化工学报 ›› 2025, Vol. 76 ›› Issue (4): 1375-1390.DOI: 10.11949/0438-1157.20240963
赵俊德1,2( ), 周爱国2, 陈彦霖1,2, 郑家乐2, 葛天舒1(
), 周爱国2, 陈彦霖1,2, 郑家乐2, 葛天舒1( )
)
收稿日期:2024-08-27
修回日期:2024-10-05
出版日期:2025-04-25
发布日期:2025-05-12
通讯作者:
葛天舒
作者简介:赵俊德(2000—),女,硕士研究生,zhaojunde@sjtu.edu.cn
基金资助:
Junde ZHAO1,2( ), Aiguo ZHOU2, Yanlin CHEN1,2, Jiale ZHENG2, Tianshu GE1(
), Aiguo ZHOU2, Yanlin CHEN1,2, Jiale ZHENG2, Tianshu GE1( )
)
Received:2024-08-27
Revised:2024-10-05
Online:2025-04-25
Published:2025-05-12
Contact:
Tianshu GE
摘要:
CO2直接空气捕集(DAC)技术相对于传统的固定源烟气捕集技术具有位置灵活、应用广泛等优势,但由于大气中CO2浓度极低(仅为0.04%左右),DAC技术的高能耗成为阻碍其商业化的首要难题。聚焦吸附法DAC技术的能耗问题,先后进行理论分析和案例引证。DAC技术的CO2分离理想最小功为19.64 kJ·mol-1(温度298.15 K,捕集率50%,纯度95%),为同等条件下烟气捕集技术的3.5倍。再生温度393 K时变温真空吸附循环(TVSA)第二定律分离效率为22.75%。吸附、排空、再生、冷凝、压缩等过程主要通过机械能和热能推动。其中排空过程机械能仅占3%左右;冷凝过程热能可以通过回热循环回收;压缩过程机械能由目标压力决定,在部分研究中计入DAC能耗。吸附过程流动机械能受反应器压降主导,床层厚度减小和吸附剂有序堆积均能够改善流动损耗问题。再生过程热能占DAC能耗的主要部分,为50%~80%,再生温度、反应器与吸附剂的质量比、吸附剂对H2O吸附性的强弱,均能造成热耗的成倍变化。在分析过程能耗的基础上,给出了吸附法DAC在反应器设计、循环方式及操作参数、自然环境及能量来源等方面的能耗优化建议。
中图分类号:
赵俊德, 周爱国, 陈彦霖, 郑家乐, 葛天舒. 吸附法CO2直接空气捕集技术能耗现状[J]. 化工学报, 2025, 76(4): 1375-1390.
Junde ZHAO, Aiguo ZHOU, Yanlin CHEN, Jiale ZHENG, Tianshu GE. Current status of energy consumption of adsorption CO2 direct air capture[J]. CIESC Journal, 2025, 76(4): 1375-1390.
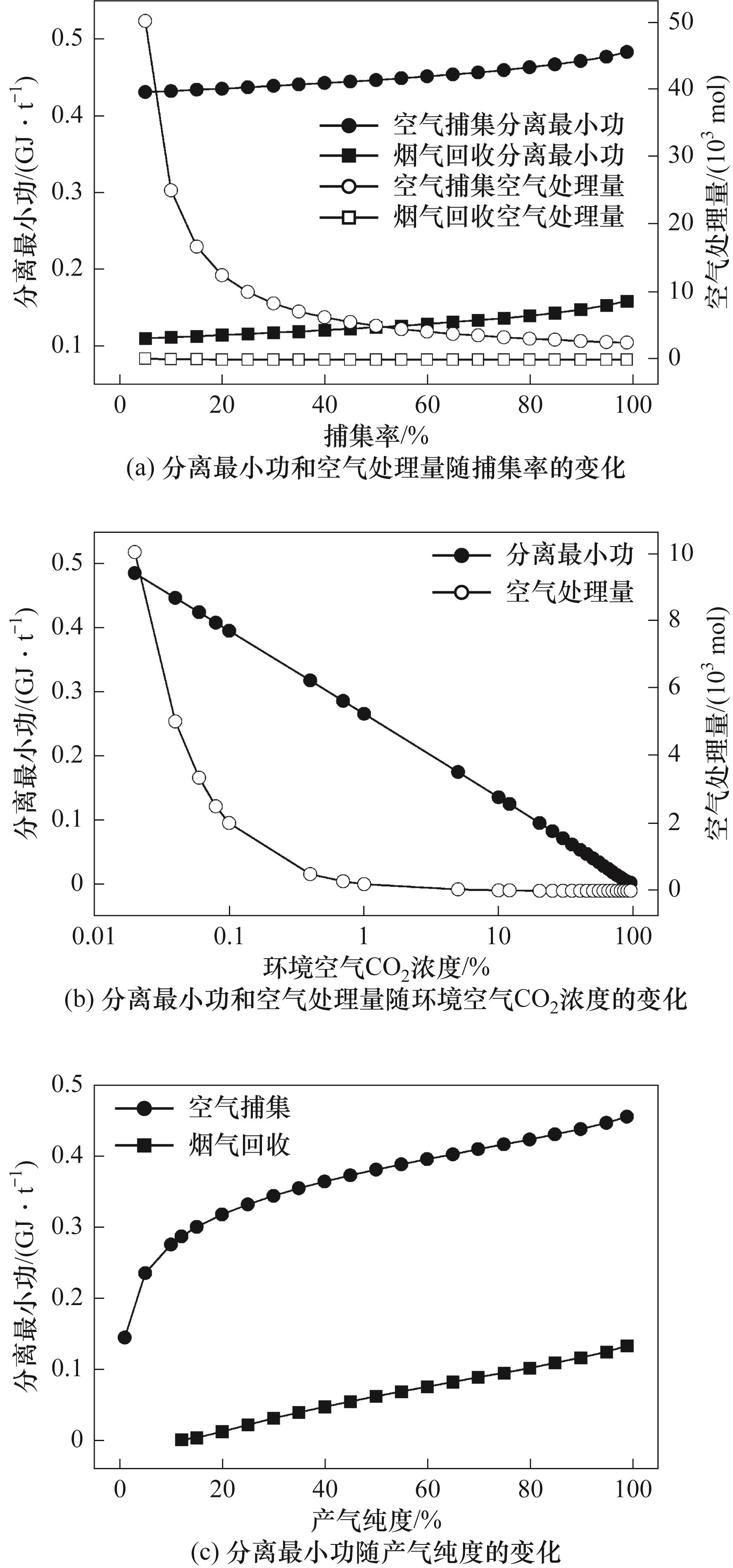
图4 空气捕集和烟气回收中分离最小功和空气处理量随捕集率、环境空气CO2浓度和产气纯度的变化
Fig.4 Variation of the minimum work of separation and air handling capacity with capture rate, ambient air CO2 concentration and product gas purity in air capture and flue gas recovery
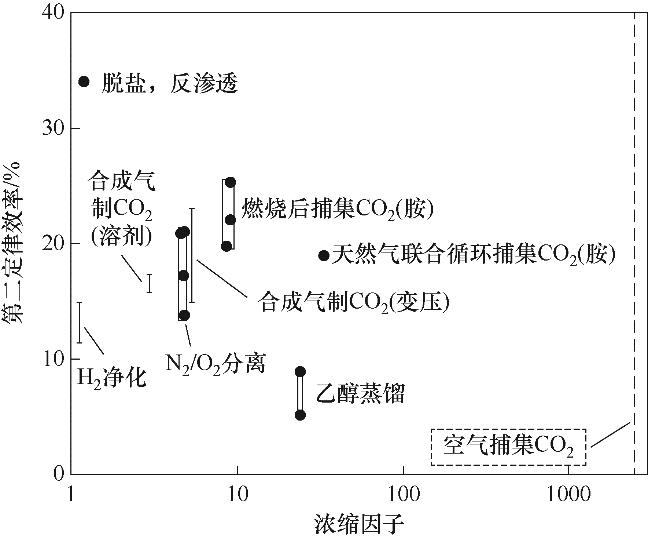
图5 工业分离过程第二定律效率与浓缩因子的经验关系[24]
Fig.5 Empirical relationship between the concentration factor of industrial separation processes vs the achieved second-law efficiency of those processes[24]
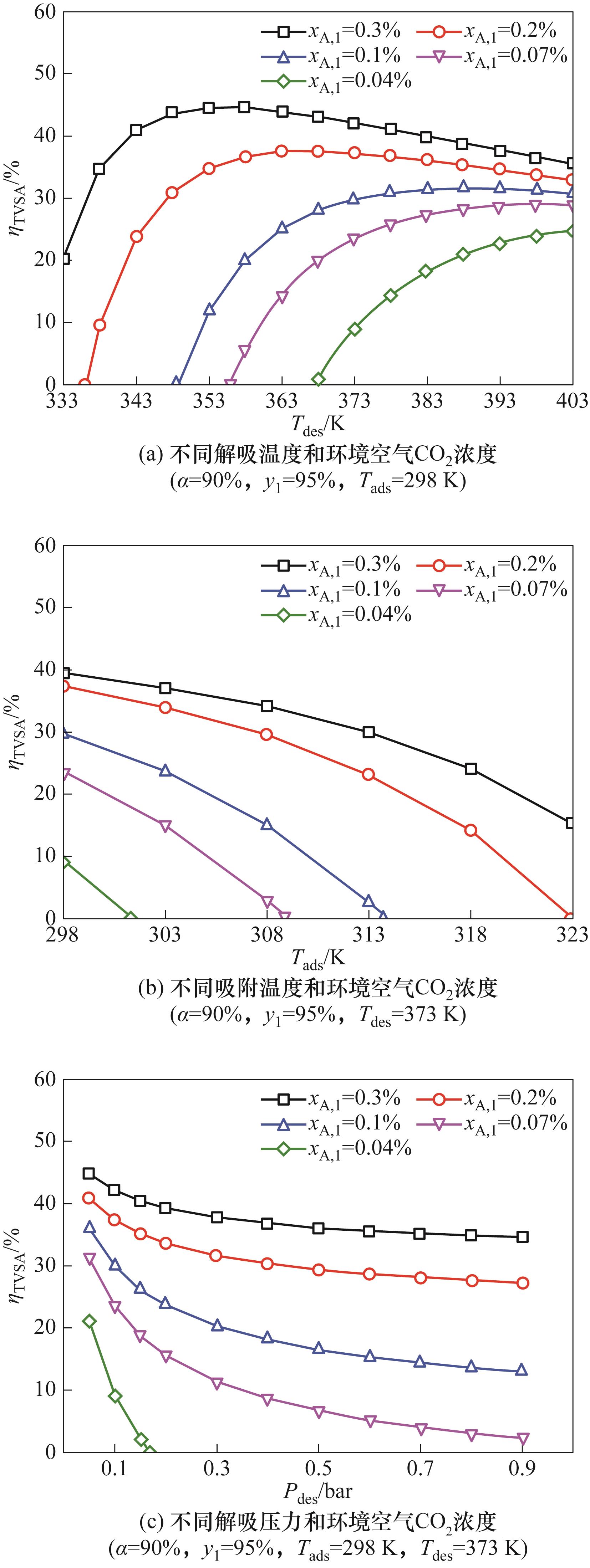
图6 不同解吸温度、吸附温度、解吸压力和环境空气CO2浓度下的TVSA的第二定律效率[25]
Fig.6 The second-law efficiency of TVSA at different desorption temperature, adsorption temperature, desorption pressure and ambient air CO2 concentration[25]

图8 床层压降随空气流速、吸附剂粒径(通道内半径)、球形度和床层厚度的变化
Fig.8 Variation of bed pressure drop with air flow rate, adsorbent pellet size (in-channel radius), sphericity and bed thickness
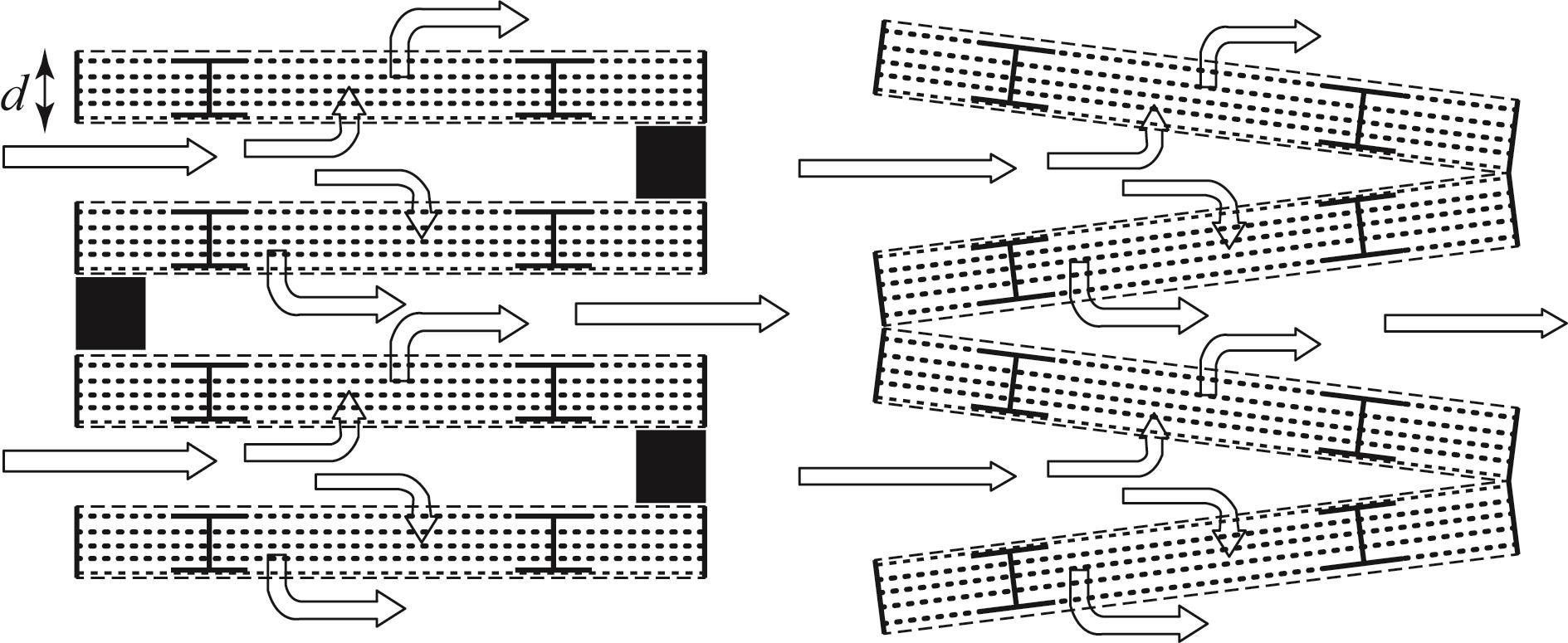
图10 Climeworks用于吸附气体分离过程的颗粒吸附床的低压降结构[40]
