化工学报 ›› 2023, Vol. 74 ›› Issue (5): 1847-1861.DOI: 10.11949/0438-1157.20230075
姚晓宇1( ), 沈俊1(
), 沈俊1( ), 李健1, 李振兴1, 康慧芳1, 唐博2,3,4, 董学强2,3,4(
), 李健1, 李振兴1, 康慧芳1, 唐博2,3,4, 董学强2,3,4( ), 公茂琼2,3,4
), 公茂琼2,3,4
收稿日期:2023-02-03
修回日期:2023-04-10
出版日期:2023-05-05
发布日期:2023-06-29
通讯作者:
沈俊,董学强
作者简介:姚晓宇(1995—),男,博士后,yaoxiaoyu22@bit.edu.cn
基金资助:
Xiaoyu YAO1( ), Jun SHEN1(
), Jun SHEN1( ), Jian LI1, Zhenxing LI1, Huifang KANG1, Bo TANG2,3,4, Xueqiang DONG2,3,4(
), Jian LI1, Zhenxing LI1, Huifang KANG1, Bo TANG2,3,4, Xueqiang DONG2,3,4( ), Maoqiong GONG2,3,4
), Maoqiong GONG2,3,4
Received:2023-02-03
Revised:2023-04-10
Online:2023-05-05
Published:2023-06-29
Contact:
Jun SHEN, Xueqiang DONG
摘要:
近、超临界流体具备优良的输运和热力学性质,可广泛应用于化工、环境、机械和热能利用等领域。由于临界点附近包括流体密度在内的热物性会发生大幅改变,因此准确确定流体的临界点,包括临界温度、临界压力和临界密度数据,对指导热力循环和系统部件设计优化有重要意义。目前实验测量是获取高精度临界参数的最直接方式。本文首先概述了气液临界点理论、临界参数的研究现状及其典型应用场景;其次,综述了目前临界参数主要的测量方法,包括定容法、变容法、流动法、脉冲加热法、密度直线中径定律法、压力-体积-温度(p-V-T)关系法、准静态热分析法和物理性质法等,总结了这些方法的优缺点、适用范围、准确性和主要研究机构;最后,探讨了临界参数测量方法当前面临的挑战和未来发展趋势。
中图分类号:
姚晓宇, 沈俊, 李健, 李振兴, 康慧芳, 唐博, 董学强, 公茂琼. 流体气液临界参数测量方法研究进展[J]. 化工学报, 2023, 74(5): 1847-1861.
Xiaoyu YAO, Jun SHEN, Jian LI, Zhenxing LI, Huifang KANG, Bo TANG, Xueqiang DONG, Maoqiong GONG. Research progress in measurement methods in vapor-liquid critical properties of mixtures[J]. CIESC Journal, 2023, 74(5): 1847-1861.

图2 变容法实验装置原理图(Burrnet膨胀法)[59]A—可视化容器;B—变容容器;C—差压零压检测器;D—铝块;E—恒温油浴;F—叶轮;G—温控器;H—铂电阻温度计;I—冷阱;J—石英晶体压力表;V1—截止阀;V2—分离阀
Fig.2 Schematic diagram of the variable-volume method apparatus (Burrnet expansion method)[59]A—optical cell; B—variable-volume vessel; C—differential null-pressure detector; D—aluminum blocks; E—constant-temperature oil bath; F—impeller; G—temperature controller; H—platinum resistance thermometer; I—cold trap; J—quartz crystal pressure gauge; V1—cutoff valve; V2—separation valve
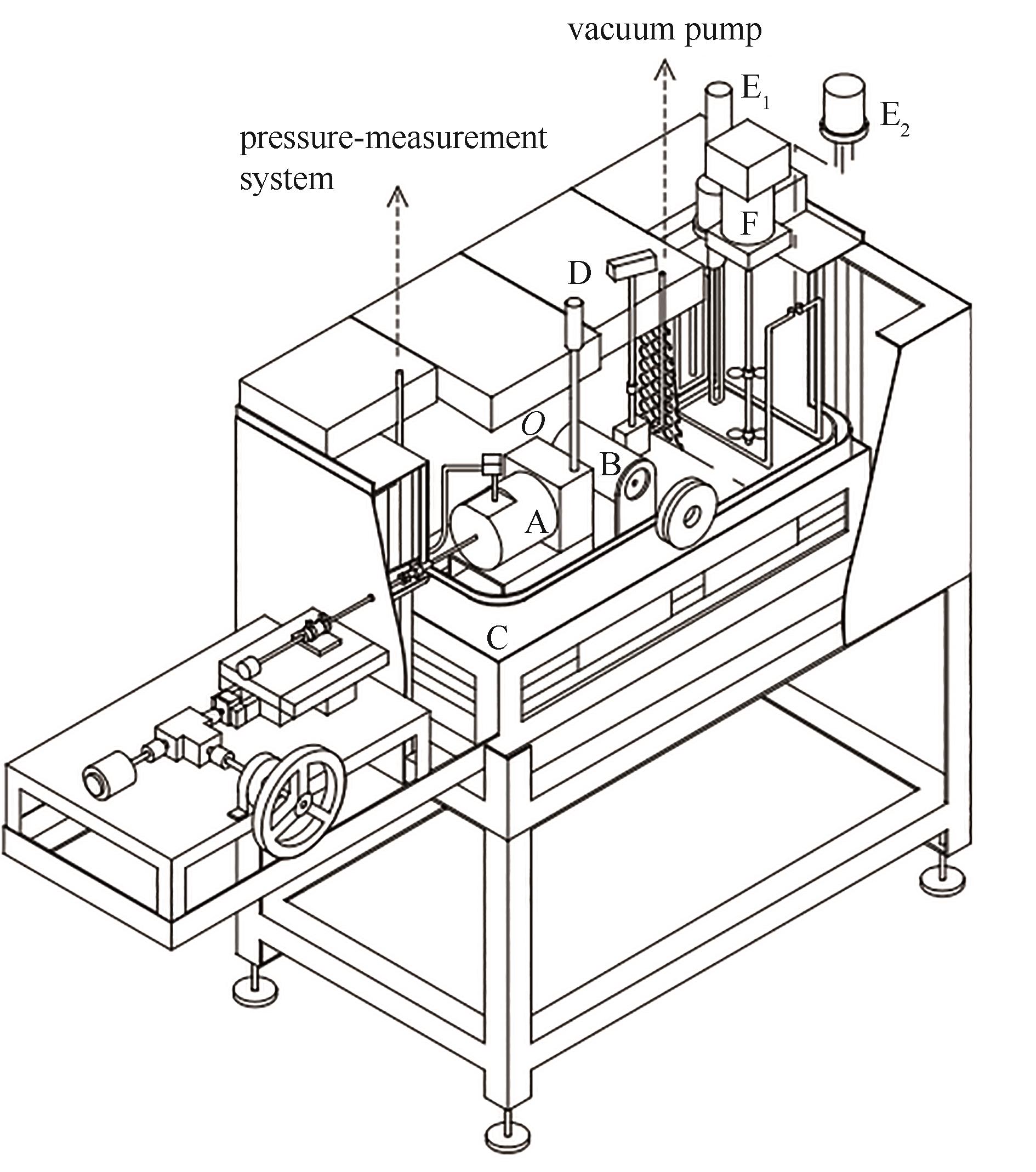
图3 变容法实验装置原理图(金属波纹管法)[60-61]A—压力容器中的金属波纹管;B—可视化容器;C—恒温油槽;D—铂电阻温度计;E1—加热器(1.2 kW);E2—加热器(0.3 kW);F—搅拌器
Fig.3 Schematic diagram of the variable-volume apparatus (metal-bellows method)[60-61]A—metal-bellows in pressure vessel; B—optical cell; C—thermostatic oil bath; D—platinum resistance thermometer; E1—heater (1.2 kW); E2—heater (0.3 kW); F—stirrer
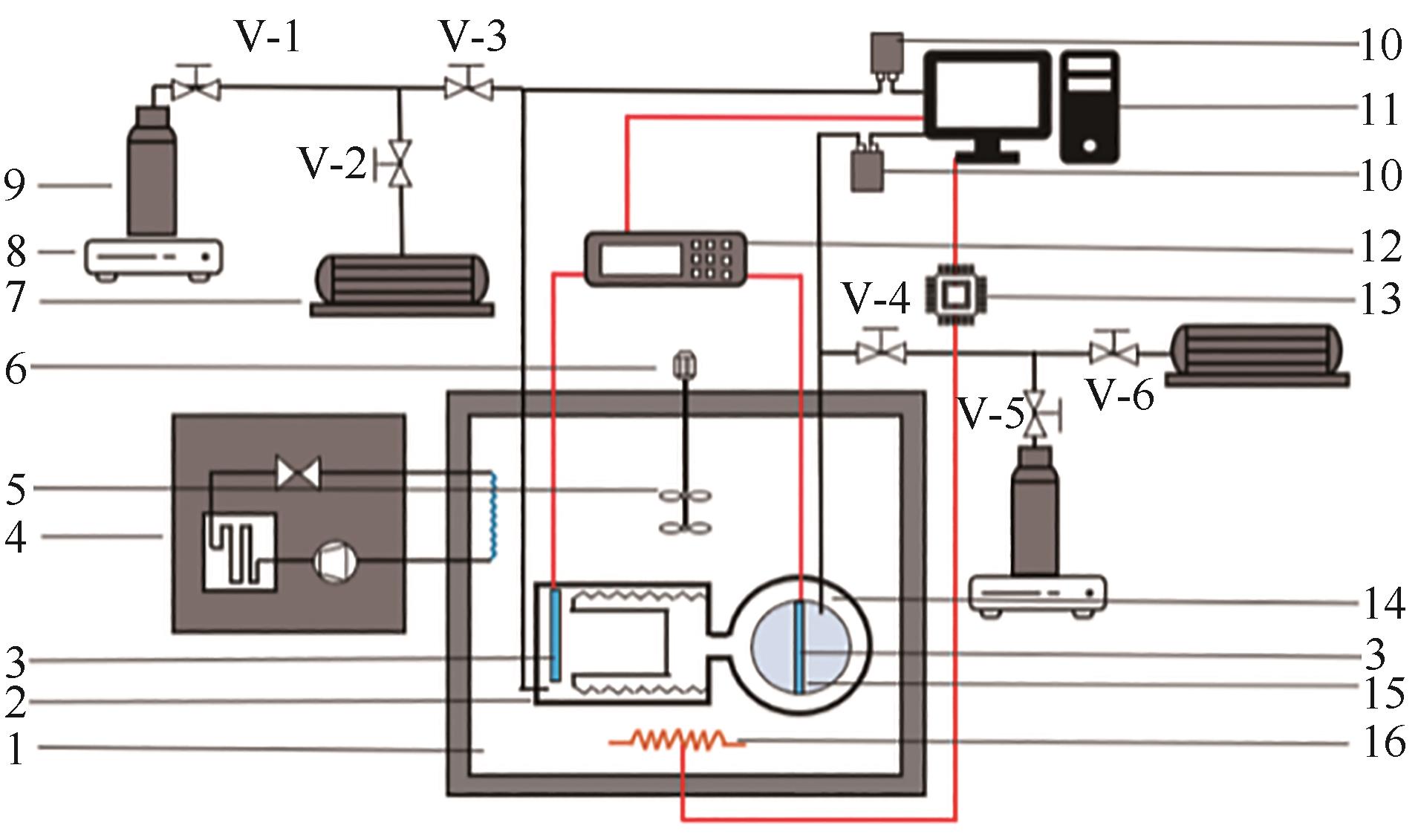
图4 中国科学院理化技术研究所变容法实验装置原理图(金属波纹管法)[62]1—恒温油浴;2—波纹管体积计;3—铂电阻温度计;4—制冷机;5—搅拌器;6—电机;7—真空泵;8—电子天平;9—气瓶;10—压力传感器;11—压力和温度读数系统;12—电桥;13—恒压电流源;14—平衡釜;15—可视化窗口;16—电加热器
Fig.4 Schematic diagram of the variable-volume apparatus (metal-bellows method) from the Technical Institute of Physics and Chemistry, Chinese Academy of Sciences[62]1—silicone oil bath; 2—metal-bellow volumeter; 3—platinum resistance thermometers; 4—refrigerator; 5—trirrer; 6—motor; 7—vacuum pump; 8—electronic balance; 9—gas cylinder; 10—pressure transducers; 11—pressure and temperature indicator; 12—bridge; 13—stabilized voltage supply; 14—equilibrium cell; 15—view windows; 16—electrical heater
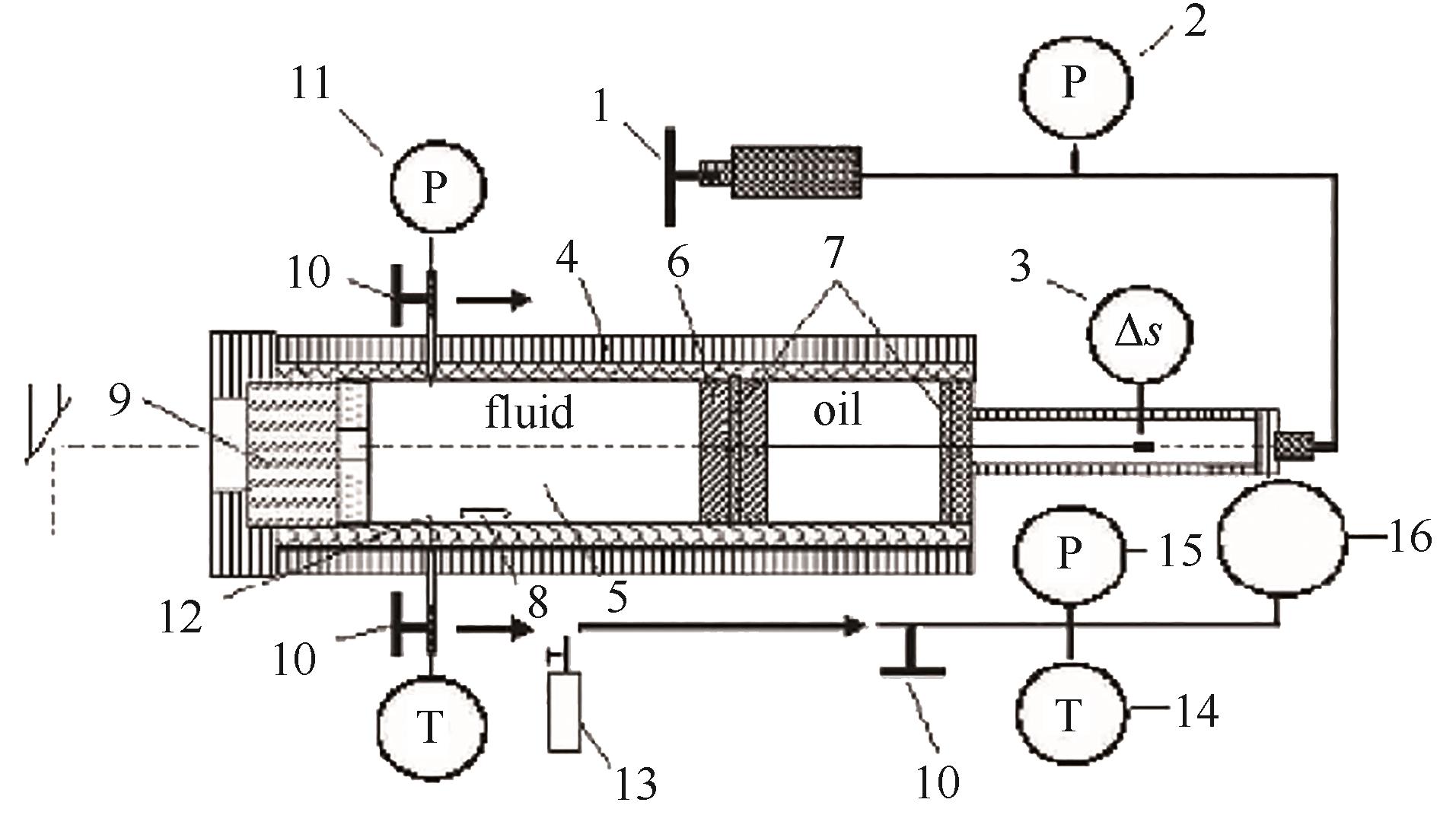
图5 天津大学变容法实验装置原理图(活塞法)[65]1—螺杆泵;2—压力表;3—霍尔探头;4—保温夹套;5—高压釜;6—位移计;7—O圈;8—搅拌器;9—石英窗;10—取样阀;11—压力传感器; 12—热电偶;13—小型不锈钢容器;14—温度计;15—真空计;16—钢球
Fig.5 Schematic diagram of the variable-volume method apparatus (piston method) from Tianjin University[65]1—screw-driven pump; 2—pressure meter; 3—hall probe; 4—heat jacket; 5—autoclave; 6—position; 7—O-ring; 8—stirrer; 9—quartz window; 10—sampling valves; 11—pressure sensor; 12—thermocouple; 13—small steel vessel; 14—thermometer; 15—vacuum meter; 16—steel bulb
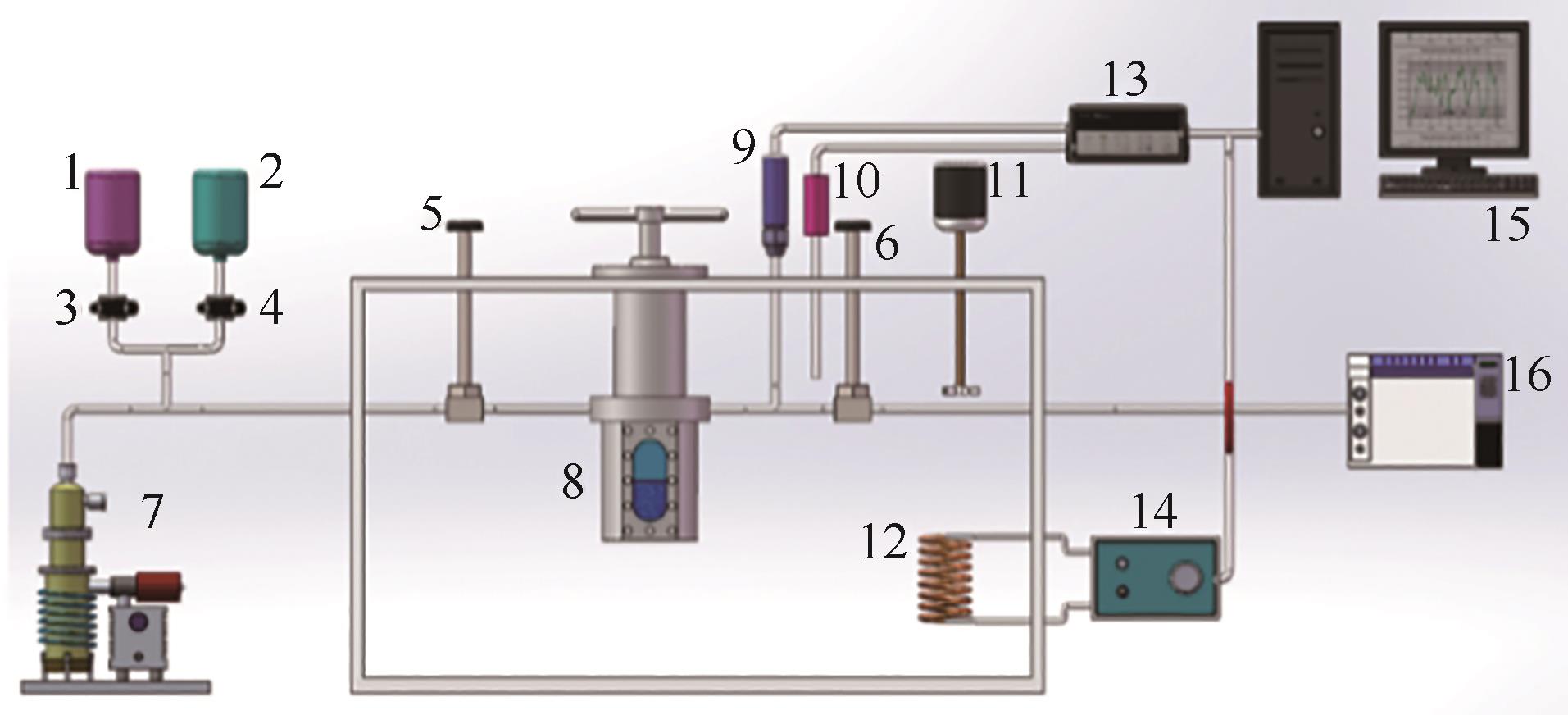
图6 中国科学技术大学变容法实验装置原理图(活塞法)[65]1—样品A容器;2—样品B容器;3~6—阀门;7—真空泵;8—可视化容器;9—压力传感器;10—铂电阻温度计;11—搅拌器;12—加热器;13—数据获取仪器;14—温度控制器;15—计算机;16—气相色谱仪
Fig.6 Schematic diagram of the variable-volume method apparatus (piston method) from University of Science and Technology of China[65]1—sample cylinder A; 2—sample cylinder B; 3-6—valves; 7—vacuum pump; 8—optical cell; 9—pressure transducer; 10—platinum resistance thermometer; 11—stirrer; 12—heater; 13—data acquisition instrument; 14—temperature controller; 15—computer; 16—gas chromatograph
