化工学报 ›› 2022, Vol. 73 ›› Issue (10): 4762-4768.DOI: 10.11949/0438-1157.20220725
收稿日期:2022-05-19
修回日期:2022-07-22
出版日期:2022-10-05
发布日期:2022-11-02
通讯作者:
钟委
作者简介:梁天水(1981—),男,博士,教授,liangtsh@zzu.edu.cn
基金资助:
Tianshui LIANG( ), Xinke WANG, Dezhi LIU, Wei ZHONG(
), Xinke WANG, Dezhi LIU, Wei ZHONG( )
)
Received:2022-05-19
Revised:2022-07-22
Online:2022-10-05
Published:2022-11-02
Contact:
Wei ZHONG
摘要:
氟胺类物质是最有希望作为哈龙替代品的含氮化合物之一,全氟三乙胺作为典型的氟胺类物质具有良好的灭火效果。为研究全氟三乙胺热解机理,在管式加热炉内对全氟三乙胺进行热分解,通过GC-MS分析全氟三乙胺在不同温度条件下的热解产物,并用Gaussian软件对其热解反应路径进行理论计算。结果表明:保持停留时间为10 s,全氟三乙胺的初始热解温度为600℃,750℃完全热解,热解产物有C4F9N、C3F7N、C2F6和C3F8,热解温度较低时C4F9N体积分数最大,热解温度较高时C3F7N体积分数最大。在全氟三乙胺热解反应路径计算中,全氟三乙胺分子中的C—C键断裂后存在1条反应路径,可生成实验产物中的C3F8;全氟三乙胺分子的C—N键断裂后存在3条反应路径,可生成实验产物中的C3F7N、 C4F9N和C2F6。全氟三乙胺热解后产生的CF3自由基可与H、OH自由基发生反应,从而产生灭火作用。此外,其热解产物C4F9N和C3F7N具有C N双键,更容易与燃烧活泼自由基·OH、·H发生化学作用,对研究全氟三乙胺的灭火机理具有十分重要的意义。
N双键,更容易与燃烧活泼自由基·OH、·H发生化学作用,对研究全氟三乙胺的灭火机理具有十分重要的意义。
中图分类号:
梁天水, 王新科, 刘德智, 钟委. 全氟三乙胺热解机理的实验与理论研究[J]. 化工学报, 2022, 73(10): 4762-4768.
Tianshui LIANG, Xinke WANG, Dezhi LIU, Wei ZHONG. Experimental and theoretical study on the pyrolysis mechanism of (C2F5)3N[J]. CIESC Journal, 2022, 73(10): 4762-4768.
| 实验条件 | 参数设置 |
|---|---|
| 毛细柱固定相 | 10%SE-54 |
| 毛细柱柱长 | 30 m |
| 毛细柱内径 | 0.25 mm |
| 进样口温度 | 280℃ |
| 检测器温度 | 240℃ |
| 柱箱升温曲线 | 50~150℃, 10℃/min |
| 分流比 | 80∶1 |
| 载气类型 | 氦气 |
| 载气流速 | 1.2 ml/min |
| 离子源温度 | 240℃ |
表1 GC-MS检测条件
Table 1 GC-MS detection conditions
| 实验条件 | 参数设置 |
|---|---|
| 毛细柱固定相 | 10%SE-54 |
| 毛细柱柱长 | 30 m |
| 毛细柱内径 | 0.25 mm |
| 进样口温度 | 280℃ |
| 检测器温度 | 240℃ |
| 柱箱升温曲线 | 50~150℃, 10℃/min |
| 分流比 | 80∶1 |
| 载气类型 | 氦气 |
| 载气流速 | 1.2 ml/min |
| 离子源温度 | 240℃ |
| 序号 | 质荷比(m/z) | 气体产物 |
|---|---|---|
| 1 | CF+(31);C2F+(43);CF2+(50);CF3+(69);C2F5+(119) | C2F6 |
| 2 | CF+(31);C2F+(43);CF2+(50);C2F2+(62);CF3+(69);C2F3+(81);C3F3+(93);C2F4+(100);C3F4+(112);C2F5+(119);C3F5+(131);C3F6+(150) | C3F8 |
| 3 | CF+(31);CF2+(50);CF3+(69);C2F4+(100);C2F4N+(114);C2F5N+(133);C3F6N+(164);C3F7N+(183) | C3F7N |
