化工学报 ›› 2022, Vol. 73 ›› Issue (9): 3940-3949.DOI: 10.11949/0438-1157.20220362
收稿日期:2022-03-11
修回日期:2022-08-12
出版日期:2022-09-05
发布日期:2022-10-09
通讯作者:
雷广平
作者简介:席国君(1997—),男,硕士研究生,15035720803@163.com
基金资助:
Guojun XI( ), Zihan LIU, Guangping LEI(
), Zihan LIU, Guangping LEI( )
)
Received:2022-03-11
Revised:2022-08-12
Online:2022-09-05
Published:2022-10-09
Contact:
Guangping LEI
摘要:
高效分离CH4/N2混合物是实现低浓度煤层气利用的关键之一。基于原位封装策略采用一锅法将FeTPPs成功封装至CuBTC的孔隙中,通过两者的协同作用达到强化CH4/N2混合气体吸附分离的目的。实验结果显示,FeTPPs的封装增强了吸附剂与CH4间的相互作用反而削弱了吸附剂与N2间的相互作用,因此FeTPPs的封装有利于CH4/N2混合气体的吸附分离。基于理想吸附溶液理论(IAST)计算发现,在几乎不损失CH4吸附量的情况下,常温常压下FeTPPs@CuBTC复合材料对CH4/N2混合气体的吸附选择性可达5.4,是CuBTC的1.28倍,也高于目前已报道的大部分zeotile和MOF材料。证实了FeTPPs封装策略在低浓度煤层气分离领域的应用潜力,也为新型吸附剂材料的开发提供了新的设计思路。
中图分类号:
席国君, 刘子涵, 雷广平. FeTPPs-CuBTC协同强化低浓度煤层气吸附分离[J]. 化工学报, 2022, 73(9): 3940-3949.
Guojun XI, Zihan LIU, Guangping LEI. Enhanced adsorption and separation of low concentration coalbed methane based on synergistic effect between FeTPPs and CuBTC[J]. CIESC Journal, 2022, 73(9): 3940-3949.
| 元素 | 含量/%(质量) |
|---|---|
| Cu | 26.229 |
| Fe | 0.339 |
表1 FeTPPs@CuBTC样品中Cu和Fe的含量
Table 1 Cu and Fe contents in FeTPPs@CuBTC samples
| 元素 | 含量/%(质量) |
|---|---|
| Cu | 26.229 |
| Fe | 0.339 |
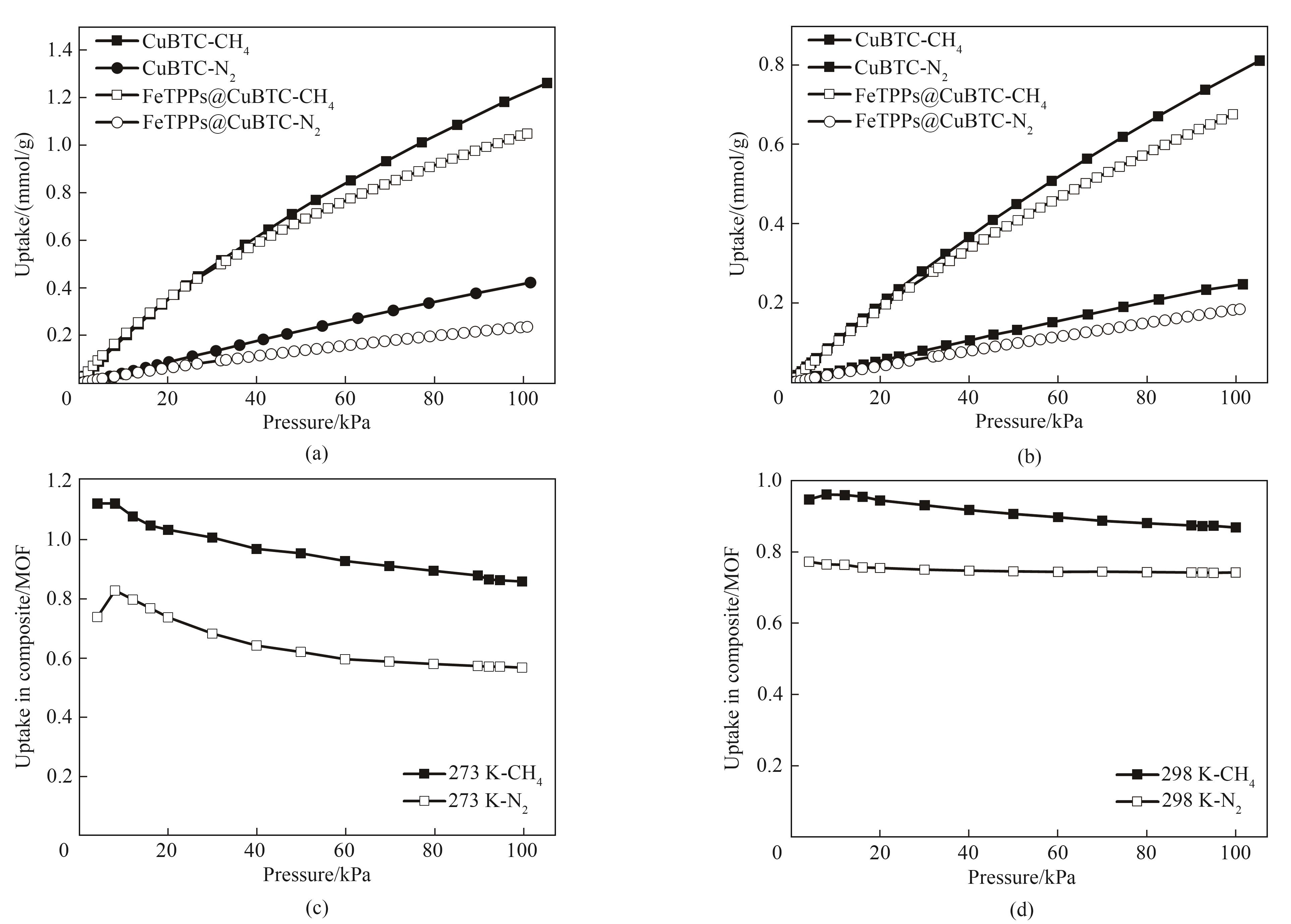
图7 CuBTC和FeTPPs@CuBTC在0℃(a)和25℃(b)下的CH4、 N2等温吸附曲线;0℃(c)和25℃(d)下FeTPPs@CuBTC对比CuBTC的CH4、N2吸附量的变化程度
Fig.7 Isothermal adsorption curves of CH4 and N2 at 0℃ (a) and 25℃ (b) for CuBTC and FeTPPs@CuBTC; The change degree of CH4 and N2 adsorption capacity of FeTPPs@CuBTC compared with CuBTC at 0℃ (c)和25℃ (d)
| 样品 | 气体 | q1/(mmol/g) | q2/(mmol/g) | a1/kPa-1 | a2/kPa-1 | k1 | k2 | R2 |
|---|---|---|---|---|---|---|---|---|
| CuBTC | CH4 | 0.035 | 3.21 | 0.095 | 0.003 | 0.801 | 1.024 | 0.99999 |