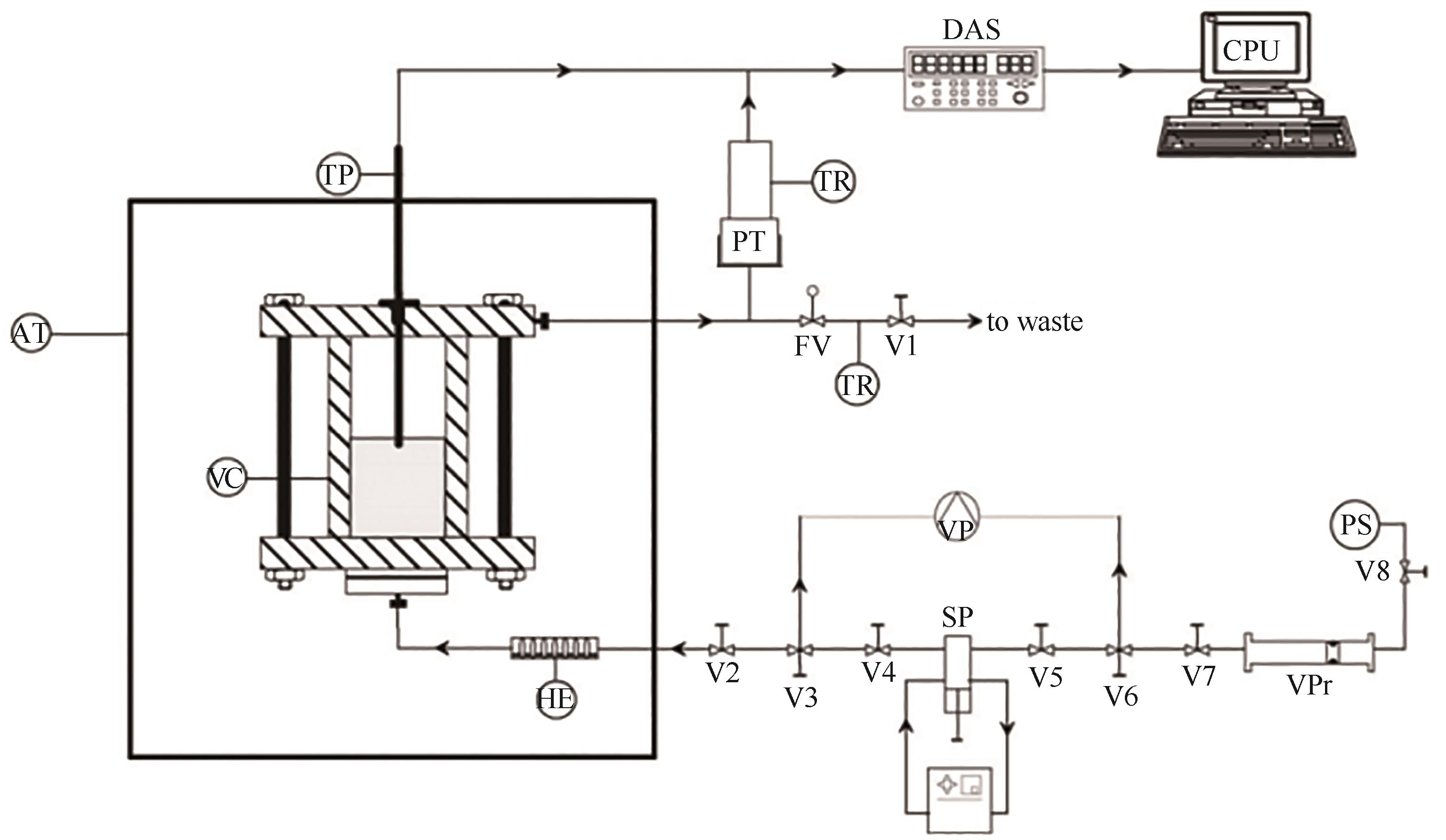
图7 流动法实验装置原理图[67]PS—压力源;VPr—容积压力;SP—注射泵;VP—真空泵;HE—换热器;VC—可视化容器;AT—空气浴;TP—铂电阻温度计套管;PT—压力传感器;TR—温度调节器;FV—流量调节阀;DAS—数据获取系统;CPU—中央处理单元;V—阀门
Fig.7 Schematic diagram of the flow method apparatus[67]PS—pressurized source; VPr—volumetric press; SP—syringe pump; VP—vacuum pump; HE—heat exchanger; VC—view cell; AT—air thermostat bath (oven); TP—platinum resistance temperature probe; PT—pressure transducer; TR—temperature regulator; FV—flow regulation valve; DAS—data acquisition system; CPU—central processor unit; V—valve
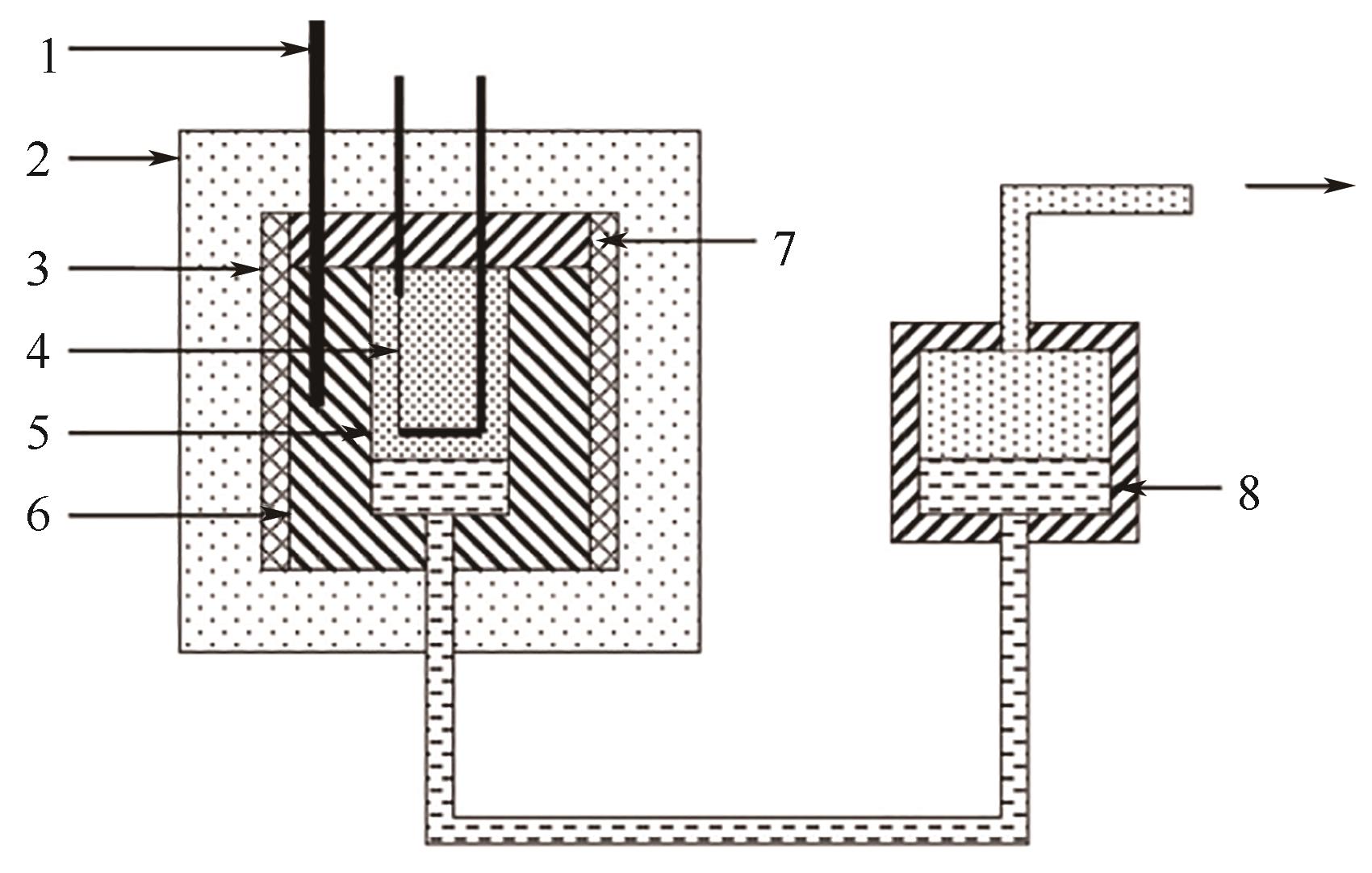
图8 脉冲加热法实验装置原理图[68]1—热电偶;2—陶瓷绝缘体;3—熔炉;4—测量管;5—待测液体;6—主体;7—法兰;8—封闭液体
Fig.8 Schematic diagram of the pulse-heating method apparatus[68]1—thermocouple; 2—ceramic thermal insulator; 3—furnace; 4—measuring probe; 5—liquid under study; 6—body; 7—flange; 8—confining liquid
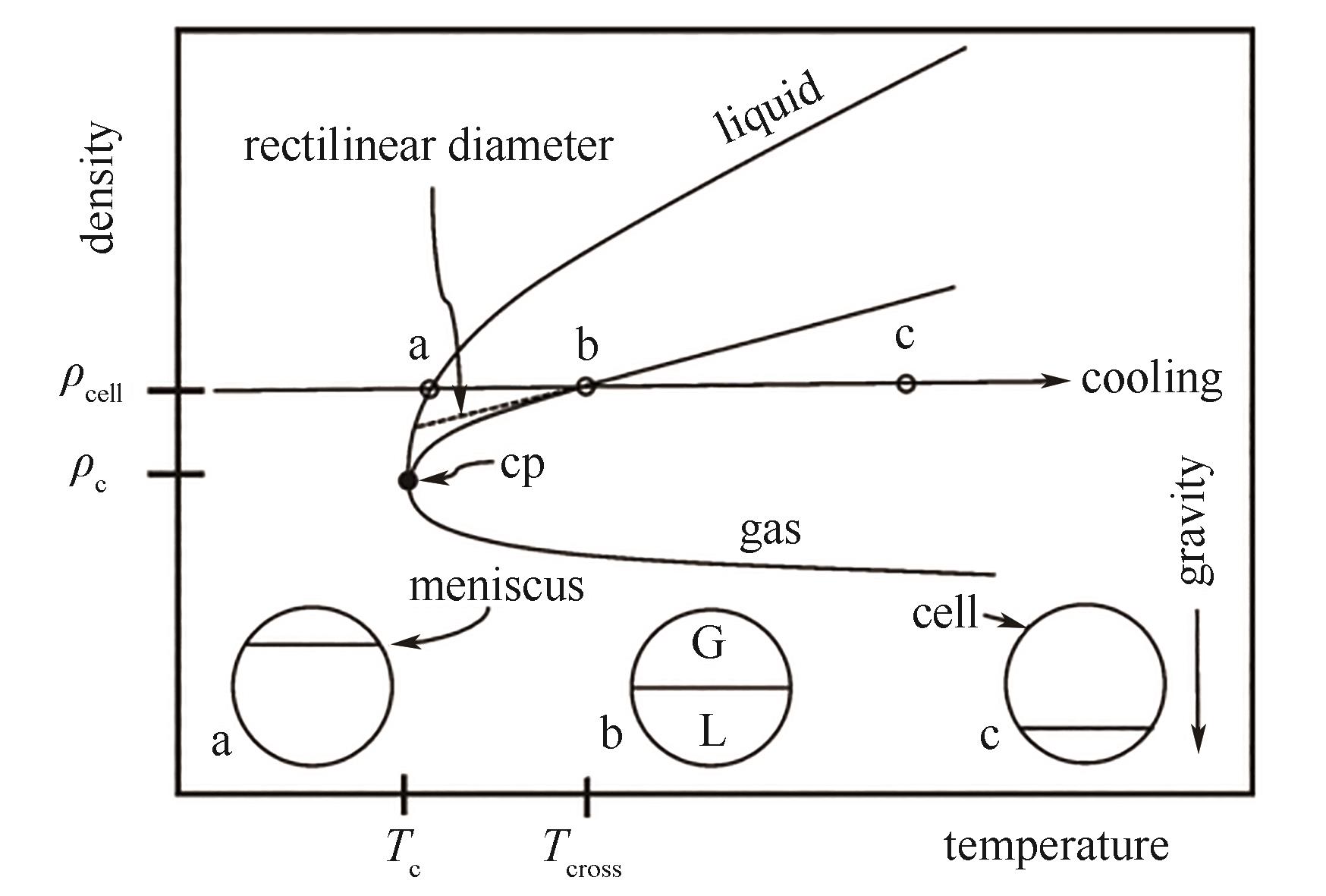
图10 密度直线中径测量密度与流体真实临界密度的偏差示意图[72]
Fig.10 A schematic diagram of the deviation between the critical density measured by rectilinear diameter law and the true critical density of fluid[72]
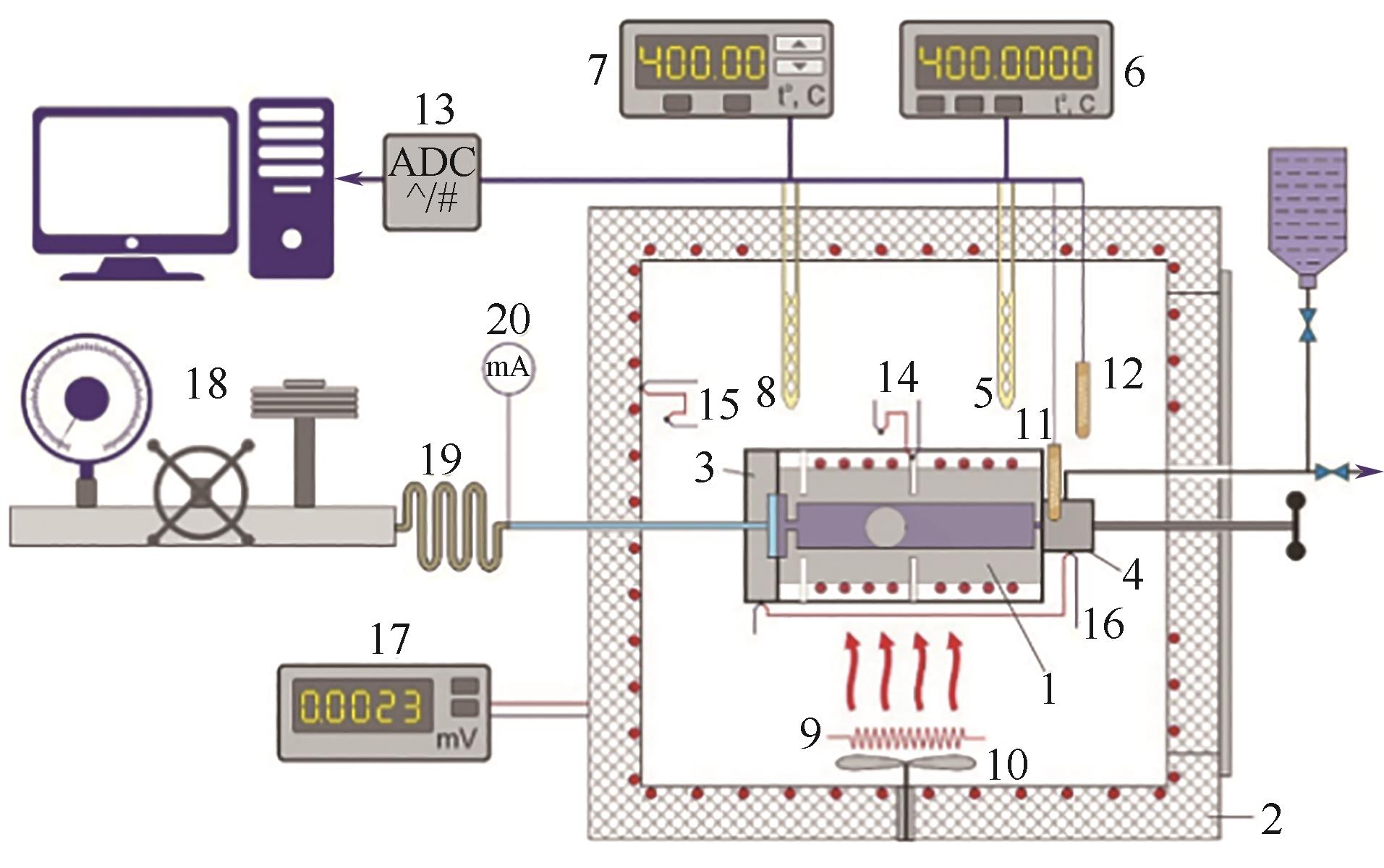
图12 准静态热分析法实验装置原理图[39]1—压力计;2—空气浴;3—差动膜分离器;4—阀门;5,8—铂电阻温度计;6—数字微欧姆表;7—数字高精度温度控制器; 9—调节加热器; 10—风扇;11,12—铂敏感元件;13—多通道模数转换器;14~16—热电偶;17—数字电压表;18—静重式压力计;19—毛细管;20—微安培表
Fig.12 Schematic diagram of the quasi-static thermograms method[39]1—piezometer; 2—air thermostat; 3—differential membrane separator; 4—valve; 5,8—platinum resistance thermometer; 6—digital micro-ohmmeter; 7—digital high precision temperature controller; 9—regulating heater; 10—fan; 11,12—platinum sensitive element; 13—multichannel analog-to-digital converter; 14-16—differential thermocouples; 17—digital voltmeter; 18—dead-weight pressure gauge; 19—capillary; 20—micro amperemeter
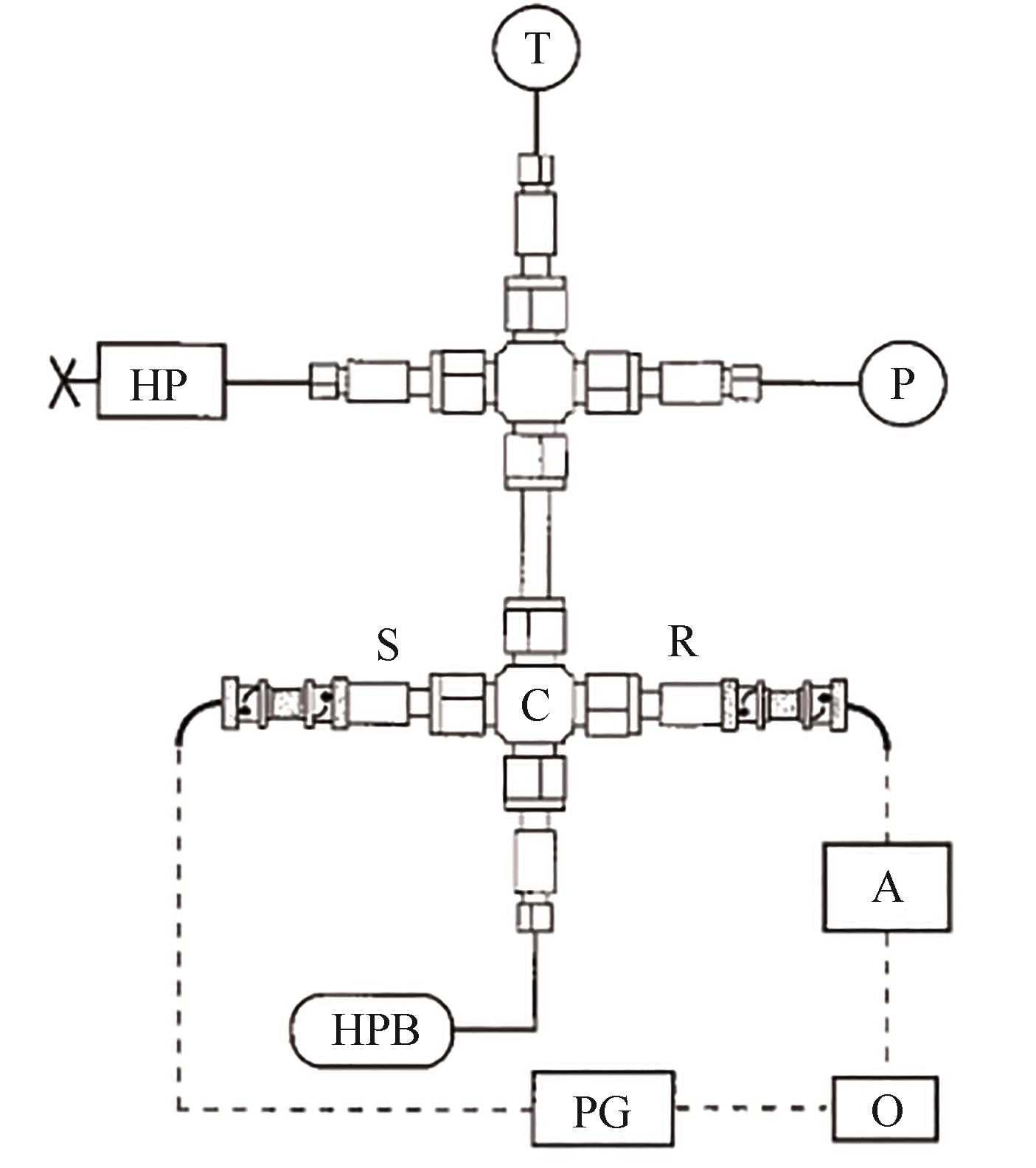
图13 声学法实验装置原理图[79]A—放大器;C—声学池;HP—手推泵;HPB—高压气体罐;O—示波器;P—压力传感器;PG—脉冲发生器;R—声波接收器;S—声波发送器; T—热电偶
Fig.13 Schematic diagram of the acoustic method apparatus[79]A—amplifier; C—acoustic cell; HP—hand pump; HPB—high-pressure bomb; O—oscilloscope; P—pressure transducer; PG—pulse generator; R—acoustic receiver; S—acoustic sender; T—thermocouple
| 测量方法 | 测量范围 | 测量精度 | 优点 | 缺点 |
|---|---|---|---|---|
| 定容法 | 热稳定物质 | 较高 | 简单、可靠 | 主观性判定临界点,实验效率低 |
| 变容法 | 热稳定物质 | 较高 | 实验效率高 | 主观性判定临界点,系统复杂度高,需精密测量或计算体积 |
| 流动法 | 热不稳定物质 | 较高 | 能测量热不稳定物质 | 主观性判定临界点,不能测量临界密度,需确保流体均匀混合和流动 |
| 脉冲加热法 | 热不稳定物质 | 较低 | 能测量易受热分解物质 | 主观性判定临界点,不能测量临界密度,需确保流体均匀混合和流动,系统复杂度高 |
| 密度直线中径定律法 | 热稳定物质 | 较低 | 简单,能以气相和液相饱和 密度数据拟合临界参数 | 近临界区数据受主观性观测影响大,拟合精度低,密度中径是否符合直线规律存在争议 |
| 压力-体积-温度 (p-V-T)关系法 | 热稳定物质 | 较低 | 拟合精度高 | 需要拟合数据较多,效率低 |
| 准静态热分析法 | 热稳定物质 | 较高 | 判断临界点准确客观,能同时 测量近临界区比定容热容 | 实验效率低 |
| 物理性质法 | 热不稳定物质 | 较低 | 判断临界点客观 | 发展不成熟,测量精度低 |
表1 临界p-ρ-T-x特性测量方法总结
Table 1 Summary of measurement methods for critical p-ρ-T-x parameters
| 测量方法 | 测量范围 | 测量精度 | 优点 | 缺点 |
|---|---|---|---|---|
| 定容法 | 热稳定物质 | 较高 | 简单、可靠 | 主观性判定临界点,实验效率低 |
| 变容法 | 热稳定物质 | 较高 | 实验效率高 | 主观性判定临界点,系统复杂度高,需精密测量或计算体积 |
| 流动法 | 热不稳定物质 | 较高 | 能测量热不稳定物质 | 主观性判定临界点,不能测量临界密度,需确保流体均匀混合和流动 |
| 脉冲加热法 | 热不稳定物质 | 较低 | 能测量易受热分解物质 | 主观性判定临界点,不能测量临界密度,需确保流体均匀混合和流动,系统复杂度高 |
| 密度直线中径定律法 | 热稳定物质 | 较低 | 简单,能以气相和液相饱和 密度数据拟合临界参数 | 近临界区数据受主观性观测影响大,拟合精度低,密度中径是否符合直线规律存在争议 |
| 压力-体积-温度 (p-V-T)关系法 | 热稳定物质 | 较低 | 拟合精度高 | 需要拟合数据较多,效率低 |
| 准静态热分析法 | 热稳定物质 | 较高 | 判断临界点准确客观,能同时 测量近临界区比定容热容 | 实验效率低 |
| 物理性质法 | 热不稳定物质 | 较低 | 判断临界点客观 | 发展不成熟,测量精度低 |
| 测量方法 | 研究机构 | 国家 | 标准不确定度 |
|---|---|---|---|
| 定容法 | 奥尔登堡大学 [ | 德国 | 10 kPa (pc)、0.1 K (Tc)、2% (ρc)、 0.0005 (x) |
| 定容法 | 卡尔斯鲁厄大学 [ | 德国 | 6 kPa (pc)、 0.06 K (Tc)、 2% (ρc)、 0.003 (x) |
| 定容法 | 华东理工大学 [ | 中国 | 30 kPa (pc)、 0.3 K (Tc)、 N/A (ρc)、 0.003 (x) |
| 定容法 | 清华大学 [ | 中国 | 0.5 kPa (pc)、 0.01 K (Tc)、 0.7% (ρc)、 N/A (x) |
| 定容法 | 马来亚大学 [ | 马来西亚 | 50 kPa (pc)、 0.2 K (Tc)、 N/A (ρc)、 0.015 (x) |
| 定容法/直线法 | 达吉斯坦州立大学/俄罗斯科学院高温联合研究所[ | 俄罗斯 | 0.05% (pc)、 0.015 K (Tc)、 0.15% (ρc)、 N/A (x) |
