化工学报 ›› 2023, Vol. 74 ›› Issue (6): 2486-2494.DOI: 10.11949/0438-1157.20230275
收稿日期:2023-03-21
修回日期:2023-05-18
出版日期:2023-06-05
发布日期:2023-07-27
通讯作者:
汪锰
作者简介:陈朝光(1997—),男,硕士研究生,1822921699@qq.com
基金资助:
Zhaoguang CHEN( ), Yuxiang JIA, Meng WANG(
), Yuxiang JIA, Meng WANG( )
)
Received:2023-03-21
Revised:2023-05-18
Online:2023-06-05
Published:2023-07-27
Contact:
Meng WANG
摘要:
低浓度废酸的资源化长期困扰着相关涉酸工艺。基于Donnan渗析原理,提出以低浓度废酸驱动中和渗析地表水脱盐,借助模拟和实验来探索耦合工艺的可行性。首先,基于Nernst-Planck、Navier-Stokes方程和电中性条件,面向中和渗析过程数学建模,研究废酸浓度对脱盐率、脱盐速度和体系pH等脱盐效果的影响。模拟结果表明,常规低浓度废酸(pH=0.5~2)足以有效驱动中和渗析脱盐过程,且提高酸浓度也未得到明显强化。进而,以均相或异相离子交换膜构建中和渗析膜堆,开展条件实验,获得了系列与模拟结果相吻合的实验规律。实验也表明,对于高浓度废酸,异相膜较显著的同离子泄漏现象会严重劣化脱盐效率。综合研究表明,耦合新工艺有望为低浓度废酸的资源化开辟新途径。
中图分类号:
陈朝光, 贾玉香, 汪锰. 以低浓度废酸驱动中和渗析脱盐的模拟与验证[J]. 化工学报, 2023, 74(6): 2486-2494.
Zhaoguang CHEN, Yuxiang JIA, Meng WANG. Modeling neutralization dialysis desalination driven by low concentration waste acid and its validation[J]. CIESC Journal, 2023, 74(6): 2486-2494.
| 膜 | ||||
|---|---|---|---|---|
| CEM | 6.77 | — | 2.88 | — |
| AEM | — | 10.11 | — | 7.12 |
表1 直流法测定异相商品膜电阻
Table 1 Resistance of commercial heterogeneous membrane measured by DC method
| 膜 | ||||
|---|---|---|---|---|
| CEM | 6.77 | — | 2.88 | — |
| AEM | — | 10.11 | — | 7.12 |
| 膜 | 厚度/ | IEC/ | WU/ | X/ | 膜面积/ |
|---|---|---|---|---|---|
| CEM | 0.48 | 2.0 | 50 | 4.0 | 100 |
| AEM | 0.48 | 1.8 | 40 | 4.5 | 100 |
表2 膜主要性能参数
Table 2 Main performance parameters of the selected membranes
| 膜 | 厚度/ | IEC/ | WU/ | X/ | 膜面积/ |
|---|---|---|---|---|---|
| CEM | 0.48 | 2.0 | 50 | 4.0 | 100 |
| AEM | 0.48 | 1.8 | 40 | 4.5 | 100 |
| 项目 | ||||
|---|---|---|---|---|
| CEM | — | — | ||
| AEM | — | — | ||
| 溶液 |
表3 离子在膜和溶液中的扩散系数
Table 3 Diffusion coefficients of ions in membrane and solution
| 项目 | ||||