| 4 | CF+(31);CF2+(50);CF3+(69);C2F4+(100);C2F4N+(114);C2F5+(119);C3F6N+(164);C4F8N+(214) | C4F9N |
表2 全氟三乙胺热解产物离子峰分布
Table 2 Distribution of ion peaks of (C2F5)3N pyrolysis products
| 序号 | 质荷比(m/z) | 气体产物 |
|---|---|---|
| 1 | CF+(31);C2F+(43);CF2+(50);CF3+(69);C2F5+(119) | C2F6 |
| 2 | CF+(31);C2F+(43);CF2+(50);C2F2+(62);CF3+(69);C2F3+(81);C3F3+(93);C2F4+(100);C3F4+(112);C2F5+(119);C3F5+(131);C3F6+(150) | C3F8 |
| 3 | CF+(31);CF2+(50);CF3+(69);C2F4+(100);C2F4N+(114);C2F5N+(133);C3F6N+(164);C3F7N+(183) | C3F7N |
| 4 | CF+(31);CF2+(50);CF3+(69);C2F4+(100);C2F4N+(114);C2F5+(119);C3F6N+(164);C4F8N+(214) | C4F9N |
| 物种 | ΔE/(kcal/mol) | (ΔE+ΔEZPVE)/(kcal/mol) |
|---|---|---|
| 全氟三乙胺:N(C2F5)3 | 0 | 0 |
P1:CF3—CF  N—C2F5+C2F6 N—C2F5+C2F6 | -11.63 | -13.28 |
P2:CF3—CF  N—C2F5+C2F6 N—C2F5+C2F6 | -7.85 | -9.52 |
| P3:N(C2F5)2CF2+CF3 | 75.73 | 72.56 |
| P4:N(C2F5)2+C2F5 | 68.60 | 65.06 |
| TS1 | 76.56 | 73.86 |
| TS2 | 79.00 | 76.27 |
表3 全氟三乙胺热解反应中所有物质相对于反应物的能量
Table 3 Energy of all substances relative to the reactants in the pyrolysis reaction of (C2F5)3N
| 物种 | ΔE/(kcal/mol) | (ΔE+ΔEZPVE)/(kcal/mol) |
|---|---|---|
| 全氟三乙胺:N(C2F5)3 | 0 | 0 |
P1:CF3—CF  N—C2F5+C2F6 N—C2F5+C2F6 | -11.63 | -13.28 |
P2:CF3—CF  N—C2F5+C2F6 N—C2F5+C2F6 | -7.85 | -9.52 |
| P3:N(C2F5)2CF2+CF3 | 75.73 | 72.56 |
| P4:N(C2F5)2+C2F5 | 68.60 | 65.06 |
| TS1 | 76.56 | 73.86 |
| TS2 | 79.00 | 76.27 |

图3 全氟三乙胺热解反应势能面[0 K,CCSD(T)/6-311++(d,p)//B3LYP/6-311++(d,p)]
Fig.3 Potential energy surface of (C2F5)3N pyrolysis reaction[0 K,CCSD(T)/6-311++(d,p)//B3LYP/6-311++(d,p)]
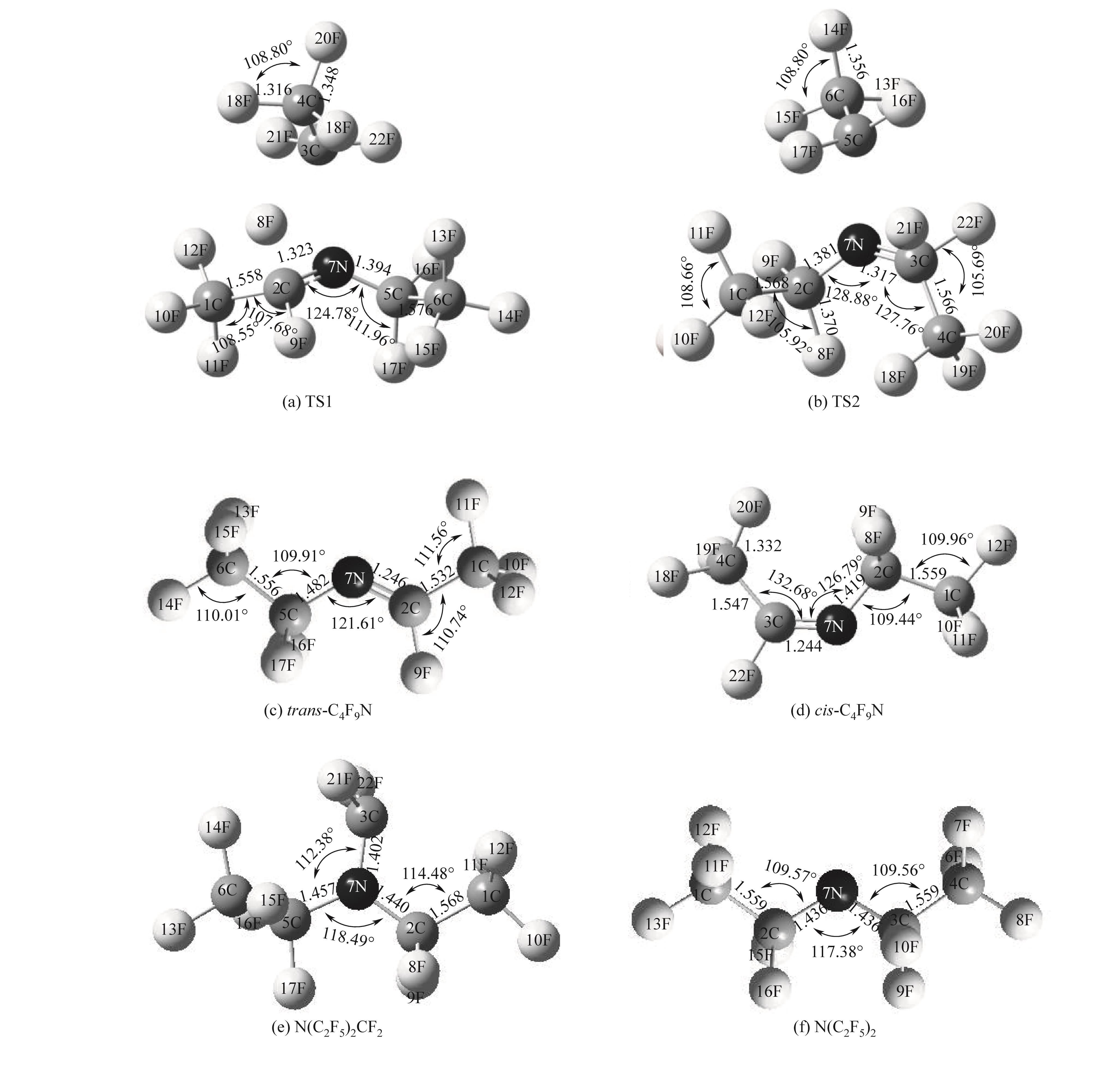
图5 全氟三乙胺主要热解产物及过渡态的优化几何结构[B3LYP/6-311++(d,p)]
Fig.5 Optimized geometry of the main pyrolysis products and transition states of (C2F5)3N [B3LYP/6-311++(d,p)]
| 5 | Zhang M L, Lin Z J. Ab initio studies of the thermal decomposition pathways of 1-bromo-3, 3, 3-trifluoropropene[J]. Journal of Molecular Structure: Theochem, 2009, 899(1/2/3): 98-110. |