| 定容法/直线法 | 西安现代化学研究所 [ | 中国 | 24 kPa (pc)、 0.21 K (Tc)、 6.4 kg/m3 (ρc)、 0.0009 (x) |
| 定容法/流体p-V-T关系法 | 俄罗斯科学院油气研究所 [ | 俄罗斯 | 70 kPa (pc)、 2 K (Tc)、 N/A (ρc)、 0.015 (x) 1.8 kPa (p)、 0.05 K (T)、 0.0003 cm3/g (V)、 0.25 (x) |
| 变容法/流体p-V-T关系法 | 九州大学 [59,41] | 日本 | 0.5 kPa (pc)、 0.01 K (Tc)、 0.15% (ρc)、 0.005 (x) |
| 变容法 | 庆应义塾大学 [61,60] | 日本 | 1.6 kPa (pc)、 0.016 K (Tc)、 0.18% (ρc)、 0.009 (x) |
| 变容法 | 中国科学技术大学 [ | 中国 | 3.1 kPa (pc)、 0.01 K (Tc)、 0.0015 (x) |
| 变容法 | 丽水国立大学 [ | 韩国 | 30 kPa (pc)、 0.1 K (Tc)、 0.001 (x) |
| 变容法 | 天津大学 [ | 中国 | 100 kPa (pc)、 0.1 K (Tc)、 N/A (ρc)、 N/A (x) |
| 变容法 | 中国科学院理化技术研究所[ | 中国 | 10.5 kPa (pc)、 0.025 K (Tc)、 0.3% (ρc)、 0.005 (x) |
| 流动法 | 洛林大学 [ | 法国 | 8 kPa (pc)、 0.05 K (Tc)、 0.000015 (x) |
| 流动法 | 西安交通大学 [ | 中国 | 5.2 kPa (pc)、 0.2 K (Tc)、 0.006 (x) |
| 流动法/流体p-V-T关系法 | 萨拉格萨大学 [ | 西班牙 | 34 kPa (pc)、 0.32 K (Tc)、 0.1% (ρc)、 0.005 (x) |
| 脉冲加热法 | 俄罗斯科学院乌拉尔分院 [ | 俄罗斯 | 80 kPa (pc)、 0.1 K (Tc)、 N/A (x) |
| 准静态热分析法 | 达吉斯坦州立大学/俄罗斯科学院高温联合研究所 [ | 俄罗斯 | 0.025% (pc)、 0.075 K (Tc)、 0.25% (ρc)、 0.00005 (x) |
| 声学法 | 诺丁汉大学 [ | 英国 | 10 kPa (pc)、 0.1 K (Tc)、 N/A (ρc)、 N/A (x) |
表2 近30年内较活跃的临界p-ρ-T-x特性测量机构及其测量原理
Table 2 Active research institutions of critical p-ρ-T-x parameters measurement in recent 30 years and their measurement principles
| 测量方法 | 研究机构 | 国家 | 标准不确定度 |
|---|---|---|---|
| 定容法 | 奥尔登堡大学 [ | 德国 | 10 kPa (pc)、0.1 K (Tc)、2% (ρc)、 0.0005 (x) |
| 定容法 | 卡尔斯鲁厄大学 [ | 德国 | 6 kPa (pc)、 0.06 K (Tc)、 2% (ρc)、 0.003 (x) |
| 定容法 | 华东理工大学 [ | 中国 | 30 kPa (pc)、 0.3 K (Tc)、 N/A (ρc)、 0.003 (x) |
| 定容法 | 清华大学 [ | 中国 | 0.5 kPa (pc)、 0.01 K (Tc)、 0.7% (ρc)、 N/A (x) |
| 定容法 | 马来亚大学 [ | 马来西亚 | 50 kPa (pc)、 0.2 K (Tc)、 N/A (ρc)、 0.015 (x) |
| 定容法/直线法 | 达吉斯坦州立大学/俄罗斯科学院高温联合研究所[ | 俄罗斯 | 0.05% (pc)、 0.015 K (Tc)、 0.15% (ρc)、 N/A (x) |
| 定容法/直线法 | 西安现代化学研究所 [ | 中国 | 24 kPa (pc)、 0.21 K (Tc)、 6.4 kg/m3 (ρc)、 0.0009 (x) |
| 定容法/流体p-V-T关系法 | 俄罗斯科学院油气研究所 [ | 俄罗斯 | 70 kPa (pc)、 2 K (Tc)、 N/A (ρc)、 0.015 (x) 1.8 kPa (p)、 0.05 K (T)、 0.0003 cm3/g (V)、 0.25 (x) |
| 变容法/流体p-V-T关系法 | 九州大学 [59,41] | 日本 | 0.5 kPa (pc)、 0.01 K (Tc)、 0.15% (ρc)、 0.005 (x) |
| 变容法 | 庆应义塾大学 [61,60] | 日本 | 1.6 kPa (pc)、 0.016 K (Tc)、 0.18% (ρc)、 0.009 (x) |
| 变容法 | 中国科学技术大学 [ | 中国 | 3.1 kPa (pc)、 0.01 K (Tc)、 0.0015 (x) |
| 变容法 | 丽水国立大学 [ | 韩国 | 30 kPa (pc)、 0.1 K (Tc)、 0.001 (x) |
| 变容法 | 天津大学 [ | 中国 | 100 kPa (pc)、 0.1 K (Tc)、 N/A (ρc)、 N/A (x) |
| 变容法 | 中国科学院理化技术研究所[ | 中国 | 10.5 kPa (pc)、 0.025 K (Tc)、 0.3% (ρc)、 0.005 (x) |
| 流动法 | 洛林大学 [ | 法国 | 8 kPa (pc)、 0.05 K (Tc)、 0.000015 (x) |
| 流动法 | 西安交通大学 [ | 中国 | 5.2 kPa (pc)、 0.2 K (Tc)、 0.006 (x) |
| 流动法/流体p-V-T关系法 | 萨拉格萨大学 [ | 西班牙 | 34 kPa (pc)、 0.32 K (Tc)、 0.1% (ρc)、 0.005 (x) |
| 脉冲加热法 | 俄罗斯科学院乌拉尔分院 [ | 俄罗斯 | 80 kPa (pc)、 0.1 K (Tc)、 N/A (x) |
| 准静态热分析法 | 达吉斯坦州立大学/俄罗斯科学院高温联合研究所 [ | 俄罗斯 | 0.025% (pc)、 0.075 K (Tc)、 0.25% (ρc)、 0.00005 (x) |
| 声学法 | 诺丁汉大学 [ | 英国 | 10 kPa (pc)、 0.1 K (Tc)、 N/A (ρc)、 N/A (x) |
| 1 | White C M, Smith D H, Jones K L, et al. Sequestration of carbon dioxide in coal with enhanced coalbed methane recovery-a review[J]. Energy and Fuels, 2005, 19(3): 659-724. |
| 2 | Queiroz J P S, Bermejo M D, Mato F, et al. Supercritical water oxidation with hydrothermal flame as internal heat source: efficient and clean energy production from waste[J]. The Journal of Supercritical Fluids, 2015, 96: 103-113. |
| 3 | Rothenfluh T, Schuler M J, Von Rohr P R. Penetration length studies of supercritical water jets submerged in a subcritical water environment using a novel optical Schlieren method[J]. The Journal of Supercritical Fluids, 2011, 57(2): 175-182. |
| 4 | Menon S K, Boettcher P A, Ventura B, et al. Hot surface ignition of n-hexane in air[J]. Combustion and Flame, 2016, 163: 42-53. |
| 5 | Kojima J J, Hegde U G, Gotti D J, et al. Flame structure of supercritical ethanol/water combustion in a co-flow air stream characterized by Raman chemical analysis[J]. The Journal of Supercritical Fluids, 2020, 166: 104995. |
