,作为关键原材料的彩色光刻胶着色剂从颜料向染料体系转变是重要的趋势。然而染料分子的光热稳定性较差,亟需从分子结构方面探索提升稳定性的有效策略。以1,4,9,10-蒽四醇为原料,合成了9种1,4-二氨基取代的蓝色蒽醌染料分子,探索了不同取代基对染料分子光物理性质、溶解性以及光热稳定性的影响。结果表明,所有染料分子在590~600 nm和630~650 nm波长范围内呈现双吸收峰性质,具有高的摩尔消光系数。其中三甘醇及单甲醚取代的染料分子热分解温度为300℃,在230℃加热0.5 h后失重约2%,365 nm波长光照射8 h后色差低于1.73,表现出优异的光热稳定性。研究为进一步制备光热稳定性优异的彩色光刻胶用染料分子奠定了基础。
中图分类号:
任玉鑫, 徐润峰, 王婉颖, 陈鹏忠, 彭孝军. 彩色光刻胶用蒽醌染料的合成及稳定性研究[J]. 化工学报, 2022, 73(5): 2251-2261.
Yuxin REN, Runfeng XU, Wanying WANG, Pengzhong CHEN, Xiaojun PENG. Synthesis and stability study of anthraquinone dyes for color photoresist[J]. CIESC Journal, 2022, 73(5): 2251-2261.
| 溶剂 | 染料 | λmax/nm | ε/(104 L/(mol·cm)) | λmax/nm | ε/(104 L/(mol·cm)) |
|---|---|---|---|---|---|
| DMF | A1 | 600 | 1.50 | 645 | 1.82 |
| A2 | 595 | 1.69 | 645 | 2.05 | |
| A3 | 600 | 1.60 | 645 | 1.98 | |
| A4 | 595 | 1.36 | 640 | 1.56 | |
| A5 | 595 | 1.14 | 640 | 1.28 | |
| A6 | 595 | 1.35 | 640 | 1.57 | |
| A7 | 595 | 1.52 | 640 | 1.78 | |
| A8 | 595 | 0.83 | 645 | 0.84 | |
| A9 | 595 | 0.72 | 645 | 0.73 | |
| 溶剂蓝78 | 600 | 1.40 | 645 | 1.58 | |
| PGME | A1 | 598 | 1.41 | 648 | 1.68 |
| A2 | 598 | 1.59 | 646 | 1.91 | |
| A3 | 598 | 1.44 | 646 | 1.76 | |
| A4 | 595 | 1.51 | 640 | 1.71 | |
| A5 | 594 | 1.11 | 638 | 1.23 | |
| A6 | 594 | 1.44 | 640 | 1.66 | |
| A7 | 594 | 1.34 | 640 | 1.54 | |
| A8 | 598 | 0.51 | 640 | 0.49 | |
| A9 | 594 | 0.40 | 642 | 0.39 | |
| 溶剂蓝78 | 598 | 1.20 | 646 | 1.38 | |
| PGMEA | A1 | 598 | 1.54 | 648 | 1.81 |
| A2 | 598 | 1.57 | 644 | 1.85 | |
| A3 | 598 | 1.56 | 646 | 1.83 | |
| A4 | 595 | 1.58 | 638 | 1.75 | |
| A5 | 592 | 1.14 | 638 | 1.23 | |
| A6 | 594 | 1.25 | 640 | 1.41 | |
| A7 | 591 | 1.57 | 638 | 1.78 | |
| A8 | 596 | 0.71 | 640 | 0.70 | |
| A9 | 598 | 0.53 | 642 | 0.51 | |
| 溶剂蓝78 | 600 | 1.33 | 643 | 1.47 |
表1 染料在不同溶剂中的光谱数据
Table 1 Spectroscopic data of dyes in organic solvents
| 溶剂 | 染料 | λmax/nm | ε/(104 L/(mol·cm)) | λmax/nm | ε/(104 L/(mol·cm)) |