Fig.10 Low-pressure drop structure of particle adsorbent bed for adsorption gas separation process from Climeworks[40]
| Volume flow/(m3·h-1) | Pressure drop/Pa |
|---|---|
| 200 | 16 |
| 400 | 31 |
| 600 | 58 |
| 800 | 98 |
表1 Climeworks吸附床结构的压降测试结果[40]
Table 1 Pressure drop test results for adsorption bed structures from Climeworks[40]
| Volume flow/(m3·h-1) | Pressure drop/Pa |
|---|---|
| 200 | 16 |
| 400 | 31 |
| 600 | 58 |
| 800 | 98 |
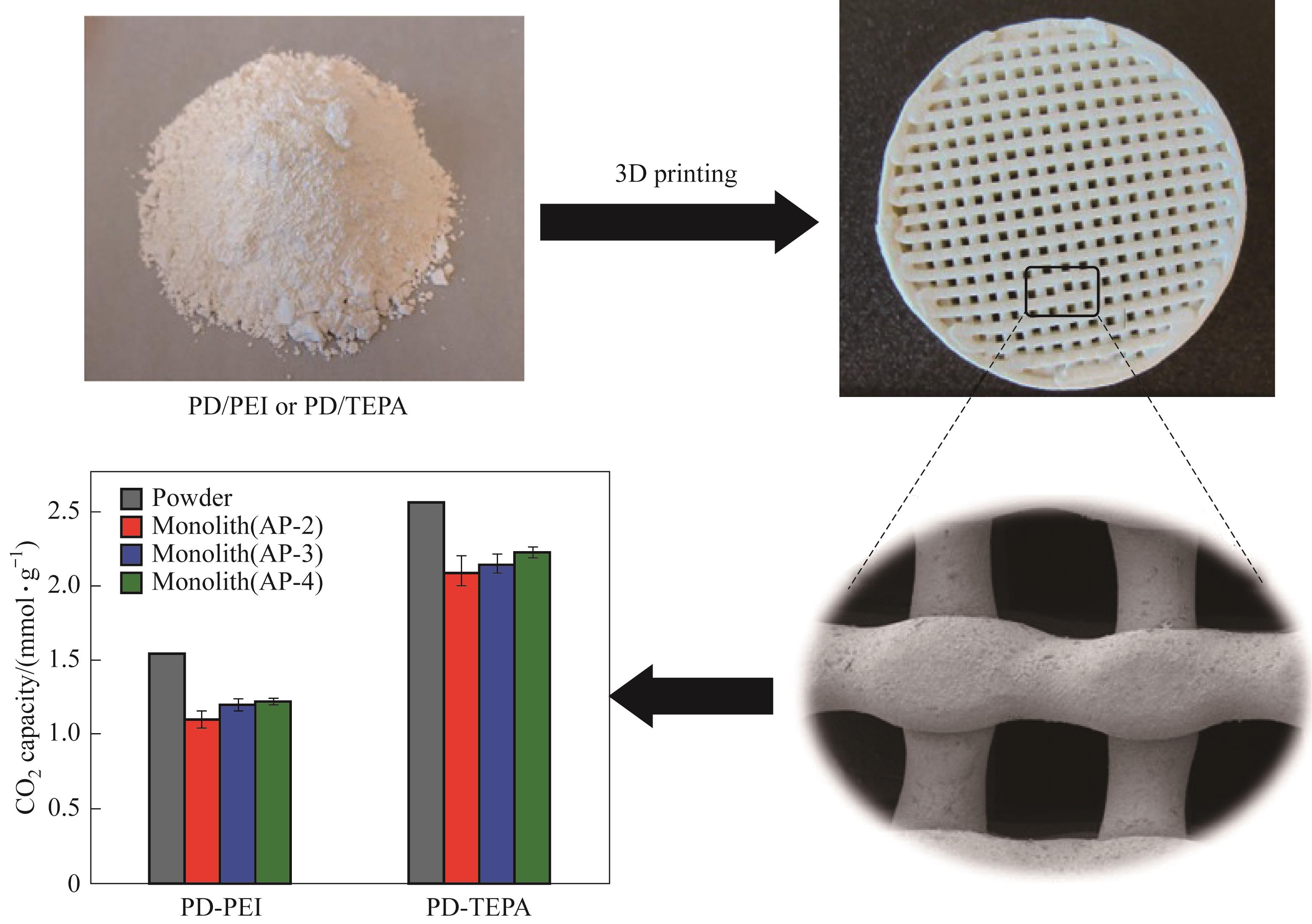
图13 3D打印胺基硅吸附剂及其与相应粉末材料在25℃、1 bar下10%CO2的N2中的CO2吸附能力对比[44]
Fig.13 3D-printed aminosilica monoliths and CO2 adsorption capacities corresponding powders obtained at 25℃ and 1 bar using 10% CO2 in N2[44]
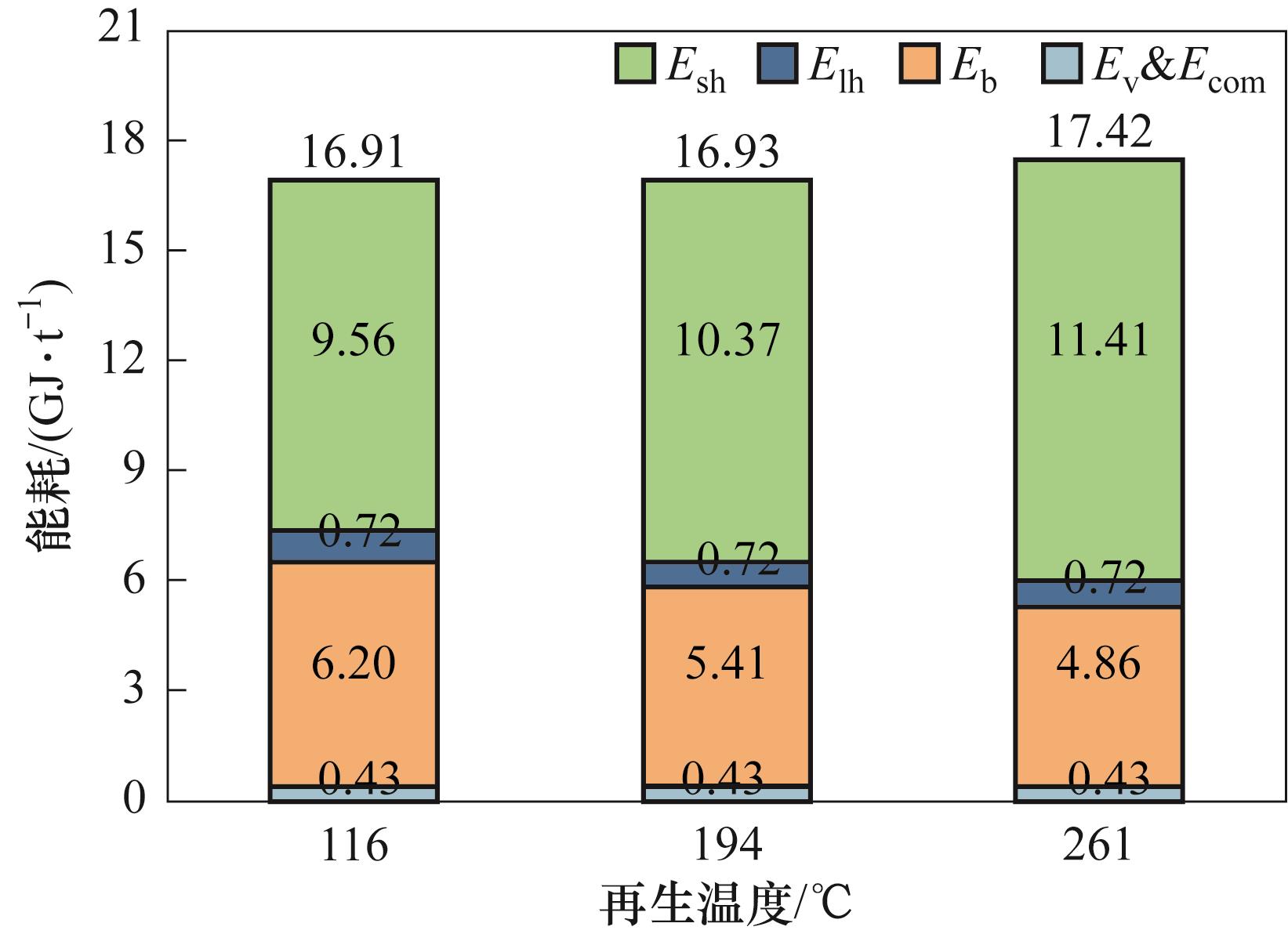
图15 不同再生温度下APG-Ⅲ捕获CO2的能耗(Tads=23.5℃,GHSV=13400 h-1)[33]
Fig.15 Energy consumption per ton of CO2 captured by APG-Ⅲ at different regeneration temperatures (Tads = 23.5℃, GHSV = 13400 h-1) [33]
| 1 | National Oceanic and Atmospheric Administration. Climate change: atmospheric carbon dioxide[EB/OL]. [2024-07-31]. . |
| 2 | Rogelj J, Schaeffer M, Meinshausen M, et al. Zero emission targets as long-term global goals for climate protection[J]. Environmental Research Letters, 2015, 10(10): 105007. |
| 3 | Smith P, Davis S J, Creutzig F, et al. Biophysical and economic limits to negative CO2 emissions[J]. Nature Climate Change, 2016, 6(1): 42-50. |
| 4 | 吴娇. 海洋升温与酸化背景下三角褐指藻光抑制及恢复研究[D]. 广州: 广州大学, 2024. |
| Wu J. Study on photoinhibition and recovery of Phaeodactylum tricornutum in the context of ocean warming and acidification[D]. Guangzhou: Guangzhou University, 2024. | |
| 5 | Matthews H D, Caldeira K. Stabilizing climate requires near‐zero emissions[J]. Geophysical Research Letters, 2008, 35(4): 032388. |
| 6 | Davis S J, Lewis N S, Shaner M, et al. Net-zero emissions energy systems[J]. Science, 2018, 360(6396): 1-9. |
| 7 | 周红军, 周颖, 徐春明. 中国碳中和目标下CO2转化的思考与实践[J]. 化工进展, 2022, 41(6): 3381-3385. |
| Zhou H J, Zhou Y, Xu C M. Exploration of the CO2 conversion under China's carbon neutrality goal[J]. Chemical Industry and Engineering Progress, 2022, 41(6): 3381-3385. | |
| 8 | Lee S Y, Park S J. A review on solid adsorbents for carbon dioxide capture[J]. Journal of Industrial and Engineering Chemistry, 2015, 23: 1-11. |
| 9 | Lackner K, Ziock H J, Grimes P. Carbon dioxide extraction from air: is it an option?[R/OL]. [2023-07-31]. . |