|---|---|---|---|---|
| CEM | — | — | ||
| AEM | — | — | ||
| 溶液 |
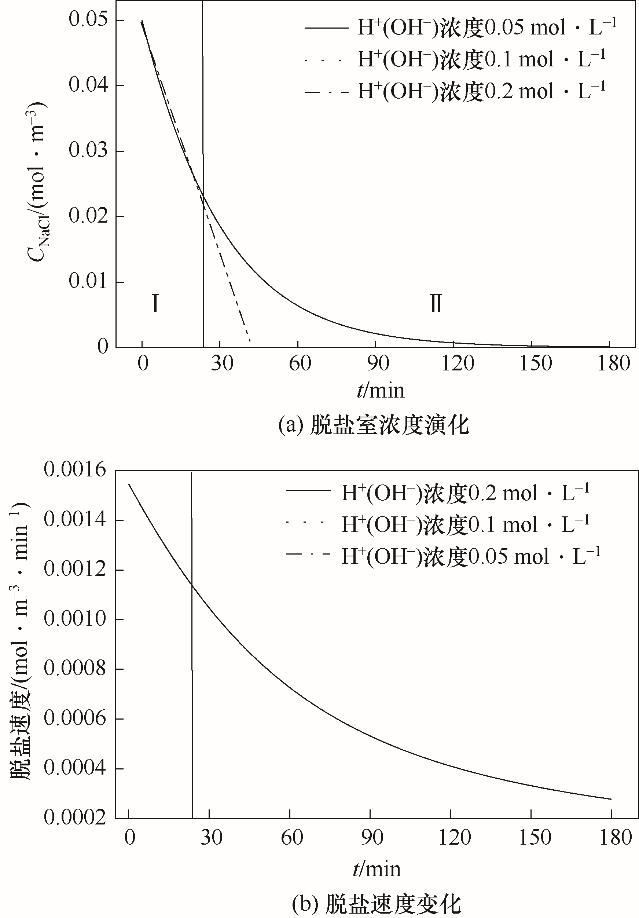
图2 酸(碱)初始浓度对盐溶液的影响(CNaClD=0.05 mol·L-1,Q=50 L·h-1)
Fig.2 Effects of initial concentration of acid (base) on salt solution (CNaClD=0.05 mol·L-1, Q=50 L·h-1)
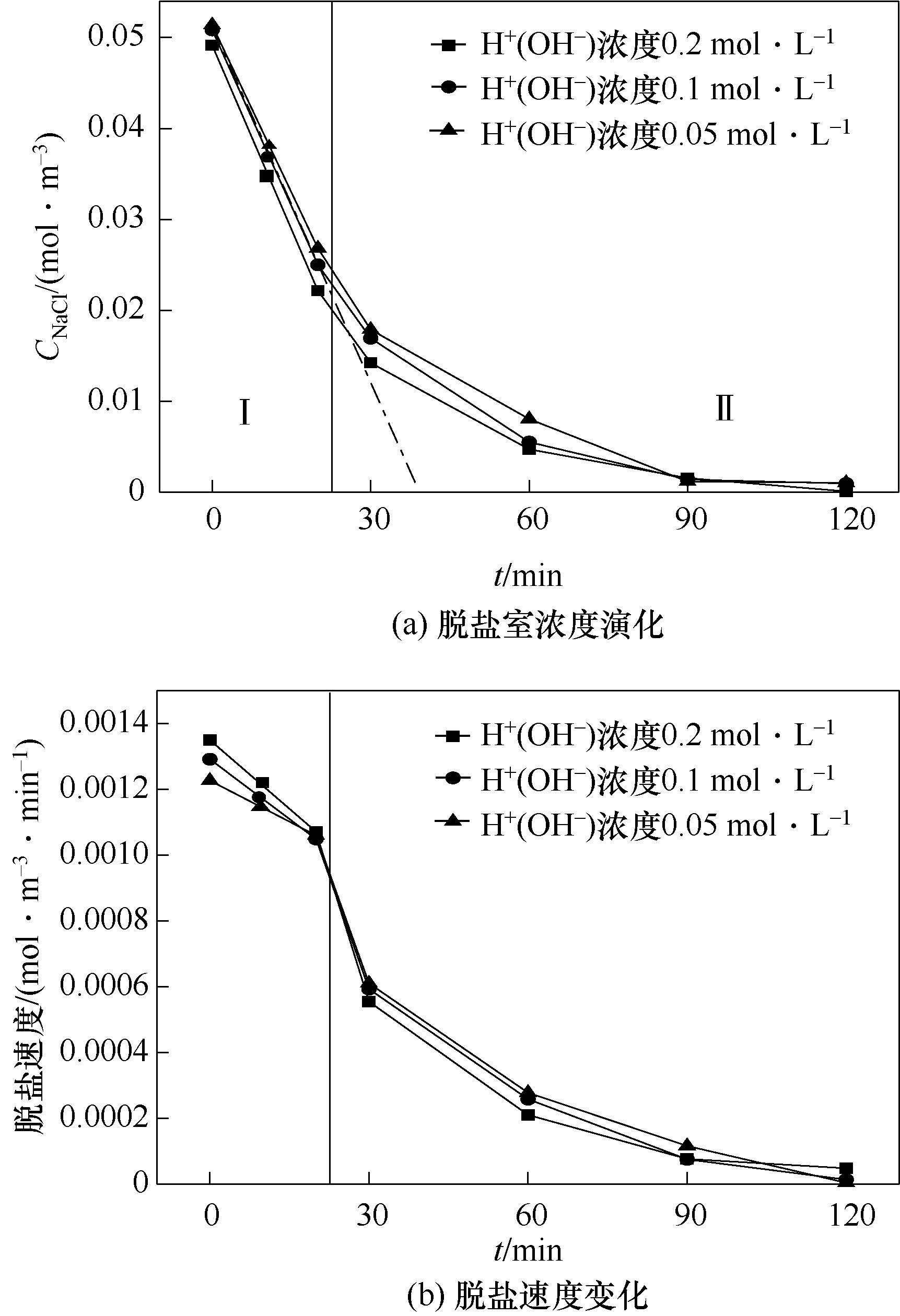
图4 酸(碱)初始浓度的影响(均相膜,CNaClD=0.05 mol·L-1,Q=50 L·h-1)
Fig.4 Effects of initial concentration of acid (base) (homogeneous IEM, CNaClD=0.05 mol·L-1,Q=50 L·h-1)
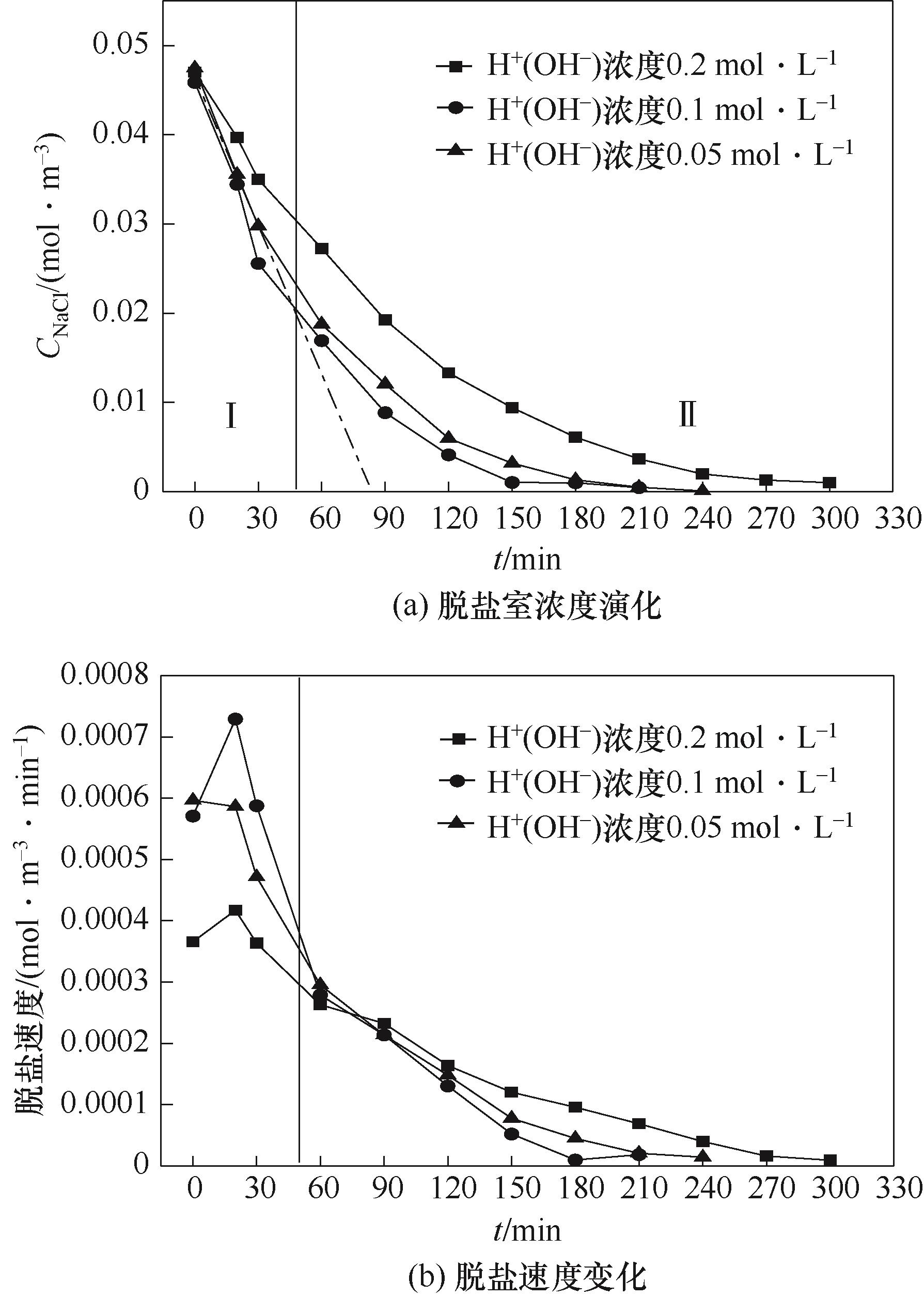
图5 酸(碱)初始浓度的影响(异相膜,CNaClD=0.05 mol·L-1,Q=50 L·h-1)
Fig.5 Effects of initial concentration of acid (base) (heterogeneous IEM, CNaClD=0.05 mol·L-1,Q=50 L·h-1)

图6 不同体系酸(碱)初始浓度对pH的影响(CNaClD=0.05 mol·L-1,Q=50 L·h-1)
Fig.6 Effects of initial concentration of acid (base) on pH in different system (CNaClD=0.05 mol·L-1,Q=50 L·h-1)
| 9 | Muhsan M A, Ilyas S, Cheema H A, et al. Recovery of nitric acid from effluent streams using solvent extraction with TBP: a comparative study in absence and presence of metal nitrates[J]. Separation and Purification Technology, 2017, 186: 90-95. |
| 10 | Kraus K A, Nelson F, Baxter J F. Anion-exchange studies(Ⅶ1, 2): Separation of sulfuric acid from metal sulfates by anion exchange[J]. Journal of the American Chemical Society, 1953, 75(11): 2768-2770. |
| 11 | Song P P, Wang M, Zhang B P, et al. Fabrication of proton permselective composite membrane for electrodialysis-based waste acid reclamation[J]. Journal of Membrane Science, 2019, 592: 117366. |
| 12 | Igawa M, Echizenya K, Hayashita T, et al. Donnan dialysis desalination[J]. Chemistry Letters, 1986, 15(2): 237-238. |
| 13 | Igawa M, Echizenya K, Hayashita T, et al. Neutralization dialysis for deionization[J]. Bulletin of the Chemical Society of Japan, 1987, 60(1): 381-383. |