|---|---|---|---|---|---|
| DMF | A1 | 600 | 1.50 | 645 | 1.82 |
| A2 | 595 | 1.69 | 645 | 2.05 | |
| A3 | 600 | 1.60 | 645 | 1.98 | |
| A4 | 595 | 1.36 | 640 | 1.56 | |
| A5 | 595 | 1.14 | 640 | 1.28 | |
| A6 | 595 | 1.35 | 640 | 1.57 | |
| A7 | 595 | 1.52 | 640 | 1.78 | |
| A8 | 595 | 0.83 | 645 | 0.84 | |
| A9 | 595 | 0.72 | 645 | 0.73 | |
| 溶剂蓝78 | 600 | 1.40 | 645 | 1.58 | |
| PGME | A1 | 598 | 1.41 | 648 | 1.68 |
| A2 | 598 | 1.59 | 646 | 1.91 | |
| A3 | 598 | 1.44 | 646 | 1.76 | |
| A4 | 595 | 1.51 | 640 | 1.71 | |
| A5 | 594 | 1.11 | 638 | 1.23 | |
| A6 | 594 | 1.44 | 640 | 1.66 | |
| A7 | 594 | 1.34 | 640 | 1.54 | |
| A8 | 598 | 0.51 | 640 | 0.49 | |
| A9 | 594 | 0.40 | 642 | 0.39 | |
| 溶剂蓝78 | 598 | 1.20 | 646 | 1.38 | |
| PGMEA | A1 | 598 | 1.54 | 648 | 1.81 |
| A2 | 598 | 1.57 | 644 | 1.85 | |
| A3 | 598 | 1.56 | 646 | 1.83 | |
| A4 | 595 | 1.58 | 638 | 1.75 | |
| A5 | 592 | 1.14 | 638 | 1.23 | |
| A6 | 594 | 1.25 | 640 | 1.41 | |
| A7 | 591 | 1.57 | 638 | 1.78 | |
| A8 | 596 | 0.71 | 640 | 0.70 | |
| A9 | 598 | 0.53 | 642 | 0.51 | |
| 溶剂蓝78 | 600 | 1.33 | 643 | 1.47 |
| 染料 | SPGMEA | SDMF |
|---|---|---|
| A1 | 8.41 | 2.50 |
| A2 | 5.79 | 2.03 |
| A3 | 1.87 | 0.94 |
| A4 | 5.60 | 6.12 |
| A5 | 5.32 | 5.92 |
| A6 | 3.76 | 4.67 |
| A7 | 3.72 | 9.90 |
| A8 | 0.25 | 0.81 |
| A9 | 0.18 | 1.20 |
表2 染料在有机溶剂中的溶解度
Table 2 Solubility of dyes in organic solvents
| 染料 | SPGMEA | SDMF |
|---|---|---|
| A1 | 8.41 | 2.50 |
| A2 | 5.79 | 2.03 |
| A3 | 1.87 | 0.94 |
| A4 | 5.60 | 6.12 |
| A5 | 5.32 | 5.92 |
| A6 | 3.76 | 4.67 |
| A7 | 3.72 | 9.90 |
| A8 | 0.25 | 0.81 |
| A9 | 0.18 | 1.20 |
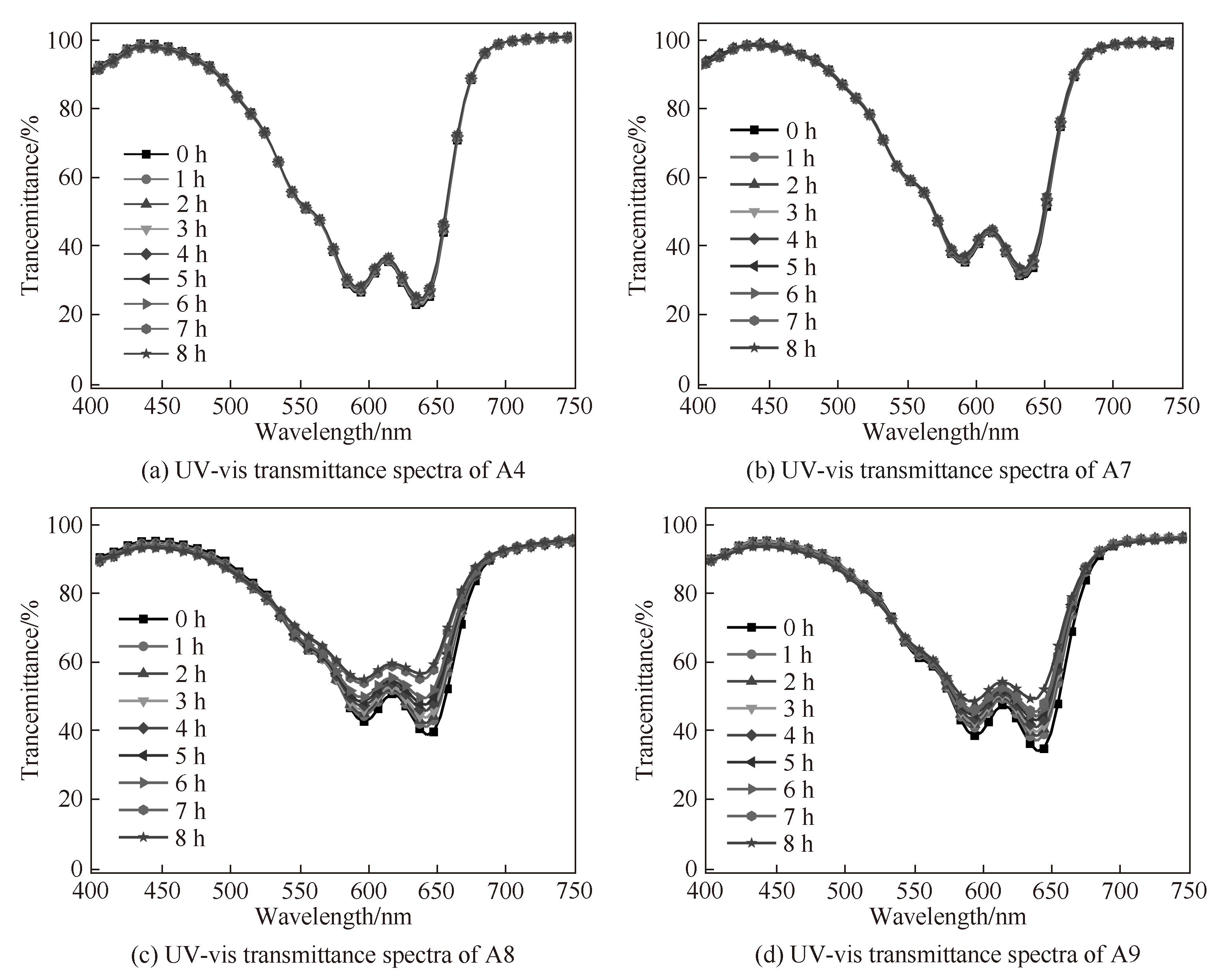
图 6 A4、A7~A9染料分子的PGMEA溶液(33 μmol/L)在365 nm波长光下连续光照8 h的透射光谱for 8 h
Fig.6 UV-vis transmittance spectra of A4,A7—A9(33 μmol/L) dyes in PGMEA upon illumination at 365 nm light
| 染料 | 光照前 | 光照后 | ΔE | ||||
|---|---|---|---|---|---|---|---|