| 6 | Reverchon E, Torino E, Dowy S, et al. Interactions of phase equilibria, jet fluid dynamics and mass transfer during supercritical antisolvent micronization[J]. Chemical Engineering Journal, 2009, 156(2): 446-458. |
| 7 | Couto R, Chambon S, Aymonier C, et al. Microfluidic supercritical antisolvent continuous processing and direct spray-coating of poly(3-hexylthiophene) nanoparticles for OFET devices[J]. Chemical Communications (Cambridge, England), 2015, 51(6): 1008-1011. |
| 8 | Wu C, Yan X J, Wang S S, et al. System optimisation and performance analysis of CO2 transcritical power cycle for waste heat recovery[J]. Energy, 2016, 100: 391-400. |
| 9 | Li M J, Zhu H H, Guo J Q, et al. The development technology and applications of supercritical CO2 power cycle in nuclear energy, solar energy and other energy industries[J]. Applied Thermal Engineering, 2017, 126: 255-275. |
| 10 | Pan L H, Freifeld B, Doughty C, et al. Fully coupled wellbore-reservoir modeling of geothermal heat extraction using CO2 as the working fluid[J]. Geothermics, 2015, 53: 100-113. |
| 11 | Hsieh J C, Lin D T W, Wei C H, et al. The heat extraction investigation of supercritical carbon dioxide flow in heated porous media[J]. Energy Procedia, 2014, 61: 262-265. |
| 12 | Crua C, Manin J, Pickett L M. On the transcritical mixing of fuels at diesel engine conditions[J]. Fuel, 2017, 208: 535-548. |
| 13 | Falgout Z, Rahm M, Sedarsky D, et al. Gas/fuel jet interfaces under high pressures and temperatures[J]. Fuel, 2016, 168: 14-21. |
| 14 | Wensing M, Vogel T, Götz G. Transition of diesel spray to a supercritical state under engine conditions[J]. International Journal of Engine Research, 2016, 17(1):108-119. |
| 15 | Polikhronidi N G, Batyrova R G, Wu J T, et al. Simultaneously measurements of the PVT and thermal-pressure coefficient of benzene in the critical and supercritical regions[J]. Journal of Molecular Liquids, 2019, 293: 111381. |
| 16 | 吴子睿, 孙瑞, 石凌峰, 等. CO2混合工质的气液相平衡的混合规则对比与预测研究[J]. 化工学报, 2022, 73(4): 1483-1492. |
| Wu Z R, Sun R, Shi L F, et al. A comparative and predictive study of the mixing rules for the vapor-liquid equilibria of CO2-based mixtures[J]. CIESC Journal, 2022, 73(4): 1483-1492. | |
| 17 | Van K P H, Scott R L. Critical lines and phase equilibria in binary van der Waals mixtures[J]. Philosophical Transactions of the Royal Society of London. Series A, Mathematical and Physical Sciences (1934—1990), 1980, 298(1442): 495-540. |
| 18 | Raju M, Banuti D T, Ma P C, et al. Widom lines in binary mixtures of supercritical fluids[J]. Scientific Reports, 2017, 7(1): 1-10. |
| 19 | Banuti D T. Crossing the Widom-line—supercritical pseudo-boiling[J]. The Journal of Supercritical Fluids, 2015, 98: 12-16. |
| 20 | Simeoni G G, Bryk T, Gorelli F A, et al. The Widom line as the crossover between liquid-like and gas-like behaviour in supercritical fluids[J]. Nature Physics, 2010, 6(7): 503-507. |
| 21 | Espinosa J R, Garaizar A, Vega C, et al. Breakdown of the law of rectilinear diameter and related surprises in the liquid-vapor coexistence in systems of patchy particles[J]. The Journal of Chemical Physics, 2019, 150(22):224510. |
| 22 | Maxim F, Contescu C, Boillat P, et al. Visualization of supercritical water pseudo-boiling at Widom line crossover[J]. Nature Communications, 2019, 10(1): 1-11. |
| 23 | Straub J, Nitsche K. Isochoric heat capacity Cv at the critical point of SF6 under micro- and earth-gravity[J]. Fluid Phase Equilibria, 1993, 88: 183-208. |
| 24 | Meyer H, Zhong F. Equilibration and other dynamic properties of fluids near the liquid-vapor critical point[J]. Comptes Rendus Mécanique, 2004, 332(5/6): 327-343. |
| 25 | Hu Z C, Zhang X R. Piston effect induced by cross-boundary mass diffusion in a binary fluid mixture near its liquid-vapor critical point[J]. International Journal of Heat and Mass Transfer, 2019, 140: 691-704. |