| 10 | Sánchez J M, Maroño M, Cillero D, et al. Laboratory- and bench-scale studies of a sweet water-gas-shift catalyst for H2 and CO2 production in pre-combustion CO2 capture[J]. Fuel, 2013, 114: 191-198. |
| 11 | Babu P, Kumar R, Linga P. A new porous material to enhance the kinetics of clathrate process: application to precombustion carbon dioxide capture[J]. Environmental Science & Technology, 2013, 47(22): 13191-13198. |
| 12 | Cotton A, Patchigolla K, Oakey J E. Minor and trace element emissions from post-combustion CO2 capture from coal: experimental and equilibrium calculations[J]. Fuel, 2014, 117: 391-407. |
| 13 | Goto K, Yogo K, Higashii T. A review of efficiency penalty in a coal-fired power plant with post-combustion CO2 capture[J]. Applied Energy, 2013, 111: 710-720. |
| 14 | Skorek-Osikowska A, Bartela L, Kotowicz J, et al. Thermodynamic and economic analysis of the different variants of a coal-fired, 460 MW power plant using oxy-combustion technology[J]. Energy Conversion and Management, 2013, 76: 109-120. |
| 15 | Wall T, Stanger R, Liu Y. Gas cleaning challenges for coal-fired oxy-fuel technology with carbon capture and storage[J]. Fuel, 2013, 108: 85-90. |
| 16 | Lackner K S. A guide to CO2 sequestration[J]. Science, 2003, 300(5626): 1677-1678. |
| 17 | Wang X, Song C. Carbon capture from flue gas and the atmosphere: a perspective[J]. Frontiers in Energy Research, 2020, 8: 560849. |
| 18 | Seipp C A, Williams N J, Kidder M K, et al. CO2 capture from ambient air by crystallization with a guanidine sorbent[J]. Angewandte Chemie, 2017, 129(4): 1062-1065. |
| 19 | Keith D W. Why capture CO2 from the atmosphere?[J]. Science, 2009, 325(5948): 1654-1655. |
| 20 | Shi X, Xiao H, Azarabadi H, et al. Sorbents for the direct capture of CO2 from ambient air[J]. Angewandte Chemie International Edition, 2020, 59(18): 6984-7006. |
| 21 | United States Department of Energy, National Energy Technology Laboratory. Carbon dioxide removal project map[EB/OL]. [2024-09-26]. . |
| 22 | Brandani S. Carbon dioxide capture from air: a simple analysis[J]. Energy & Environment, 2012, 23(2/3): 319-328. |
| 23 | Kulkarni A R, Sholl D S. Analysis of equilibrium-based TSA processes for direct capture of CO2 from air[J]. Industrial & Engineering Chemistry Research, 2012, 51(25): 8631-8645. |