| 6 | Takahashi F, Katta V R, Linteris G T, et al. A computational study of extinguishment and enhancement of propane cup-burner flames by halon and alternative agents[J]. Fire Safety Journal, 2017, 91: 688-694. |
| 7 | Takahashi F, Katta V R, Linteris G T, et al. Combustion inhibition and enhancement of cup-burner flames by CF3Br, C2HF5, C2HF3Cl2, and C3H2F3Br[J]. Proceedings of the Combustion Institute, 2015, 35(3): 2741-2748. |
| 8 | Linteris G T, Takahashi F, Katta V R. Cup-burner flame extinguishment by CF3Br and Br2 [J]. Combustion and Flame, 2007, 149(1/2): 91-103. |
| 9 | 余彬彬, 蒋新生, 禹进, 等. 全氟己酮抑制航空煤油燃烧实验及化学动力学研究[J]. 化工学报, 2022, 73(4): 1834-1844. |
| Yu B B, Jiang X S, Yu J, et al. Experimental and chemical dynamics study on the inhibition of combustion of aviation kerosene by C6F12O[J]. CIESC Journal, 2022, 73(4): 1834-1844. | |
| 10 | Tapscott R E, Sheinson R S, Babushok V I, et al. Alternative fire suppressant chemicals [R]. National Institute of Standards and Technology, 2001. |
| 11 | Heinonen E W, Lifke J L, Tapscott R E. Advanced streaming agent development(volume Ⅳ): Tropodegradable halocarbons[R]. New Mexico Engineering Research Inst Albuquerque, 1996. |
| 12 | 梁天水, 刘德智, 王永锦, 等. 全氟三乙胺和全氟己酮混合气体的灭火效果研究[J]. 化工学报, 2020, 71(7): 3387-3392. |
| Liang T S, Liu D Z, Wang Y J, et al. Study on fire extinguishing efficiency of the mixtures of C6F12O and (C2F5)3N[J]. CIESC Journal, 2020, 71(7): 3387-3392. | |
| 13 | Sheinson R S, Driscoll D C. Fire suppression mechanisms: agent testing by cup burner[C]//International Conference on CFC and Halon Alternatives. Washington, DC, 1989: 10-11. |
| 14 | Takahashi K, Sekiuji Y, Yamamori Y, et al. Kinetic studies on the reactions of CF3 with O(3P) and H atoms at high temperatures[J]. The Journal of Physical Chemistry A, 1998, 102(43): 8339-8348. |