| N2 | 0.03 | 0.805 | 0.051 | 9.89×10-4 | 1.099 | 1.288 | 0.9999 | |
| FeTPPs@CuBTC | CH4 | 0.4 | 0.369 | 1.19×10-5 | 0.006 | 2.000 | 1.02 | 0.99997 |
| N2 | 0.05 | 2.769 | 0.014 | 0.004 | 1.304 | 0.959 | 1 |
表2 CuBTC和FeTPPs@CuBTC 的 CH4、N2在25℃下等温吸附线的拟合参数
Table 2 Fitted parameters of CH4 and N2 at 25 ℃ for CuBTC and FeTPPs@CuBTC
| 样品 | 气体 | q1/(mmol/g) | q2/(mmol/g) | a1/kPa-1 | a2/kPa-1 | k1 | k2 | R2 |
|---|---|---|---|---|---|---|---|---|
| CuBTC | CH4 | 0.035 | 3.21 | 0.095 | 0.003 | 0.801 | 1.024 | 0.99999 |
| N2 | 0.03 | 0.805 | 0.051 | 9.89×10-4 | 1.099 | 1.288 | 0.9999 | |
| FeTPPs@CuBTC | CH4 | 0.4 | 0.369 | 1.19×10-5 | 0.006 | 2.000 | 1.02 | 0.99997 |
| N2 | 0.05 | 2.769 | 0.014 | 0.004 | 1.304 | 0.959 | 1 |
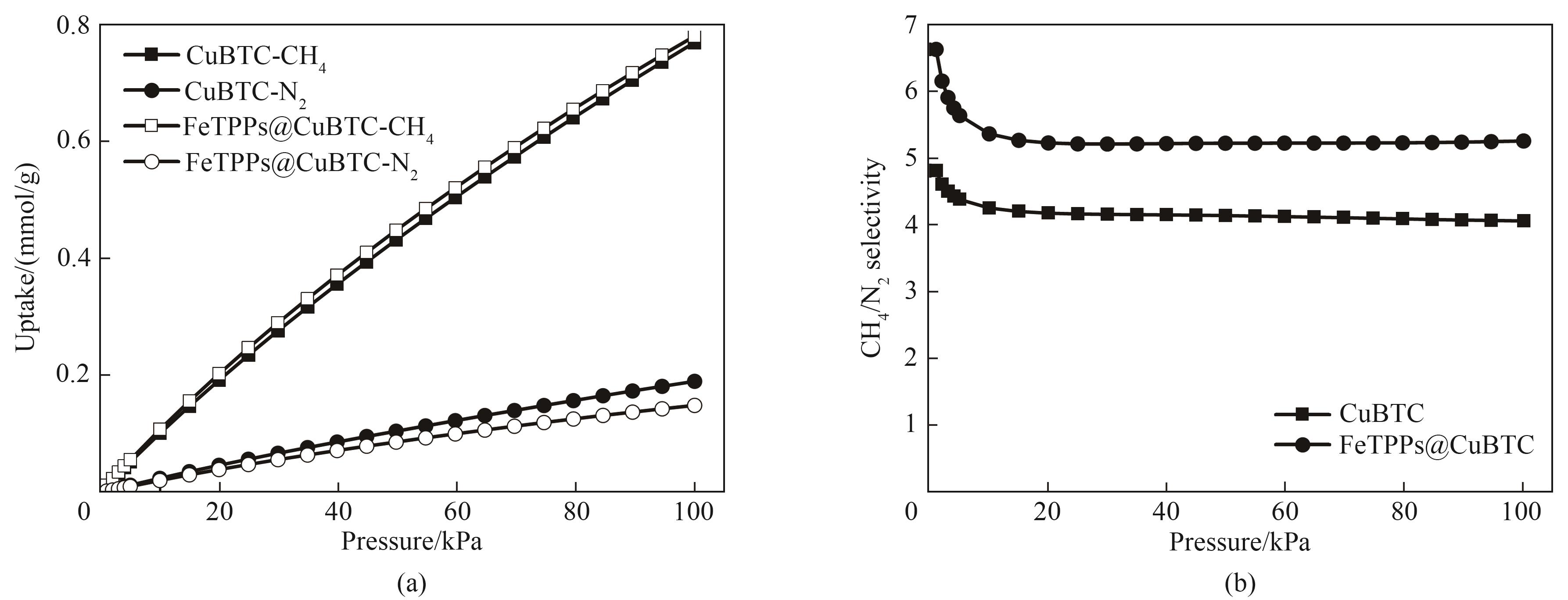
图9 CuBTC和FeTPPs@CuBTC对CH4/N2的各组分吸附量(a)和IAST吸附选择性(b)(CH4∶N2=50∶50, 25℃)(CH4∶N2= 50∶50 at 25℃)
Fig.9 Adsorption capacity (a) and IAST-predicted CH4/N2 selectivity (b) of CuBTC and FeTPPs@CuBTC for CH4/N2 mixture

图10 FeTPPs@CuBTC与文献[12,20-21,24,35-39]报道的部分吸附剂在CH4吸附容量和CH4/N2吸附选择性的对比(CH4∶N2=50∶50, 25℃)
Fig.10 Comparison between FeTPPs@CuBTC and some adsorbents reported in literatures[12,20-21,24,35-39] in CH4 adsorption capacity and CH4/N2 adsorption selectivity (CH4∶N2=50∶50, 25℃)
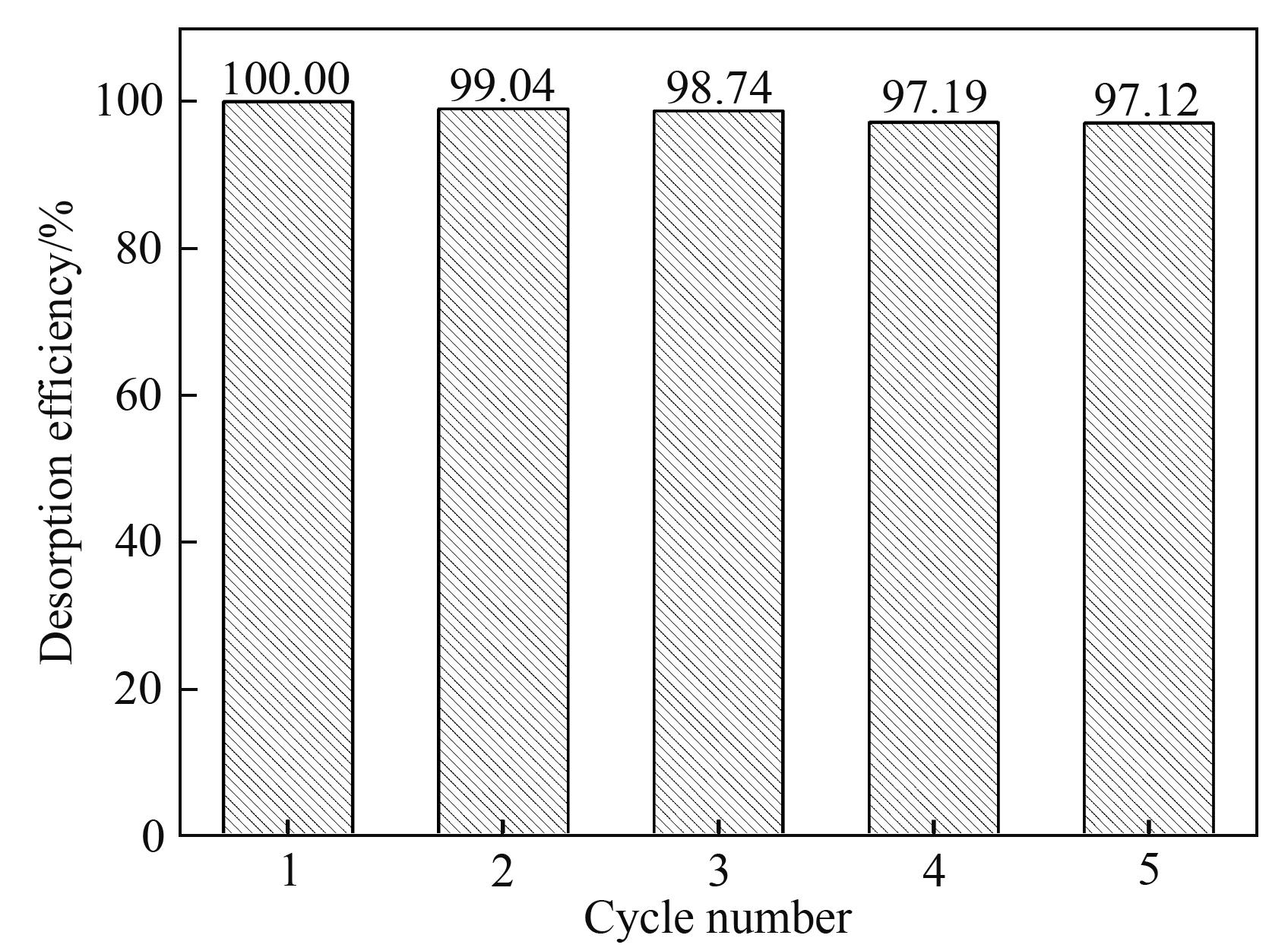
图13 常温常压下五次吸附解吸过程中FeTPPs@CuBTC对N2吸附能力的变化
Fig.13 Change of N2 adsorption capacity of FeTPPs@CuBTC during the fifth adsorption and desorption process at 298 K and 100 kPa
| 1 | 门相勇, 韩征, 宫厚健, 等. 新形势下中国煤层气勘探开发面临的挑战与机遇[J]. 天然气工业, 2018, 38(9): 10-16. |
| Men X Y, Han Z, Gong H J, et al. Challenges and opportunities of CBM exploration and development in China under new situations[J]. Natural Gas Industry, 2018, 38(9): 10-16. | |