| 26 | Khmelinskii I, Woodcock L V. Supercritical fluid gaseous and liquid states: a review of experimental results[J]. Entropy (Basel, Switzerland), 2020, 22(4):437. |
| 27 | Lee S, Lee J, Kim Y, et al. Quasi-equilibrium phase coexistence in single component supercritical fluids[J]. Nature Communications, 2021, 12(1): 1-7. |
| 28 | Abdulagatov A I, Stepanov G V, Abdulagatov I M. The critical properties of binary mixtures containing carbon dioxide: Krichevskii parameter and related thermodynamic properties[J]. High Temperature, 2007, 45(3): 408-424. |
| 29 | Jacob J, Kumar A, Anisimov M A, et al. Crossover from Ising to mean-field critical behavior in an aqueous electrolyte solution[J]. Physical Review E-Statistical Physics, Plasmas, Fluids, and Related Interdisciplinary Topics, 1998, 58(2): 2188-2200. |
| 30 | Berche B, Henkel M, Kenna R. Critical phenomena: 150 years since cagniard de la tour[J]. Journal of Physical Studies, 2009, 13(3):3001. |
| 31 | Lopez-Echeverry J S, Reif-Acherman S, Araujo-Lopez E. Peng-Robinson equation of state: 40 years through cubics[J]. Fluid Phase Equilibria, 2017, 447: 39-71. |
| 32 | Sadowski G. Special issue celebrating 30 years of SAFT[J]. Journal of Chemical & Engineering Data, 2020, 65(12): 5627. |
| 33 | Chou G F, Prausnitz J M. A phenomenological correction to an equation of state for the critical region[J]. AIChE Journal, 1989, 35(9): 1487-1496. |
| 34 | Kiselev S B, Friend D G. Cubic crossover equation of state for mixtures[J]. Fluid Phase Equilibria, 1999, 162(1/2): 51-82. |
| 35 | Wilson L C, Jasperson L V, VonNiederhausern D, et al. DIPPR project 851—thirty years of vapor-liquid critical point measurements and experimental technique development[J]. Journal of Chemical & Engineering Data, 2018, 63(9): 3408-3417. |
| 36 | Chirico R D, Frenkel M, Magee J W, et al. Improvement of quality in publication of experimental thermophysical property data: challenges, assessment tools, global implementation, and online support[J]. Journal of Chemical & Engineering Data, 2013, 58(10): 2699-2716. |
| 37 | Bell I H, Wronski J, Quoilin S, et al. Pure and pseudo-pure fluid thermophysical property evaluation and the open-source thermophysical property library coolprop[J]. Industrial & Engineering Chemistry Research, 2014, 53(6): 2498-2508. |
| 38 | Onken U, Rarey-Nies J, Gmehling J. The Dortmund data bank: a computerized system for retrieval, correlation, and prediction of thermodynamic properties of mixtures[J]. International Journal of Thermophysics, 1989, 10(3): 739-747. |
| 39 | Abdulagatov I M, Bazaev A R, Bazaev E A, et al. Experimental study of the critical and supercritical phenomena in ternary mixture of water+1-propanol+n-hexane[J]. Journal of Molecular Liquids, 2020, 316: 113789. |
| 40 | Higashi Y, Sakoda N. Measurements of PvT properties, saturated densities, and critical parameters for 3, 3, 3-trifluoropropene (HFO1243zf)[J]. Journal of Chemical & Engineering Data, 2018, 63(10): 3818-3822. |
| 41 | Sakoda N, Higashi Y. Measurements of PvT properties, vapor pressures, saturated densities, and critical parameters for cis-1-chloro-2,3,3,3-tetrafluoropropene (R1224yd(Z))[J]. Journal of Chemical & Engineering Data, 2019, 64(9): 3983-3987. |
| 42 | Higashi Y, Tanaka K, Ichikawa T. Critical parameters and saturated densities in the critical region for trans-1, 3, 3, 3-tetrafluoropropene (HFO-1234ze(E))[J]. Journal of Chemical & Engineering Data, 2010, 55(4): 1594-1597. |
| 43 | Tanaka K, Akasaka R, Sakaue E, et al. Measurements of the critical parameters for cis-1,1,1,4,4,4-hexafluoro-2-butene[J]. Journal of Chemical & Engineering Data, 2017, 62(3): 1135-1138. |
| 44 | Duan Y Y, Shi L, Zhu M S, et al. Critical parameters and saturated density of trifluoroiodomethane (CF3I)[J]. Journal of Chemical & Engineering Data, 1999, 44(3): 501-504. |
| 45 | Fu Y D, Han L Z, Zhu M S. PVT properties, vapor pressures and critical parameters of HFC-32[J]. Fluid Phase Equilibria, 1995, 111(2): 273-286. |