| 15 | Linteris G T, Truett L. Inhibition of premixed methane-air flames by fluoromethanes[J]. Combustion and Flame, 1996, 105(1/2): 15-27. |
| 16 | Linteris G T, Burgess D R Jr, Babushok V, et al. Inhibition of premixed methane-air flames by fluoroethanes and fluoropropanes[J]. Combustion and Flame, 1998, 113(1/2): 164-180. |
| 17 | Hynes R G, Mackie J C, Masri A R. Inhibition of premixed hydrogen-air flames by 2-H heptafluoropropane[J]. Combustion and Flame, 1998, 113(4): 554-565. |
| 18 | Babushok V I, Linteris G T, Meier O C. Combustion properties of halogenated fire suppressants[J]. Combustion and Flame, 2012, 159(12): 3569-3575. |
| 19 | Takahashi K, Inomata T, Fukaya H, et al. New halon replacements based on perfluoroalkylamines[M]//ACS Symposium Series. Washington, DC: American Chemical Society, 1997: 139-150. |
| 20 | Takahashi K, Sekiuji Y, Inomata T, et al. Inhibition of combustion by bromine-free polyfluorocarbons(Ⅰ): Burning velocities of methane flames containing polyfluoroalkylamines[J]. Combustion Science and Technology, 1994, 102(1/2/3/4/5/6): 213-230. |
| 21 | Fukaya H, Ono T, Abe T. New fire suppression mechanism of perfluoroalkylamines[J]. Journal of the Chemical Society, Chemical Communications, 1995(12): 1207. |
| 22 | Yamamoto T, Yasuhara A, Shiraishi F, et al. Thermal decomposition of halon alternatives[J]. Chemosphere, 1997, 35(3): 643-654. |
| 23 | Lu D Y, Chao M Y, Zhou X M. Theoretical studies on the reactions of 1, 1, 2, 2, 3, 3, 4-heptafluorocyclopentane with hydroxyl and hydrogen free radicals[J]. Chinese Journal of Chemistry, 2014, 32(9): 897-908. |
| 24 | 梁天水, 王宗莹, 高坤, 等. 基于cup burner的含铁基添加剂超细水雾灭火有效性分析[J]. 化工学报, 2019, 70(3): 1236-1242. |
| Liang T S, Wang Z Y, Gao K, et al. Analysis of fire suppression effectiveness of ultra-fine water mist containing iron compounds additives in cup burner[J]. CIESC Journal, 2019, 70(3): 1236-1242. | |