| 14 | Bleha M, Tishchenko G A. Neutralization dialysis for desalination[J]. Journal of Membrane Science, 1992, 73(2/3): 305-311. |
| 15 | 赵宜江, 邢卫红, 徐南平. 扩散渗析法从钛白废酸中回收硫酸[J]. 高校化学工程学报, 2002, 16(2): 217-221. |
| Zhao Y J, Xing W H, Xu N P. Recovery of sulfuric acid from titanium white waste acid by diffusion dialysis[J]. Journal of Chemical Engineering of Chinese Universities, 2002, 16(2): 217-221. | |
| 16 | Nikonenko V V, Kovalenko A V, Urtenov M K, et al. Desalination at overlimiting currents: state-of-the-art and perspectives[J]. Desalination, 2014, 342: 85-106. |
| 17 | Strathmann H. Electrodialysis, a mature technology with a multitude of new applications[J]. Desalination, 2010, 264(3): 268-288. |
| 18 | Kozmai A, Nikonenko V, Pismenskaya N, et al. Effect of anion exchange membrane capacity loss on pH and electric conductivity of saline solution during neutralization dialysis[J]. Journal of Membrane Science, 2020, 595: 117573. |
| 19 | Velizarov S. Transport of arsenate through anion-exchange membranes in donnan dialysis[J]. Journal of Membrane Science, 2013, 425/426: 243-250. |
| 20 | Matos C T, Velizarov S, Crespo J G, et al. Simultaneous removal of perchlorate and nitrate from drinking water using the ion exchange membrane bioreactor concept[J]. Water Research, 2006, 40(2): 231-240. |
| 21 | Chérif M, Mkacher I, Dammak L, et al. Water desalination by neutralization dialysis with ion-exchange membranes: flow rate and acid/alkali concentration effects[J]. Desalination, 2015, 361: 13-24. |
| 22 | Palatý Z, Bendová H. Neutralisation dialysis of oxalic acid in a batch cell[J]. Chemical Engineering and Processing-Process Intensification, 2020, 154: 108007. |
| 23 | Wang G Q, Tanabe H, Igawa M. Transport of glycine by neutralization dialysis[J]. Journal of Membrane Science, 1995, 106(3): 207-211. |