| 46 | Liu X Y, Lan T, Wang C J, et al. Measurement of critical temperature and critical pressure of tert-butanol and alkane mixtures[J]. Journal of Molecular Liquids, 2020, 302: 112582. |
| 47 | Xin N, Liu Y, Guo X D, et al. Determination of critical properties for binary and ternary mixtures containing propanol and alkanes using a flow view-type apparatus[J]. The Journal of Supercritical Fluids, 2016, 108: 35-44. |
| 48 | Liu Y, Zhang Y, He M G, et al. Determination of the critical properties of C6-C10 n-alkanes and their binary systems using a flow apparatus[J]. Journal of Chemical & Engineering Data, 2014, 59(11): 3852-3857. |
| 49 | Lan T, Yao C Q, Liu X Y, et al. Measurements of critical properties of ethyl propionate with the gasoline components[J]. Journal of Molecular Liquids, 2021, 322: 114801. |
| 50 | Wu J T, Liu Z G, Wang B, et al. Measurement of the critical parameters and the saturation densities of dimethyl ether[J]. Journal of Chemical & Engineering Data, 2004, 49(3): 704-708. |
| 51 | Vostrikov S V, Pimerzin A A, Konnova M E, et al. Plotting of phase (vapor-liquid) transition surface near the critical point out of data from isochoric experiment. Experimental procedure[J]. Fluid Phase Equilibria, 2018, 462: 118-129. |
| 52 | Haynes W M. Hand Book of Chemistry and Physics[M]. 97th ed. London: Taylor & Francis Group, 2017: 67-91. |
| 53 | Bell Ian H, Demian R, Ala B, et al. Survey of data and models for refrigerant mixtures containing halogenated olefins[J]. Journal of Chemical & Engineering Data, 2021, 66(6): 2335-2354. |
| 54 | Ambrose D, Tsonopoulos C, Nikitin E D, et al. Vapor-liquid critical properties of elements and compounds (12): Review of recent data for hydrocarbons and non-hydrocarbons[J]. Journal of Chemical & Engineering Data, 2015, 60(12): 3444-3482. |
| 55 | Ambrose D, Young C L. Vapor-liquid critical properties of elements and compounds (1): An introductory survey[J]. Journal of Chemical & Engineering Data, 1995, 40(2): 345-357. |
| 56 | Bobbo S, Di Nicola G, Zilio C, et al. Low GWP halocarbon refrigerants: a review of thermophysical properties[J]. International Journal of Refrigeration, 2018, 90: 181-201. |
| 57 | Christian I, Sven H, Andreas G. Vapor pressures and vapor-liquid critical properties of four pentene isomers[J]. Journal of Chemical & Engineering Data, 2017, 62(9): 2837-2841. |
| 58 | Horstmann S, Fischer K, Gmehling J, et al. Experimental determination of the critical line for (carbon dioxide + ethane) and calculation of various thermodynamic properties for (carbon dioxide + n-alkane) using the PSRK model[J]. The Journal of Chemical Thermodynamics, 2000, 32(4): 451-464. |
| 59 | Uchida Y, Yasumoto M, Yamada Y, et al. Critical properties of four HFE + HFC binary systems: trifluoromethoxymethane (HFE-143m) + pentafluoroethane (HFC-125), + 1, 1, 1, 2-tetrafluoroethane (HFC-134a), + 1, 1, 1, 2, 3, 3, 3-heptafluoropropane (HFC-227ea), and + 1, 1, 1, 2, 3, 3-hexafluoropropane (HFC-236ea)[J]. Journal of Chemical & Engineering Data, 2004, 49: 1615-1621. |
| 60 | Sakabe A, Arai D, Miyamoto H, et al. Measurements of the critical parameters for {xNH3+(1-x)H2O} with x=(0.9098, 0.7757, 0.6808)[J]. The Journal of Chemical Thermodynamics, 2008, 40(10): 1527-1530. |
| 61 | Sato M, Masui G, Uematsu M. Critical parameters for ammonia[J]. The Journal of Chemical Thermodynamics, 2005, 37(9): 931-934. |
| 62 | Yao X Y, Dong X Q, Zhao Y X, et al. A new apparatus for measurement of the critical p-ρ-T properties based on the variable-volume method[J]. International Journal of Refrigeration, 2022, 136: 220-228. |
| 63 | Yao X Y, Dong X Q, Zhao Y X, et al. Measurement of critical parameters for the binary mixture of R744 (carbon dioxide) + R1243zf (3,3,3-trifluoropropene)[J]. Journal of Chemical & Engineering Data, 2022, 67(9): 2128-2135. |