| 24 | House K Z, Baclig A C, Ranjan M, et al. Economic and energetic analysis of capturing CO2 from ambient air[J]. Proceedings of the National Academy of Sciences, 2011, 108(51): 20428-20433. |
| 25 | Zhao R, Liu L, Zhao L, et al. Thermodynamic exploration of temperature vacuum swing adsorption for direct air capture of carbon dioxide in buildings[J]. Energy Conversion and Management, 2019, 183: 418-426. |
| 26 | Zhao R, Deng S, Liu Y, et al. Carbon pump: fundamental theory and applications[J]. Energy, 2017, 119: 1131-1143. |
| 27 | Jiang L, Roskilly A P, Wang R Z. Performance exploration of temperature swing adsorption technology for carbon dioxide capture[J]. Energy Conversion and Management, 2018, 165: 396-404. |
| 28 | Clausse M, Merel J, Meunier F. Numerical parametric study on CO2 capture by indirect thermal swing adsorption[J]. International Journal of Greenhouse Gas Control, 2011, 5(5): 1206-1213. |
| 29 | Pirngruber G D, Guillou F, Gomez A, et al. A theoretical analysis of the energy consumption of post-combustion CO2 capture processes by temperature swing adsorption using solid sorbents[J]. International Journal of Greenhouse Gas Control, 2013, 14: 74-83. |
| 30 | Veneman R, Frigka N, Zhao W, et al. Adsorption of H2O and CO2 on supported amine sorbents[J]. International Journal of Greenhouse Gas Control, 2015, 41: 268-275. |
| 31 | Shen C, Liu Z, Li P, et al. Two-stage VPSA process for CO2 capture from flue gas using activated carbon beads[J]. Industrial & Engineering Chemistry Research, 2012, 51(13): 5011-5021. |
| 32 | Oreggioni G D, Brandani S, Luberti M, et al. CO2 capture from syngas by an adsorption process at a biomass gasification CHP plant: its comparison with amine-based CO2 capture[J]. International Journal of Greenhouse Gas Control, 2015, 35: 71-81. |
| 33 | Wilson S M W, Tezel F H. Direct dry air capture of CO2 using VTSA with faujasite zeolites[J]. Industrial & Engineering Chemistry Research, 2020, 59(18): 8783-8794. |
| 34 | Mazzotti M, Baciocchi R, Desmond M J, et al. Direct air capture of CO2 with chemicals: optimization of a two-loop hydroxide carbonate system using a countercurrent air-liquid contactor[J]. Climatic Change, 2013, 118(1): 119-135. |
| 35 | Ergun S. Fluid flow through packed columns[J]. Chemical Engineering Progress, 1952, 48: 89-94. |