| 24 | Biesheuvel P M. Two-fluid model for the simultaneous flow of colloids and fluids in porous media[J]. Journal of Colloid and Interface Science, 2011, 355(2): 389-395. |
| 25 | Mareev S A, Nikonenko V V. A numerical experiment approach to modeling impedance: application to study a Warburg-type spectrum in a membrane system with diffusion coefficients depending on concentration[J]. Electrochimica Acta, 2012, 81: 268-274. |
| 26 | Nikolskii B P. Theory of the glass electrode. I.Theoretical[J]. J.Phys. Chem., 1937, 10: 495-503. |
| 27 | Galama A H, Post J W, Stuart M A C, et al. Validity of the Boltzmann equation to describe Donnan equilibrium at the membrane-solution interface[J]. Journal of Membrane Science, 2013, 442: 131-139. |
| 28 | Wang Q, Chen G Q, Kentish S E. Sorption and diffusion of organic acid ions in anion exchange membranes: acetate and lactate ions as a case study[J]. Journal of Membrane Science, 2020, 614: 118534. |
| 29 | Zabolotsky V I, Nikonenko V V. Effect of structural membrane inhomogeneity on transport properties[J]. Journal of Membrane Science, 1993, 79(2/3): 181-198. |
| 30 | Dammak L, Lteif R, Bulvestre G, et al. Determination of the diffusion coefficients of ions in cation-exchange membranes, supposed to be homogeneous, from the electrical membrane conductivity and the equilibrium quantity of absorbed electrolyte[J]. Electrochimica Acta, 2001, 47(3): 451-457. |
| 31 | Kozmai A, Chérif M, Dammak L, et al. Modelling non-stationary ion transfer in neutralization dialysis[J]. Journal of Membrane Science, 2017, 540: 60-70. |
| 1 | 胡术刚, 马术文, 王之静, 等. 钛白废酸废水治理及副产石膏应用探讨[J]. 中国资源综合利用, 2003, 21(9): 2-8. |