| 64 | Yao X Y, Shen J, Kang H H, et al. Measurement of critical parameters for the binary mixture of R744 (carbon dioxide) + R1234yf (2,3,3,3-tetrafluoropro-1-ene)[J]. The Journal of Chemical Thermodynamics, 2023, 178: 106978. |
| 65 | 朱虎刚. 高压下单组分流体pVT性质和二元系统相平衡和临界曲线[D]. 天津: 天津大学, 2007: 34. |
| Zhu H G. pVT properties of one-component fluid at high pressure and phase equilibrium and critical curve of binary system[D]. Tianjin: Tianjin University, 2007: 34. | |
| 66 | Zhang N, Hu P, Chen L X, et al. Measurements of critical properties of the binary mixture of 1,1,1-trifluoroethane (HFC-143a) +trans-1,3,3,3-tetrafluoropropene (HFO-1234ze(E))[J]. Journal of Chemical & Engineering Data, 2021, 66(7): 2717-2722. |
| 67 | Juntarachat N, Valtz A, Coquelet C, et al. Experimental measurements and correlation of vapor-liquid equilibrium and critical data for the CO2+R1234yf and CO2+R1234ze(E) binary mixtures[J]. International Journal of Refrigeration, 2014, 47: 141-152. |
| 68 | Nikitin E D, Popov A P. Vapor-liquid critical point measurements of fifteen compounds by the pulse-heating method[J]. Fluid Phase Equilibria, 2014, 380: 11-17. |
| 69 | Cornfeld A B, Carr H Y. Experimental evidence concerning the law of rectilinear diameter[J]. Physical Review Letters, 1972, 29(5): 320. |
| 70 | Nicoll J F. Critical phenomena of fluids: asymmetric Landau-Ginzburg-Wilson model[J]. Physical Review A, 1981, 24(4): 2203-2220. |
| 71 | Abdulagatov I M, Levina L N, Zakaryaev Z R, et al. The two-phase specific heat at constant volume of propane in the critical region[J]. Fluid Phase Equilibria, 1997, 127(1/2): 205-236. |
| 72 | Garrabos Y, Lecoutre C, Marre S, et al. Liquid-vapor rectilinear diameter revisited[J]. Physical Review. E, 2018, 97(2): 020101. |
| 73 | Young S. The vapor pressures, specific volumes, heat of vaporization, and critical constants of 300 pure organic substances[J]. Proc. Roy. Soc. Dublin NS, 1910, 21: 374. |
| 74 | Van Poolen L J, Jacobsen R T, Jahangiri M. Comparison of the critical liquid volume fraction to rectilinear-diameter methods for prediction of the critical density of ethylene and oxygen[J]. International Journal of Thermophysics, 1986, 7(3): 513-524. |
| 75 | Rzoska S J, Kalabiński J, Drozd-Rzoska A. Critical concentration in binary mixtures of limited miscibility[J]. Fluid Phase Equilibria, 2021, 540:112979. |
| 76 | Duschek W, Kleinrahm R, Wagner W. Measurement and correlation of the (pressure, density, temperature) relation of carbon dioxide (Ⅱ): Saturated-liquid and saturated-vapour densities and the vapour pressure along the entire coexistence curve[J]. The Journal of Chemical Thermodynamics, 1990, 22(9): 841-864. |
| 77 | Andreas K, Robertson Duncan G, Aguiar Ricardo Ana I, et al. Probing vapor/liquid equilibria of near-critical binary gas mixtures by acoustic measurements[J]. The Journal of Physical Chemistry, 1996, 100(22): 9522-9526. |
| 78 | Kordikowski A, Robertson D G, Poliakoff M, et al. Acoustic determination of the critical surfaces in the ternary systems CO2 + CH2F2 + CF3CH2F and CO + C2H4 + CH3CHCH2 and in their binary subsystems[J]. Journal of Physical Chemistry B, 1997, 101(30): 5853-5862. |
| 79 | Ke J, Parrott A J, Sanchez-Vicente Y, et al. New phase equilibrium analyzer for determination of the vapor-liquid equilibrium of carbon dioxide and permanent gas mixtures for carbon capture and storage[J]. The Review of Scientific Instruments, 2014, 85(8): 085110. |
| 80 | Ke J, King P J, George M W, et al. Method for locating the vapor–liquid critical point of multicomponent fluid mixtures using a shear mode piezoelectric sensor[J]. Analytical Chemistry, 2005, 77(1): 85-92. |
| 81 | Belyakov M Y, Gorodetskii E E, Kulikov V D, et al. Light-scattering anomaly in the vicinity of liquid-vapor critical point of multicomponent mixtures[J]. Chemical Physics, 2011, 379(1/2/3): 123-127. |
| 82 | Deng B L, Kanda Y, Chen L, et al. Visualization study of supercritical fluid convection and heat transfer in weightlessness by interferometry: a brief review[J]. Microgravity Science and Technology, 2017, 29(4): 275-295. |