| 36 | 曹伟波. 壁流蜂窝式微填充床的制备及其在变压吸附过程的应用[D]. 杭州: 浙江大学, 2017. |
| Cao W B. Fabrication of honeycomb wall-flow micro packed bed and application in PSA process[D]. Hangzhou: Zhejiang University, 2017. | |
| 37 | Sinha A, Darunte L A, Jones C W, et al. Systems design and economic analysis of direct air capture of CO2 through temperature vacuum swing adsorption using MIL-101(Cr)-PEI-800 and mmen-Mg2 (dobpdc) MOF adsorbents[J]. Industrial & Engineering Chemistry Research, 2017, 56(3): 750-764. |
| 38 | Yang R T. Gas Separation by Adsorption Processes[M]. Butterworth-Heinemann, 1987. |
| 39 | Yasushi A, Tatsuji M. Filter for purifying exhaust gases: EP0766993A2[P]. 1997-04-09. |
| 40 | Gebald C, Piatkowski N, Rüesch T, et al. Low-pressure drop structure of particle adsorbent bed for adsorption gas separation process: WO2014170184[P]. 2014-10-23. |
| 41 | Couck S, Lefevere J, Mullens S, et al. CO2, CH4 and N2 separation with a 3DFD-printed ZSM-5 monolith[J]. Chemical Engineering Journal, 2017, 308: 719-726. |
| 42 | Couck S, Cousin-Saint-Remi J, van der Perre S, et al. 3D-printed SAPO-34 monoliths for gas separation[J]. Microporous and Mesoporous Materials, 2018, 255: 185-191. |
| 43 | Thakkar H, Eastman S, Hajari A, et al. 3D-printed zeolite monoliths for CO2 removal from enclosed environments[J]. ACS Applied Materials & Interfaces, 2016, 8(41): 27753-27761. |
| 44 | Thakkar H, Eastman S, Al-Mamoori A, et al. Formulation of aminosilica adsorbents into 3D-printed monoliths and evaluation of their CO2 capture performance[J]. ACS Applied Materials & Interfaces, 2017, 9(8): 7489-7498. |
| 45 | Bollini P, Choi S, Drese J H, et al. Oxidative degradation of aminosilica adsorbents relevant to postcombustion CO2 capture[J]. Energy & Fuels, 2011, 25(5): 2416-2425. |
| 46 | Heydari-Gorji A, Sayari A. Thermal, oxidative, and CO2-induced degradation of supported polyethylenimine adsorbents[J]. Industrial & Engineering Chemistry Research, 2012, 51(19): 6887-6894. |
| 47 | Ahmadalinezhad A, Sayari A. Oxidative degradation of silica-supported polyethylenimine for CO2 adsorption: insights into the nature of deactivated species[J]. Physical Chemistry Chemical Physics, 2013, 16(4): 1529-1535. |
| 48 | Choi S, Drese J H, Jones C W. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources[J]. ChemSusChem, 2009, 2(9): 796-854. |