| L | a | b | L | a | b | ||
| A1 | 84.518 | -18.715 | -23.367 | 85.577 | -16.489 | -21.677 | 2.99 |
| A2 | 81.446 | -21,160 | -30.003 | 82.039 | -19.813 | -28.698 | 1.96 |
| A3 | 84.845 | -17.406 | -24.473 | 85.031 | -16.055 | -23.718 | 1.56 |
| A4 | 79.305 | -19.392 | -33.490 | 79.678 | -18.186 | -32.311 | 1.73 |
| A5 | 86.429 | -12.869 | -19.214 | 87.066 | -11.554 | -19.437 | 1.48 |
| A6 | 82.322 | -18.314 | -27.970 | 82.493 | -16.874 | -26.368 | 2.16 |
| A7 | 83.177 | -15.160 | -26.529 | 83.442 | -13.973 | -25.931 | 1.36 |
| A8 | 85.299 | -14.146 | -21.558 | 87.386 | -7.471 | -16.721 | 8.50 |
| A9 | 84.699 | -16.060 | -23.370 | 86.129 | -9.713 | -19.805 | 7.41 |
| 溶剂蓝78 | 83.923 | -14.011 | -23.774 | 84.963 | -13.864 | -22.040 | 1.40 |
表3 光照前后染料L、a、b数值以及色差变化(ΔE)
Table 3 L,a,b data and color difference value (ΔE) of dyes before and after accelerated irradiation
| 染料 | 光照前 | 光照后 | ΔE | ||||
|---|---|---|---|---|---|---|---|
| L | a | b | L | a | b | ||
| A1 | 84.518 | -18.715 | -23.367 | 85.577 | -16.489 | -21.677 | 2.99 |
| A2 | 81.446 | -21,160 | -30.003 | 82.039 | -19.813 | -28.698 | 1.96 |
| A3 | 84.845 | -17.406 | -24.473 | 85.031 | -16.055 | -23.718 | 1.56 |
| A4 | 79.305 | -19.392 | -33.490 | 79.678 | -18.186 | -32.311 | 1.73 |
| A5 | 86.429 | -12.869 | -19.214 | 87.066 | -11.554 | -19.437 | 1.48 |
| A6 | 82.322 | -18.314 | -27.970 | 82.493 | -16.874 | -26.368 | 2.16 |
| A7 | 83.177 | -15.160 | -26.529 | 83.442 | -13.973 | -25.931 | 1.36 |
| A8 | 85.299 | -14.146 | -21.558 | 87.386 | -7.471 | -16.721 | 8.50 |
| A9 | 84.699 | -16.060 | -23.370 | 86.129 | -9.713 | -19.805 | 7.41 |
| 溶剂蓝78 | 83.923 | -14.011 | -23.774 | 84.963 | -13.864 | -22.040 | 1.40 |
| 染料 | 失重率(230℃)/% | Td/℃ |
|---|---|---|
| A1 | 14.56 | 250 |
| A2 | 55.24 | 250 |
| A3 | 1.18 | 300 |
| A4 | 2.13 | 300 |
| A5 | 15.00 | 250 |
| A6 | 5.63 | 300 |
| A7 | 1.92 | 300 |
| A8 | 5.77 | 200 |
| A9 | 9.15 | 200 |