| Hu S G, Ma S W, Wang Z J, et al. Application research on titanium dioxide waste acid and acid waste water treatment and the byproduct-gypsum[J]. China Resources Comprehensive Utilization, 2003, 21(9): 2-8. | |
| 2 | 孙安妮, 孙根行. 废盐酸再生利用研究进展[J]. 当代化工, 2011, 40(11): 1178-1181. |
| Sun A N, Sun G X. Research progress in regeneration and utilization of waste hydrochloric acid[J]. Contemporary Chemical Industry, 2011, 40(11): 1178-1181. | |
| 3 | 钱钧. 废酸集中处理资源化利用模式[C]//2019中国工业水处理大会暨第39届年会. 泰安, 2019. |
| Qian J. Centralized treatment model of waste acid reclamation[C]//China Industrial Water Treatment Conference and the 39th Annual Meeting. Tai'an, 2019. | |
| 4 | 樊盛春, 李越湘. 钢铁酸洗废液的处理与综合利用[J]. 江西冶金, 2000, 20(3): 12-14 |
| Fan C C, Li Y X. Treatment and comprehensive utilization of pickling waste liquid of iron and steel[J]. Jiangxi Metallurgy, 2000, 20(3): 12-14. | |
| 5 | Zhang Y L, Song G L, Luo T, et al. Acid-triggered polyether sulfone—polyvinyl pyrrolidone blend anion exchange membranes for the recovery of titania waste acid via diffusion dialysis[J]. Journal of Membrane Science, 2022, 662: 120980. |
| 6 | Kladnig W. New development of acid regeneration in steel pickling plants[J]. Journal of Iron and Steel Research, International, 2008, 15(4): 1-6. |
| 7 | 陈文松, 宁寻安, 白晓燕. 废酸液的资源化处理技术[J]. 工业水处理, 2008, 28(3): 20-22, 80. |
| Chen W S, Ning X A, Bai X Y. Treatment technologies of waste acid liquid for resource recycling[J]. Industrial Water Treatment, 2008, 28(3): 20-22, 80. | |
| 8 | Song K, Meng Q Q, Shu F, et al. Recovery of high purity sulfuric acid from the waste acid in toluene nitration process by rectification[J]. Chemosphere, 2013, 90(4): 1558-1562. |
| 32 | Larchet C, Dammak L, Auclair B, et al. A simplified procedure for ion-exchange membrane characterization[J]. New Journal of Chemistry, 2004, 28(10): 1260-1267. |