| 49 | Samanta A, Zhao A, Shimizu G K H, et al. Post-combustion CO2 capture using solid sorbents: a review[J]. Industrial & Engineering Chemistry Research, 2012, 51(4): 1438-1463. |
| 50 | Quang D V, Dindi A, Rayer A V, et al. Impregnation of amines onto porous precipitated silica for CO2 capture[J]. Energy Procedia, 2014, 63: 2122-2128. |
| 51 | Sinha A, Realff M J. A parametric study of the techno‐economics of direct CO2 air capture systems using solid adsorbents[J]. AIChE Journal, 2019, 65(7): e16607. |
| 52 | Zhu X C, Ge T S, Yang F, et al. Design of steam-assisted temperature vacuum-swing adsorption processes for efficient CO2 capture from ambient air[J]. Renewable and Sustainable Energy Reviews, 2021, 137: 110651. |
| 53 | Min Y J, Ganesan A, Realff M J, et al. Direct air capture of CO2 using poly(ethyleneimine)-functionalized expanded poly(tetrafluoroethylene)/silica composite structured sorbents[J]. ACS Applied Materials & Interfaces, 2022, 14(36): 40992-41002. |
| 54 | Sujan A R, Pang S H, Zhu G, et al. Direct CO2 capture from air using poly(ethylenimine)-loaded polymer/silica fiber sorbents[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(5): 5264-5273. |
| 55 | Didas S A, Sakwa-Novak M A, Foo G S, et al. Effect of amine surface coverage on the co-adsorption of CO2 and water: spectral deconvolution of adsorbed species[J]. The Journal of Physical Chemistry Letters, 2014, 5(23): 4194-4200. |
| 56 | Wurzbacher J A, Gebald C, Piatkowski N, et al. Concurrent separation of CO2 and H2O from air by a temperature-vacuum swing adsorption/desorption cycle[J]. Environmental Science & Technology, 2012, 46(16): 9191-9198. |
| 57 | Sehaqui H, Gálvez M E, Becatinni V, et al. Fast and reversible direct CO2 capture from air onto all-polymer nanofibrillated cellulose-polyethylenimine foams[J]. Environmental Science & Technology, 2015, 49(5): 3167-3174. |
| 58 | Elfving J, Bajamundi C, Kauppinen J, et al. Modelling of equilibrium working capacity of PSA, TSA and TVSA processes for CO2 adsorption under direct air capture conditions[J]. Journal of CO2 Utilization, 2017, 22: 270-277. |
| 59 | Kumar D R, Rosu C, Sujan A R, et al. Alkyl-aryl amine-rich molecules for CO2 removal via direct air capture[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(29): 10971-10982. |