| 2 | Liang W G, Yan J W, Zhang B N, et al. Review on coal bed methane recovery theory and technology: recent progress and perspectives[J]. Energy & Fuels, 2021, 35(6): 4633-4643. |
| 3 | 刘见中, 孙海涛, 雷毅, 等. 煤矿区煤层气开发利用新技术现状及发展趋势[J]. 煤炭学报, 2020, 45(1): 258-267. |
| Liu J Z, Sun H T, Lei Y, et al. Current situation and development trend of coalbed methane development and utilization technology in coal mine area[J]. Journal of China Coal Society, 2020, 45(1): 258-267. | |
| 4 | 贾晓霞, 王丽, 元宁, 等. 二价铬/钼/镍空配位MOFs的CH4/N2吸附分离研究[J]. 化工学报, 2018, 69(9): 3896-3904. |
| Jia X X, Wang L, Yuan N, et al. CH4/N2 adsorption separation research of MOFs with divalent Cr/Mo/Ni unsaturated metal sites[J]. CIESC Journal, 2018, 69(9): 3896-3904. | |
| 5 | Águeda Maté V I, Delgado Dobladez J A, Álvarez-Torrellas S, et al. Modeling and simulation of the efficient separation of methane/nitrogen mixtures with [Ni3(HCOO)6] MOF by PSA[J]. Chemical Engineering Journal, 2019, 361: 1007-1018. |
| 6 | Liang D, Hu Y F, Bao Q, et al. A suitable zeolite Rho for separating CO2/CH4 in pressure swing adsorption (PSA) process[J]. Inorganic Chemistry Communications, 2021, 127: 108547. |
| 7 | Feng W R, Wu H, Jin J S, et al. Transformation of Al-CDC from 3D crystals to 2D nanosheets in macroporous polyacrylates with enhanced CH4/N2 separation efficiency and stability[J]. Chemical Engineering Journal, 2022, 429: 132285. |
| 8 | Ali Abd A, Roslee Othman M. Biogas upgrading to fuel grade methane using pressure swing adsorption: parametric sensitivity analysis on an industrial scale[J]. Fuel, 2022, 308: 121986. |
| 9 | Shang H, Li Y P, Liu J Q. CH4/N2 separation on methane molecules grade diameter channel molecular sieves with a CHA-type structure[J]. Chinese Journal of Chemical Engineering, 2019, 27(5): 1044-1049. |