| 33 | 张维润. 电渗析工程学[M]. 北京: 科学出版社, 1995. |
| Zhang W R. Electrodialysis Engineering[M]. Beijing: Science Press, 1995. | |
| 34 | 佐田俊胜. 离子交换膜: 制备, 表征, 改性和应用[M]. 汪锰, 任庆春, 译. 北京: 化学工业出版社, 2015. |
| Toshikatsu S. Ion Exchange Membranes: Preparation, Characterization and Application[M]. Wang M, Ren Q C, trans. Beijing: Chemical Industry Press, 2015. |
| [1] | 韩晨, 司徒友珉, 朱斌, 许建良, 郭晓镭, 刘海峰. 协同处理废液的多喷嘴粉煤气化炉内反应流动研究[J]. 化工学报, 2023, 74(8): 3266-3278. |
| [2] | 张曼铮, 肖猛, 闫沛伟, 苗政, 徐进良, 纪献兵. 危废焚烧处理耦合有机朗肯循环系统工质筛选与热力学优化[J]. 化工学报, 2023, 74(8): 3502-3512. |
| [3] | 屈园浩, 邓文义, 谢晓丹, 苏亚欣. 活性炭/石墨辅助污泥电渗脱水研究[J]. 化工学报, 2023, 74(7): 3038-3050. |
| [4] | 顾浩, 张福建, 刘珍, 周文轩, 张鹏, 张忠强. 力电耦合作用下多孔石墨烯膜时间维度的脱盐性能及机理研究[J]. 化工学报, 2023, 74(5): 2067-2074. |
| [5] | 罗来明, 张劲, 郭志斌, 王海宁, 卢善富, 相艳. 1~5 kW高温聚合物电解质膜燃料电池堆的理论模拟与组装测试[J]. 化工学报, 2023, 74(4): 1724-1734. |
| [6] | 刘定平, 陈爱桦, 张向阳, 何文浩, 王海. 铝灰半干法水解脱氮研究[J]. 化工学报, 2023, 74(3): 1294-1302. |
| [7] | 闫军营, 王皝莹, 李瑞瑞, 符蓉, 蒋晨啸, 汪耀明, 徐铜文. 选择性电渗析:机遇与挑战[J]. 化工学报, 2023, 74(1): 224-236. |
| [8] | 罗欣宜, 冯超, 刘晶, 乔瑜. 污泥不同热处理工艺产物磷的浸出回收实验研究[J]. 化工学报, 2022, 73(9): 4034-4044. |
| [9] | 黄陆月, 刘畅, 许勇毅, 邢浩若, 王峰, 马双忱. CDI二维浓度传质模型的建立以及实验验证[J]. 化工学报, 2022, 73(7): 2933-2943. |
| [10] | 朱嫣然, 葛亮, 李兴亚, 徐铜文. 三相结构离子交换膜的构筑及应用研究[J]. 化工学报, 2022, 73(6): 2397-2414. |
| [11] | 郭志强, 燕可洲, 张吉元, 柳丹丹, 高阳艳, 郭彦霞. 煤矸石/粉煤灰对赤泥钠化还原焙烧反应的影响机制[J]. 化工学报, 2022, 73(5): 2194-2205. |
| [12] | 许世佩, 王超, 李庆远, 张炳康, 许世伟, 张雪琴, 王诗颖, 丛梦晓. 氧化钙对油基钻屑热脱附产物影响的研究[J]. 化工学报, 2022, 73(4): 1724-1731. |
| [13] | 迟子怡, 刘成伟, 张欲凌, 李学刚, 肖文德. CO氧化偶联反应器模拟与优化[J]. 化工学报, 2022, 73(11): 4974-4986. |
| [14] | 王慧艳, 陈怡沁, 周静红, 曹约强, 周兴贵. 锂离子电池正极涂层孔隙结构优化的数值模拟[J]. 化工学报, 2022, 73(1): 376-383. |
| [15] | 王乾浩, 赵璐, 孙付琳, 房克功. ZSM-5催化剂与低温等离子体协同转化H2S-CO2制合成气[J]. 化工学报, 2022, 73(1): 255-265. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号