| 60 | Gebald C, Repond N, Wurzbacher J A. Steam assisted vacuum desorption process for carbon dioxide capture: US201515324775[P]. 2019-05-07. |
| 61 | Stampi-Bombelli V, van der Spek M, Mazzotti M. Analysis of direct capture of CO2 from ambient air via steam-assisted temperature-vacuum swing adsorption[J]. Adsorption, 2020, 26(7): 1183-1197. |
| 62 | van der Giesen C, Meinrenken C J, Kleijn R, et al. A life cycle assessment case study of coal-fired electricity generation with humidity swing direct air capture of CO2 versus MEA-based postcombustion capture[J]. Environmental Science & Technology, 2017, 51(2): 1024-1034. |
| 63 | 葛天舒, 徐静, 赵俊德, 等. 一种热泵驱动的回热型直接空气碳捕集系统: 202311163513.X[P]. 2023-12-22. |
| Ge T S, Xu J, Zhao J D, et al. A heat pump driven regenerative direct air carbon capture system: 202311163513. X[P] 2023-12-22. | |
| 64 | 葛天舒, 徐静, 赵俊德. CO2再循环吹扫的热泵驱动直接空气碳捕集系统及其方法: 202310897457.6[P]. 2023-10-27. |
| Ge T S, Xu J, Zhao J D. Direct air carbon capture system driven by heat pump for CO2 recirculation blowing and its method: 202310897457.6[P] 2023-10-27. | |
| 65 | National Academies of Sciences E, Medicine. Negative Emissions Technologies and Reliable Sequestration: A Research Agenda[M]. Washington, D.C.: National Academies Press, 2019. |
| 66 | Gebald C, Wurzbacher J A, Borgschulte A, et al. Single-component and binary CO2 and H2O adsorption of amine-functionalized cellulose[J]. Environmental Science & Technology, 2014, 48(4): 2497-2504. |
| 67 | Gutknecht V, Snæbjörnsdóttir S Ó, Sigfússon B, et al. Creating a carbon dioxide removal solution by combining rapid mineralization of CO2 with direct air capture[J]. Energy Procedia, 2018, 146: 129-134. |
| 68 | Beuttler C, Charles L, Wurzbacher J. The role of direct air capture in mitigation of anthropogenic greenhouse gas emissions[J]. Frontiers in Climate, 2019, 1: 10. |
| 69 | Ji Y, Liu W, Yong J Y, et al. Techno-economic analysis on temperature vacuum swing adsorption system integrated with pre-dehumidification for direct air capture[J]. Carbon Capture Science & Technology, 2024, 12: 100199. |
| 70 | Sanz-Pérez E S, Murdock C R, Didas S A, et al. Direct capture of CO2 from ambient air[J]. Chemical Reviews, 2016, 116(19): 11840-11876. |
| 71 | 赵洁. CO2/H2O竞争吸附碳捕集基础及应用研究[D]. 天津: 天津大学, 2022. |