| 10 | Krishna R. Methodologies for evaluation of metal-organic frameworks in separation applications[J]. RSC Advances, 2015, 5(64): 52269-52295. |
| 11 | Yuan S, Qin J S, Li J L, et al. Retrosynthesis of multi-component metal-organic frameworks[J]. Nature Communications, 2018, 9: 808. |
| 12 | Wang X Q, Li L B, Yang J F, et al. CO2/CH4 and CH4/N2 separation on isomeric metal organic frameworks[J]. Chinese Journal of Chemical Engineering, 2016, 24(12): 1687-1694. |
| 13 | Ullah S, Bustam M A, Assiri M A, et al. Synthesis, and characterization of metal-organic frameworks-177 for static and dynamic adsorption behavior of CO2 and CH4 [J]. Microporous and Mesoporous Materials, 2019, 288: 109569. |
| 14 | Saha D, Bao Z B, Jia F, et al. Adsorption of CO2, CH4, N2O, and N2 on MOF-5, MOF-177, and zeolite 5A[J]. Environmental Science & Technology, 2010, 44(5): 1820-1826. |
| 15 | Liu H, Li B R, Zhao Y Y, et al. Investigation on a Zr-based metal-organic framework (MOF-801) for the high-performance separation of light alkanes[J]. Chemical Communications, 2021, 57(96): 13008-13011. |
| 16 | Li J M, Yang J F, Li L B, et al. Separation of CO2/CH4 and CH4/N2 mixtures using MOF-5 and Cu3(BTC)2 [J]. Journal of Energy Chemistry, 2014, 23(4): 453-460. |
| 17 | Pan R, Guo Y N, Tang Y N, et al. Dicationic liquid containing alkenyl modified CuBTC improves the performance of the composites: increasing the CO2 adsorption effect[J]. Chemical Engineering Journal, 2022, 430: 132127. |
| 18 | Kloutse F A, Gauthier W, Hourri A, et al. Study of competitive adsorption of the N2O-CO2-CH4-N2 quaternary mixture on CuBTC[J]. Separation and Purification Technology, 2020, 235: 116211. |
| 19 | Kloutse F A, Hourri A, Natarajan S, et al. Hydrogen separation by adsorption: experiments and modelling of H2-N2-CO2 and H2-CH4-CO2 mixtures adsorption on CuBTC and MOF-5[J]. Microporous and Mesoporous Materials, 2018, 271: 175-185. |