| 溶剂蓝78 | 43.95 | 250 |
表4 染料在230℃加热30 min后的失重率及分解温度
Table 4 The weight loss of dyes at 230℃ for 30 min and decomposition temperature
| 染料 | 失重率(230℃)/% | Td/℃ |
|---|---|---|
| A1 | 14.56 | 250 |
| A2 | 55.24 | 250 |
| A3 | 1.18 | 300 |
| A4 | 2.13 | 300 |
| A5 | 15.00 | 250 |
| A6 | 5.63 | 300 |
| A7 | 1.92 | 300 |
| A8 | 5.77 | 200 |
| A9 | 9.15 | 200 |
| 溶剂蓝78 | 43.95 | 250 |
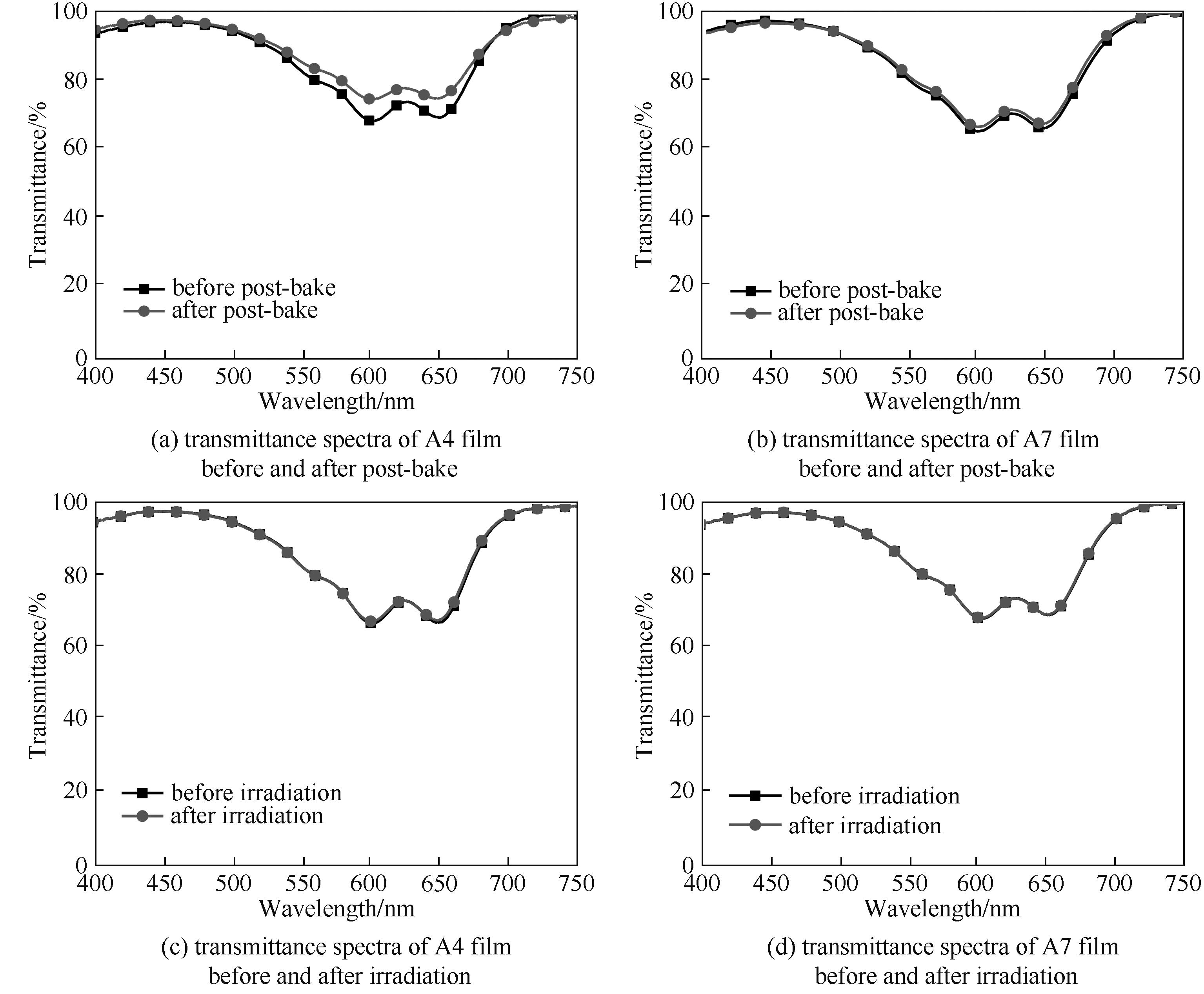
图9 后烘前后染料薄膜的透射光谱及光照前后染料薄膜的透射光谱
Fig.9 Transmittance spectra of dye film before and after post-bake and transmittance spectra of dye film before and after irradiation
| 染料 | 后烘前 | 后烘后 | ΔE | ||||
|---|---|---|---|---|---|---|---|
| L | a | b | L | a | b | ||
| A4 | 97.17 | -3.06 | -4.42 | 98.43 | -1.68 | -2.52 | 2.66 |
| A7 | 94.61 | -4.61 | -7.84 | 94.73 | -4.41 | -7.81 | 0.24 |
表5 后烘前后染料薄膜的色差
Table 5 The color difference of the dye film before and after post-bake
| 染料 | 后烘前 | 后烘后 | ΔE | ||||
|---|---|---|---|---|---|---|---|
| L | a | b | L | a | b | ||
| A4 | 97.17 | -3.06 | -4.42 | 98.43 | -1.68 | -2.52 | 2.66 |
| A7 | 94.61 | -4.61 | -7.84 | 94.73 | -4.41 | -7.81 | 0.24 |
| 染料 | 光照前 | 光照后 | ΔE | ||||
|---|---|---|---|---|---|---|---|
| L | a | b | L | a | b | ||
| A4 | 97.03 | -3.19 | -4.83 | 96.99 | -3.22 | -4.67 | 0.16 |
| A7 | 94.74 | -4.51 | -8.02 | 94.71 | -4.37 | -7.93 | 0.17 |
表6 光照前后染料薄膜的色差
Table 6 The color difference of the dye film before and after irradiation
| 染料 | 光照前 | 光照后 | ΔE | ||||
|---|---|---|---|---|---|---|---|
| L | a | b | L | a | b | ||
| A4 | 97.03 | -3.19 | -4.83 | 96.99 | -3.22 | -4.67 | 0.16 |
| A7 | 94.74 | -4.51 | -8.02 | 94.71 | -4.37 | -7.93 | 0.17 |
| 1 | Kim Y D, Kim J P, Kwon O S, et al. The synthesis and application of thermally stable dyes for ink-jet printed LCD color filters[J]. Dyes and Pigments, 2009, 81(1): 45-52. |