| 25 | Scott A P, Radom L. Harmonic vibrational frequencies: an evaluation of Hartree-Fock, Møller–Plesset, quadratic configuration interaction, density functional theory, and semiempirical scale factors[J]. The Journal of Physical Chemistry, 1996, 100(41): 16502-16513. |
| 26 | Liu Y, Yang G C, Sun S L, et al. Density functional theory investigation on the second-order nonlinear optical properties of chlorobenzyl-o-carborane derivatives[J]. Chinese Journal of Chemistry, 2012, 30(10): 2349-2355. |
| 27 | Zhou Y, Liu D J, Fu Y, et al. Accurate prediction of Ir—H bond dissociation enthalpies by density functional theory methods[J]. Chinese Journal of Chemistry, 2014, 32(3): 269-275. |
| 28 | Bai Q L, Zhang C H, Cheng C H, et al. Synthesis, photophysical properties and near infrared electroluminescence of 1(4), 8(11), 15(18), 22(25)-tetra-(methoxy-phenoxy)phthalocyanine[J]. Chinese Journal of Chemistry, 2012, 30(3): 689-694. |
| 29 | Gottlieb A D, Weishäupl R M. Strongly separated pairs of core electrons in computed ground states of small molecules[J]. Computational and Theoretical Chemistry, 2013, 1007: 82-89. |
| 30 | Zhang M H, Li R Z, Yu Y Z. A DFT study on the structure and properties of Cu/Cr2O3 catalyst[J]. Chinese Journal of Chemistry, 2012, 30(4): 771-778. |
| 31 | Raghavachari K, Trucks G W, Pople J A, et al. Reprint of: a fifth-order perturbation comparison of electron correlation theories[J]. Chemical Physics Letters, 2013, 589: 37-40. |
| 32 | Gaensslen M, Gross U, Oberhammer H, et al. Perfluorotriethylamine: an amine with unusual structure and reactivity[J]. Angewandte Chemie International Edition in English, 1992, 31(11): 1467-1468. |
| 33 | Cobos C J, Hintzer K, Sölter L, et al. Shock wave and modelling study of the dissociation pathways of (C2F5)3N[J]. Physical Chemistry Chemical Physics: PCCP, 2019, 21(19): 9785-9792. |
| 1 | Burkholder J B, Cox R A, Ravishankara A R. Atmospheric degradation of ozone depleting substances, their substitutes, and related species[J]. Chemical Reviews, 2015, 115(10): 3704-3759. |