| 20 | Nozari V, Zeeshan M, Keskin S, et al. Effect of methylation of ionic liquids on the gas separation performance of ionic liquid/metal-organic framework composites[J]. Crystengcomm, 2018, 20(44): 7137-7143. |
| 21 | Wu Y Q, Yuan D H, Zeng S, et al. Significant enhancement in CH4/N2 separation with amine-modified zeolite Y[J]. Fuel, 2021, 301: 121077. |
| 22 | Chen Y W, Wu H X, Yuan Y N, et al. Highly rapid mechanochemical synthesis of a pillar-layer metal-organic framework for efficient CH4/N2 separation[J]. Chemical Engineering Journal, 2020, 385: 123836. |
| 23 | Sezginel K B, Keskin S, Uzun A. Tuning the gas separation performance of CuBTC by ionic liquid incorporation[J]. Langmuir, 2016, 32(4): 1139-1147. |
| 24 | Chang M, Zhao Y J, Yang Q Y, et al. Microporous metal-organic frameworks with hydrophilic and hydrophobic pores for efficient separation of CH4/N2 mixture[J]. ACS Omega, 2019, 4(11): 14511-14516. |
| 25 | Pirngruber G D, Hamon L, Bourrelly S, et al. A method for screening the potential of MOFs as CO2 adsorbents in pressure swing adsorption processes[J]. ChemSusChem, 2012, 5(4): 762-776. |
| 26 | Kondo A, Noguchi H, Ohnishi S, et al. Novel expansion/shrinkage modulation of 2D layered MOF triggered by clathrate formation with CO2 molecules[J]. Nano Letters, 2006, 6(11): 2581-2584. |
| 27 | Ribeiro R P P L, Mota J P B. Surface area and porosity of Co3(ndc)3(dabco) metal-organic framework and its methane storage capacity: a combined experimental and simulation study[J]. The Journal of Physical Chemistry C, 2021, 125(4): 2411-2423. |