| 2 | Kim Y D, Cho J H, Park C R, et al. Synthesis, application and investigation of structure-thermal stability relationships of thermally stable water-soluble azo naphthalene dyes for LCD red color filters[J]. Dyes and Pigments, 2011, 89(1): 1-8. |
| 3 | Choi J, Lee W, Chun S K, et al. Facile synthesis and characterization of novel coronene chromophores and their application to LCD color filters[J]. Dyes and Pigments, 2012, 94(1): 34-39. |
| 4 | Kim S H, Namgoong J W, Yuk S B, et al. Synthesis and characteristics of metal-phthalocyanines tetra-substituted at non-peripheral (α) or peripheral (β) positions, and their applications in LCD color filters[J]. Journal of Inclusion Phenomena and Macrocyclic Chemistry, 2015, 82(1/2): 195-202. |
| 5 | 郭景. 生产彩色滤光片用材料简介[J]. 科技创新导报, 2019, 16(31): 94, 96. |
| Guo J. Brief introduction of materials used in producing color filter [J]. Science and Technology Innovation Herald, 2019, 16(31): 94, 96. | |
| 6 | 付学勇. 颜料分散机理的探讨及新的分散方法[J]. 涂料工业, 2010, 40(7): 67-68, 72. |
| Fu X Y. Discussion on pigment dispersion mechanism and new dispersion method[J]. Paint & Coatings Industry, 2010, 40(7): 67-68, 72. | |
| 7 | Sugiura T. Development of pigment-dispersed-type color filters for LCDs[J]. Journal of the Society for Information Display, 1993, 1(3): 341. |
| 8 | Sabnis R W. Color filter technology for liquid crystal displays[J]. Displays, 1999, 20(3): 119-129. |
| 9 | Fagelman K E, Guthrie J T. A study of pigment solubility in model compounds that represent a polycarbonate-poly(butylene terephthalate) blend[J]. Surface Coatings International Part B: Coatings Transactions, 2006, 89(2): 109-116. |
| 10 | Choi S H, Kil G H, Kim N R, et al. Preparation of red dyes derived from diketo-pyrrolo-pyrrole based pigment and their properties for LCD color filters[J]. Bulletin of the Korean Chemical Society, 2010, 31(11): 3427-3430. |
| 11 | 张卓, 何璇, 李琳. 颜料分散用树脂的合成及性能研究[J]. 液晶与显示, 2010, 25(4): 481-485. |