| 2 | Molina M J, Rowland F S. Stratospheric sink for chlorofluoromethanes: chlorine atom-catalysed destruction of ozone[J]. Nature, 1974, 249(5460): 810-812. |
| 3 | Kennedy E M, Li K, Moghtaderi B, et al. A process for disposal of halon 1301 (CBrF3)[J]. Chemical Engineering Communications, 1999, 176(1): 195-200. |
| 4 | Wan D, Xu J H, Zhang J B, et al. Historical and projected emissions of major halocarbons in China[J]. Atmospheric Environment, 2009, 43(36): 5822-5829. |
| [1] | 吴雷, 刘姣, 李长聪, 周军, 叶干, 刘田田, 朱瑞玉, 张秋利, 宋永辉. 低阶粉煤催化微波热解制备含碳纳米管的高附加值改性兰炭末[J]. 化工学报, 2023, 74(9): 3956-3967. |
| [2] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [3] | 杨峥豪, 何臻, 常玉龙, 靳紫恒, 江霞. 生物质快速热解下行式流化床反应器研究进展[J]. 化工学报, 2023, 74(6): 2249-2263. |
| [4] | 龙臻, 王谨航, 何勇, 梁德青. 离子液体与动力学抑制剂作用下混合气体水合物生成特性研究[J]. 化工学报, 2023, 74(4): 1703-1711. |
| [5] | 衣思敏, 马亚丽, 刘伟强, 张金帅, 岳岩, 郑强, 贾松岩, 李雪. 微晶菱镁矿蒸氨及水化动力学研究[J]. 化工学报, 2023, 74(4): 1578-1586. |
| [6] | 包嘉靖, 别洪飞, 王子威, 肖睿, 刘冬, 吴石亮. 正庚烷对冲扩散火焰中添加长链醚类对碳烟前体生成特性的影响[J]. 化工学报, 2023, 74(4): 1680-1692. |
| [7] | 蹇建, 张嘉明, 佘祥, 周虎, 游奎一, 罗和安. V4+和V5+比例对钒磷氧催化NO2氧化环己烷性能的影响[J]. 化工学报, 2023, 74(4): 1570-1577. |
| [8] | 禹进, 余彬彬, 蒋新生. 一种基于虚拟组分的燃烧调控化学作用量化及分析方法研究[J]. 化工学报, 2023, 74(3): 1303-1312. |
| [9] | 徐银, 蔡洁, 陈露, 彭宇, 刘夫珍, 张晖. 异相可见光催化耦合过硫酸盐活化技术在水污染控制中的研究进展[J]. 化工学报, 2023, 74(3): 995-1009. |
| [10] | 陈瑞哲, 程磊磊, 顾菁, 袁浩然, 陈勇. 纤维增强树脂复合材料化学回收技术研究进展[J]. 化工学报, 2023, 74(3): 981-994. |
| [11] | 周璇, 李孟亚, 孙杰, 岑振凯, 吕强三, 周立山, 王海涛, 韩丹丹, 龚俊波. 添加剂对氨基酸晶体生长的影响[J]. 化工学报, 2023, 74(2): 500-510. |
| [12] | 张娜, 潘鹤林, 牛波, 张亚运, 龙东辉. 酚醛树脂热裂解反应机理的密度泛函理论研究[J]. 化工学报, 2023, 74(2): 843-860. |
| [13] | 李鑫, 曾少娟, 彭奎霖, 袁磊, 张香平. CO2电催化还原制合成气研究进展及趋势[J]. 化工学报, 2023, 74(1): 313-329. |
| [14] | 郝泽光, 张乾, 高增林, 张宏文, 彭泽宇, 杨凯, 梁丽彤, 黄伟. 生物质与催化裂化油浆共热解协同作用研究[J]. 化工学报, 2022, 73(9): 4070-4078. |
| [15] | 邵健, 冯军宗, 柳凤琦, 姜勇刚, 李良军, 冯坚. 酚醛树脂基炭微球结构调控与功能化制备研究进展[J]. 化工学报, 2022, 73(9): 3787-3801. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号