| 28 | Tu T N, Nguyen H T D, Tran N T. Tailoring the pore size and shape of the one-dimensional channels in iron-based MOFs for enhancing the methane storage capacity[J]. Inorganic Chemistry Frontiers, 2019, 6(9): 2441-2447. |
| 29 | Ye Y X, Lin R B, Cui H, et al. A microporous metal-organic framework with naphthalene diimide groups for high methane storage[J]. Dalton Transactions, 2020, 49(12): 3658-3661. |
| 30 | Larsen R W, Wojtas L, Perman J, et al. Mimicking heme enzymes in the solid state: metal-organic materials with selectively encapsulated heme[J]. Journal of the American Chemical Society, 2011, 133(27): 10356-10359. |
| 31 | Luo F Q, Lin Y L, Zheng L Y, et al. Encapsulation of hemin in metal-organic frameworks for catalyzing the chemiluminescence reaction of the H2O2-luminol system and detecting glucose in the neutral condition[J]. ACS Applied Materials & Interfaces, 2015, 7(21): 11322-11329. |
| 32 | Yan T T, Guo J H, Liu Z Q, et al. Metalloporphyrin encapsulation for enhanced conversion of CO2 to C2H4 [J]. ACS Applied Materials & Interfaces, 2021, 13(22): 25937-25945. |
| 33 | 叶振华. 化工吸附分离过程[M]. 北京: 中国石化出版社, 1992. |
| Ye Z H. Chemical Adsorption Separation Process [M]. Beijing: China Petrochemical Press, 1992. | |
| 34 | Zhou X, Huang W Y, Miao J P, et al. Enhanced separation performance of a novel composite material GrO@MIL-101 for CO2/CH4 binary mixture[J]. Chemical Engineering Journal, 2015, 266: 339-344. |
| 35 | Hu J L, Sun T J, Liu X W, et al. Separation of CH4/N2 mixtures in metal-organic frameworks with 1D micro-channels[J]. RSC Advances, 2016, 6(68): 64039-64046. |
| 36 | Liu J Q, Shang H, Yang J F, et al. Novel zeolite/carbon monolith adsorbents for efficient CH4/N2 separation[J]. Chemical Engineering Journal, 2021, 426: 130163. |