| Zhang Z, He X, Li L. Synthesis and performance of polyacrylate resin used on pigment-dispersion[J]. Chinese Journal of Liquid Crystals and Displays, 2010, 25(4): 481-485. | |
| 12 | Chun S K, Kim Y D, Choi J H, et al. The synthesis of thermally-stable red dyes for LCD color filters and analysis of their aggregation and spectral properties[J]. Dyes and Pigments, 2011, 88(2): 166-173. |
| 13 | 李宏彦, 杨久霞, 吕艳英, 等. TFT-LCD用彩色滤光片[J]. 现代显示, 2005(6): 41-44. |
| Li H Y, Yang J X, Lyu Y Y, et al. Color filter for TFT-LCD[J]. Advanced Display, 2005(6): 41-44. | |
| 14 | 朱昌昌, 陈嵘. LCD用彩色滤色器的现状和进展[J]. 光电子技术, 1996, 16(4): 280-285. |
| Zhu C C, Chen R. State-of-art and development trend of color filter for LCDs[J]. Optoelecfronic Technology, 1996, 16(4): 280-285. | |
| 15 | 张天庆, 杜健军, 陈鹏, 等. 1, 4-二氨基-2, 3-邻苯二甲酰亚胺蒽醌染料的合成与应用[J]. 精细化工, 2019, 36(9): 1949-1955. |
| Zhang T Q, Du J J, Chen P, et al. Synthesis and application of 1, 4-diamino-2, 3-phthalimide-anthraquinone dyes[J]. Fine Chemicals, 2019, 36(9): 1949-1955. | |
| 16 | Galagan Y, Su W F. Fadable ink for time-temperature control of food freshness: novel new time-temperature indicator[J]. Food Research International, 2008, 41(6): 653-657. |
| 17 | 段孝宁, 潘鑫, 邓宏坡. 一种蓝色水暂溶性分散染料及其制法和应用: 102199364[P]. 2011-09-28. |
| Duan X N, Pan X, Deng H P. A blue water temporarily solubilized disperse dye and preparation method and application: 102199364[P]. 2011-09-28. | |
| 18 | 盛明, 刘慧霞, 张燕柳. 米托蒽醌治疗进展型多发性硬化症的疗效观察[J]. 慢性病学杂志, 2014, 15(2): 143-144. |
| Sheng M, Liu H X, Zhang Y L. Clinical observation of mitoxantrone in the treatment of progressive multiple sclerosis[J]. Chronic Pathematology Journal, 2014, 15(2): 143-144. | |
| 19 | Qi F F, Zhang W, Xue Y Y, et al. Bienzyme-catalytic and dioxygenation-mediated anthraquinone ring opening[J]. Journal of the American Chemical Society, 2021, 143(40): 16326-16331. |
| 20 | 宋晓燕, 何国庆, 毕艳兰, 等. 大豆油基印刷油墨连结料的制备研究[J]. 中国粮油学报, 2006, 21(4): 71-75. |