| 83 | Lemmon E W, Huber M L, Mclinden M O. REFPROP 9.1[DB]. NIST Standard Reference Database, 2013. |
| 84 | Diefenbacher A, Türk M. Critical properties (pc, Tc, and ρc) and phase equilibria of binary mixtures of CO2, CHF3, CH2F2, and SF6 [J]. Fluid Phase Equilibria, 2001, 182(1/2): 121-131. |
| 85 | Li J F, Qin Z F, Wang G F, et al. Critical temperatures and pressures of several binary and ternary mixtures concerning the alkylation of 2-methylpropane with 1-butene in the presence of methane or carbon dioxide[J]. Journal of Chemical and Engineering Data, 2007, 52(5): 1736-1740. |
| 86 | Singh H, Lucien F P, Foster N R. Critical properties for binary mixtures of ethane containing low concentrations of n-alkane[J]. Journal of Chemical & Engineering Data, 2000, 45(1): 131-135. |
| 87 | Bezgomonova E I, Abdulagatov I M, Stepanov G V. Experimental study of the one-, two-, and three-phase isochoric heat capacities of n-hexane + water mixtures near the lower critical line (Ⅰ): Experimental results[J]. Journal of Molecular Liquids, 2012, 175: 121-134. |
| 88 | Yang Z Q, Valtz A, Coquelet C, et al. Critical properties and vapor-liquid equilibrium of two near-azeotropic mixtures containing HFOs[J]. International Journal of Refrigeration, 2022, 138:133-147. |
| 89 | Belyakov M Y, Kulikov V D, Muratov A R, et al. Thermodynamic properties of a model hydrocarbon ternary mixture in the vicinity of critical point: measurements and modeling with crossover equation of state[J]. Fluid Phase Equilibria, 2020, 518: 112630. |
| 90 | Byun H S, Choi M Y, Lim J S. High-pressure phase behavior and modeling of binary mixtures for alkyl acetate in supercritical carbon dioxide[J]. The Journal of Supercritical Fluids, 2006, 37(3): 323-332. |
| 91 | Gil L, Blanco S T, Rivas C, et al. Experimental determination of the critical loci for {n-C6H14 or CO2+ alkan-1-ol} mixtures. Evaluation of their critical and subcritical behavior using PC-SAFT EoS[J]. The Journal of Supercritical Fluids, 2012, 71: 26-44. |
| 92 | McLinden M O, Brown J S, Brignoli R, et al. Limited options for low-global-warming-potential refrigerants[J]. Nature Communications, 2017, 8(1): 1-9. |
| 93 | Yang Z, Feng B, Ma H Y, et al. Analysis of lower GWP and flammable alternative refrigerants[J]. International Journal of Refrigeration, 2021, 126: 12-22. |
| [1] | 汪尔奇, 彭书舟, 杨震, 段远源. 含HFO混合体系气液相平衡的理论模型评价[J]. 化工学报, 2023, 74(8): 3216-3225. |
| [2] | 陈科, 杜理, 曾英, 任思颖, 于旭东. 四元体系LiCl+MgCl2+CaCl2+H2O 323.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(5): 1896-1903. |
| [3] | 毛元敬, 杨智, 莫松平, 郭浩, 陈颖, 罗向龙, 陈健勇, 梁颖宗. C6~C10烷醇的SAFT-VR Mie状态方程参数回归及其热物性研究[J]. 化工学报, 2023, 74(3): 1033-1041. |
| [4] | 程文婷, 李杰, 徐丽, 程芳琴, 刘国际. AlCl3·6H2O在FeCl3、CaCl2、KCl及KCl–FeCl3溶液中溶解度的实验及预测[J]. 化工学报, 2023, 74(2): 642-652. |
| [5] | 唐茹意, 潘罕骞, 郑侠俊, 张广欣, 汪星平, 崔希利, 邢华斌. Z型全氟聚醚的结构表征[J]. 化工学报, 2023, 74(1): 479-486. |
| [6] | 杨克, 王辰升, 纪虹, 郑凯, 邢志祥, 毕海普, 蒋军成. 聚多巴胺包覆混合粉体抑制甲烷爆炸的实验研究[J]. 化工学报, 2022, 73(9): 4245-4254. |
| [7] | 杨松涛, 李东洋, 牛玉清, 李鑫钢, 康绍辉, 李洪, 叶开凯, 周志全, 高鑫. 氟化物势能函数和热力学性质的分子模拟研究进展[J]. 化工学报, 2022, 73(9): 3828-3840. |
| [8] | 席国君, 刘子涵, 雷广平. FeTPPs-CuBTC协同强化低浓度煤层气吸附分离[J]. 化工学报, 2022, 73(9): 3940-3949. |
| [9] | 孙哲, 金华强, 李康, 顾江萍, 黄跃进, 沈希. 基于知识数据化表达的制冷空调系统故障诊断方法[J]. 化工学报, 2022, 73(7): 3131-3144. |
| [10] | 任嘉辉, 刘豫, 刘朝, 刘浪, 李莹. 基于分子指纹和拓扑指数的工质临界温度理论预测[J]. 化工学报, 2022, 73(4): 1493-1500. |
| [11] | 高欢, 丁国良, 周发贤, 庄大伟. R410A制冷剂在润滑油中的动态析出特性的研究[J]. 化工学报, 2022, 73(3): 1054-1062. |
| [12] | 孙铭泽, 马宁, 李浩然, 姜海峰, 洪文鹏, 牛晓娟. 中低温超临界CO2及其混合工质布雷顿循环热力学分析[J]. 化工学报, 2022, 73(3): 1379-1388. |
| [13] | 李明宴, 李进龙, 彭昌军, 刘洪来. 基于COSMO-SAC模型研究离子液体对氨水溶液汽液平衡的影响[J]. 化工学报, 2022, 73(3): 1044-1053. |
| [14] | 王洁冰, 高金彤, 徐震原. 基于不同类型溶液蒸气压特性的太阳能界面蒸发实验研究[J]. 化工学报, 2022, 73(2): 663-671. |
| [15] | 李怀旭, 孙晓岩, 陶少辉, 夏力, 项曙光. 基于分子热力学性质和密度峰聚类的脱硫汽油集总[J]. 化工学报, 2022, 73(12): 5449-5460. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号