| 37 | Li L B, Yang J F, Li J M, et al. Separation of CO2/CH4 and CH4/N2 mixtures by M/DOBDC: a detailed dynamic comparison with MIL-100(Cr) and activated carbon[J]. Microporous and Mesoporous Materials, 2014, 198: 236-246. |
| 38 | Wu Y Q, Yuan D H, He D W, et al. Decorated traditional zeolites with subunits of metal-organic frameworks for CH4/N2 separation[J]. Angewandte Chemie International Edition, 2019, 58(30): 10241-10244. |
| 39 | Nguyen P T K, Nguyen H T D, Pham H Q, et al. Synthesis and selective CO2 capture properties of a series of hexatopic linker-based metal-organic frameworks[J]. Inorganic Chemistry, 2015, 54(20): 10065-10072. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [3] | 赵亚欣, 张雪芹, 王荣柱, 孙国, 姚善泾, 林东强. 流穿模式离子交换层析去除单抗聚集体[J]. 化工学报, 2023, 74(9): 3879-3887. |
| [4] | 张佳怡, 何佳莉, 谢江鹏, 王健, 赵鹬, 张栋强. 渗透汽化技术用于锂电池生产中N-甲基吡咯烷酮回收的研究进展[J]. 化工学报, 2023, 74(8): 3203-3215. |
| [5] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [6] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [7] | 邢雷, 苗春雨, 蒋明虎, 赵立新, 李新亚. 井下微型气液旋流分离器优化设计与性能分析[J]. 化工学报, 2023, 74(8): 3394-3406. |
| [8] | 刘爽, 张霖宙, 许志明, 赵锁奇. 渣油及其组分黏度的分子层次组成关联研究[J]. 化工学报, 2023, 74(8): 3226-3241. |
| [9] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [10] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [11] | 张缘良, 栾昕奇, 苏伟格, 李畅浩, 赵钟兴, 周利琴, 陈健民, 黄艳, 赵祯霞. 离子液体复合萃取剂选择性萃取尼古丁的研究及DFT计算[J]. 化工学报, 2023, 74(7): 2947-2956. |
| [12] | 高金明, 郭玉娇, 鄂承林, 卢春喜. 一种封闭罩内顺流多旋臂气液分离器的分离特性研究[J]. 化工学报, 2023, 74(7): 2957-2966. |
| [13] | 文兆伦, 李沛睿, 张忠林, 杜晓, 侯起旺, 刘叶刚, 郝晓刚, 官国清. 基于自热再生的隔壁塔深冷空分工艺设计及优化[J]. 化工学报, 2023, 74(7): 2988-2998. |
| [14] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [15] | 韩奎奎, 谭湘龙, 李金芝, 杨婷, 张春, 张永汾, 刘洪全, 于中伟, 顾学红. 四通道中空纤维MFI分子筛膜用于二甲苯异构体分离[J]. 化工学报, 2023, 74(6): 2468-2476. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号