| Song X Y, He G Q, Bi Y L, et al. Preparation of soybean oil based printing ink vehicle[J]. Journal of the Chinese Cereals and Oils Association, 2006, 21(4): 71-75. | |
| 21 | 杨涛, 马传国. 蒽醌催化大豆油热聚合反应的研究[J]. 中国油脂, 2007, 32(2): 78-81. |
| Yang T, Ma C G. Heat polymerization of soybean oil catalyzed by anthraquinone[J]. China Oils and Fats, 2007, 32(2): 78-81. | |
| 22 | Jing Y, Wu M, Wong A A, et al. In situ electrosynthesis of anthraquinone electrolytes in aqueous flow batteries[J]. Green Chemistry, 2020, 22(18): 6084-6092. |
| 23 | 贾红玲, 周振勇, 杨倩, 等. 基于数字图像中LAB颜色空间的肉色质量评价方法研究[J]. 现代农业科技, 2016(2): 277, 281. |
| Jia H L, Zhou Z Y, Yang Q, et al. Research on meat color quality evaluation method based on LAB color space in digital image [J]. Modern Agricultural Science and Technology, 2016(2): 277, 281. | |
| 24 | Conway B R, Eskew R T, Martin P R, et al. A tour of contemporary color vision research[J]. Vision Research, 2018, 151: 2-6. |
| 25 | Park J, Park Y, Park J. Synthesis and physical property measurement of new red pigment based on anthraquinone derivatives for color filter pigments[J]. Molecular Crystals and Liquid Crystals, 2011, 551(1): 116-122. |
| 26 | Hansen C M. Hansen Solubility Parameters: A User’s Handbook[M]. 2nd ed. Boca Raton: CRC Press, 2012. |
| 27 | Gu K Z, Zhu W H, Peng X J. Enhancement strategies of targetability, response and photostability for in vivo bioimaging[J]. Science China-Chemistry, 2019, 6(2): 189-198. |
| 28 | Wu X M, Zhu W H. Stability enhancement of fluorophores for lighting up practical application in bioimaging[J]. Chemical Society Reviews, 2015, 44(13): 4179-4184. |
| 29 | 胡永静. 醚链取代/桥联聚噻吩的热电及电致变色性能[D]. 南昌: 江西科技师范大学, 2017. |
| Hu Y J. Thermoelectric and electrochromic properties of oligo(oxyethylene)-substituted/bridged polythiophene[D]. Nanchang: Jiangxi Science and Technology Normal University, 2017. | |