| Zhao J. Fundamental and application research of CO2 and H2O competitive adsorption on carbon capture[D]. Tianjin: Tianjin University, 2022. | |
| 72 | National Renewable Energy Laboratory. Life cycle greenhouse gas emissions from electricity generation: update[R/OL]. [2024-07-09]. . |
| 73 | Sabatino F, Grimm A, Gallucci F, et al. A comparative energy and costs assessment and optimization for direct air capture technologies[J]. Joule, 2021, 5(8): 2047-2076. |
| 74 | Hong W Y. A techno-economic review on carbon capture, utilisation and storage systems for achieving a net-zero CO2 emissions future[J]. Carbon Capture Science & Technology, 2022, 3: 100044. |
| 75 | Erans M, Sanz-Pérez E S, Hanak D P, et al. Direct air capture: process technology, techno-economic and socio-political challenges[J]. Energy & Environmental Science, 2022, 15(4): 1360-1405. |
| [1] | 蔡天姿, 张海丰, 林海丹, 张子龙, 周鹏宇, 王柏林, 李小年. 硼掺杂氮基石墨烯检测变压器油中溶解气体CO和CO2的密度泛函理论研究[J]. 化工学报, 2025, 76(4): 1841-1851. |
| [2] | 何正磊, 胡丁丁. 基于多智能体强化学习的造纸污水多目标优化[J]. 化工学报, 2025, 76(4): 1617-1634. |
| [3] | 晋伊浩, 罗俊欣, 胡章茂, 王唯, 殷谦. 亲水改性硫酸镁/膨胀蛭石复合材料的吸附储热性能[J]. 化工学报, 2025, 76(4): 1852-1862. |
| [4] | 朱峰, 赵跃, 马凤翔, 刘伟. 改性UIO-66对SF6/N2混合气体及其分解产物的吸附特性[J]. 化工学报, 2025, 76(4): 1604-1616. |
| [5] | 张履胜, 王治红, 柳青, 李雪雯, 谭仁敏. 液-液相变吸收剂捕集二氧化碳研究进展[J]. 化工学报, 2025, 76(3): 933-950. |
| [6] | 伏遥, 邵应娟, 钟文琪. TiO2掺杂钙基材料加压碳酸化循环储热性能实验研究[J]. 化工学报, 2025, 76(3): 1180-1190. |
| [7] | 常斐, 师人博, 刘士花, 高文倩, 王一飞, 郑镔, 焦怡萱, 蓝兴英, 徐春明, 韩晔华. 石化行业产品生命周期碳足迹评价研究现状及展望[J]. 化工学报, 2025, 76(2): 419-437. |
| [8] | 姚佳逸, 张东辉, 唐忠利, 李文彬. 基于二级双回流的变压吸附捕碳工艺研究[J]. 化工学报, 2025, 76(2): 744-754. |
| [9] | 杨晋宁, 王卫凡, 徐冬, 刘毅, 翁小涵, 原野, 王志. 工业烟道气碳捕集膜技术放大研究进展[J]. 化工学报, 2025, 76(2): 504-518. |
| [10] | 贾晶宇, 孔德齐, 沈圆辉, 张东辉, 李文彬, 唐忠利. 合成氨反应器尾气变压吸附氨分离工艺的模拟与分析[J]. 化工学报, 2025, 76(2): 718-730. |
| [11] | 王佳欣, 韦艳红, 农顺洋, 熊艳舒, 李楣, 李文. 多重量子化学理论计算解析多胺修饰壳聚糖气凝胶吸附美拉德色素分子机制[J]. 化工学报, 2025, 76(1): 107-119. |
| [12] | 杨勇, 祖子轩, 李煜坤, 王东亮, 范宗良, 周怀荣. T型圆柱形微通道内CO2碱液吸收数值模拟[J]. 化工学报, 2024, 75(S1): 135-142. |
| [13] | 陈彦霖, 周爱国, 郑家乐, 杨川箬, 葛天舒. 载体对于胺浸渍类DAC吸附剂性能的影响[J]. 化工学报, 2024, 75(S1): 217-222. |
| [14] | 唐宇昊, 张迎迎, 赵智伟, 鲁梦悦, 张飞飞, 王小青, 杨江峰. 弱极性超微孔Sc/In-CPM-66A用于CH4/N2吸附分离性能[J]. 化工学报, 2024, 75(9): 3210-3220. |
| [15] | 马林峰, 欧爱彤, 李志远, 李垚, 刘润泽, 吴晓乐, 徐景涛. Na2S改性生物炭高效吸附重金属离子:制备及吸附机理[J]. 化工学报, 2024, 75(7): 2594-2603. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号