| 30 | 单斌. 高光稳定性聚乙烯胺型大分子自交联染料的合成及应用[D]. 大连: 大连理工大学, 2016. |
| Shan B. Synthesis and application of polyvinylamine macromolecule self-crosslinking dyes with high light stability[D]. Dalian: Dalian University of Technology, 2016. | |
| 31 | Kim T H, Lee B J, An S O, et al. The synthesis of red dyes based on diketo-pyrrolo-pyrrole chromophore to improve heat stability and solubility for colour filter fabrication[J]. Dyes and Pigments, 2020, 174: 108053. |
| [1] | 江河, 袁俊飞, 王林, 邢谷雨. 均流腔结构对微细通道内相变流动特性影响的实验研究[J]. 化工学报, 2023, 74(S1): 235-244. |
| [2] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [3] | 郑玉圆, 葛志伟, 韩翔宇, 王亮, 陈海生. 中高温钙基材料热化学储热的研究进展与展望[J]. 化工学报, 2023, 74(8): 3171-3192. |
| [4] | 孟令玎, 崇汝青, 孙菲雪, 孟子晖, 刘文芳. 改性聚乙烯膜和氧化硅固定化碳酸酐酶[J]. 化工学报, 2023, 74(8): 3472-3484. |
| [5] | 于旭东, 李琪, 陈念粗, 杜理, 任思颖, 曾英. 三元体系KCl + CaCl2 + H2O 298.2、323.2及348.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(8): 3256-3265. |
| [6] | 李彬, 徐正虎, 姜爽, 张天永. 双氧水催化氧化法清洁高效合成促进剂CBS[J]. 化工学报, 2023, 74(7): 2919-2925. |
| [7] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [8] | 蔡斌, 张效林, 罗倩, 党江涛, 左栗源, 刘欣梅. 导电薄膜材料的研究进展[J]. 化工学报, 2023, 74(6): 2308-2321. |
| [9] | 徐文超, 孙志高, 李翠敏, 李娟, 黄海峰. 静态条件下表面活性剂E-1310对HCFC-141b水合物生成的影响[J]. 化工学报, 2023, 74(5): 2179-2185. |
| [10] | 陈科, 杜理, 曾英, 任思颖, 于旭东. 四元体系LiCl+MgCl2+CaCl2+H2O 323.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(5): 1896-1903. |
| [11] | 王子健, 柯明, 李佳涵, 李舒婷, 孙巾茹, 童燕兵, 赵治平, 刘加英, 任璐. 短b轴ZSM-5分子筛制备方法及应用研究进展[J]. 化工学报, 2023, 74(4): 1457-1473. |
| [12] | 刘润竹, 储甜甜, 张孝阿, 王成忠, 张军营. α,ω-端羟基亚苯基氟硅聚合物的合成及性能[J]. 化工学报, 2023, 74(3): 1360-1369. |
| [13] | 陈向上, 马振杰, 任希华, 贾悦, 吕晓龙, 陈华艳. 三维网络萃取膜的制备及传质效率研究[J]. 化工学报, 2023, 74(3): 1126-1133. |
| [14] | 程文婷, 李杰, 徐丽, 程芳琴, 刘国际. AlCl3·6H2O在FeCl3、CaCl2、KCl及KCl–FeCl3溶液中溶解度的实验及预测[J]. 化工学报, 2023, 74(2): 642-652. |
| [15] | 杨宏欣, 李兴亚, 葛亮, 徐铜文. 含哌啶阳离子侧长链型一/二价阴离子选择性分离膜的制备[J]. 化工学报, 2022, 73(8): 3739-3748. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号