化工学报 ›› 2025, Vol. 76 ›› Issue (3): 1180-1190.DOI: 10.11949/0438-1157.20240922
收稿日期:2024-08-13
修回日期:2024-09-28
出版日期:2025-03-25
发布日期:2025-03-28
通讯作者:
邵应娟
作者简介:伏遥(1999—),女,硕士研究生,fuyao_hhr@163.com
基金资助:
Yao FU( ), Yingjuan SHAO(
), Yingjuan SHAO( ), Wenqi ZHONG
), Wenqi ZHONG
Received:2024-08-13
Revised:2024-09-28
Online:2025-03-25
Published:2025-03-28
Contact:
Yingjuan SHAO
摘要:
在CaCO3/CaO热化学储能循环的释热(碳酸化)过程中,加压可显著提高钙基材料的循环储热性能。本文对TiO2掺杂钙基材料(CaCO3-TiO2)在加压条件下的碳酸化循环储热性能展开研究,重点讨论了TiO2掺杂量、碳酸化压力、循环次数等对CaCO3-TiO2储热性能的影响。结果表明:掺杂5%(质量分数)TiO2时,CaCO3-5TiO2比纯CaCO3碱性更强,碳酸化反应更易进行,循环储热性能最好。碳酸化压力升高可以增强CaCO3-5TiO2的储热性能,但增强幅度随着压力升高而减小。在最佳工况(0.8 MPa,850℃)下循环30次后,CaCO3-5TiO2储热密度是常压下纯CaCO3的2.9倍。SEM/TEM和BET表征显示,TiO2与CaO反应生成的CaTiO3有效缓解了材料的烧结与团聚,循环30次后,煅烧CaCO3-5TiO2的比表面积和孔容是煅烧纯CaCO3的2倍和1.4倍,具有更稳定的加压碳酸化循环储热性能。
中图分类号:
伏遥, 邵应娟, 钟文琪. TiO2掺杂钙基材料加压碳酸化循环储热性能实验研究[J]. 化工学报, 2025, 76(3): 1180-1190.
Yao FU, Yingjuan SHAO, Wenqi ZHONG. Experimental study on cyclic heat storage performance of TiO2-doped calcium based materials under pressurized carbonation[J]. CIESC Journal, 2025, 76(3): 1180-1190.
| 实验组 | TiO2掺杂量/% | 碳酸化 温度/℃ | 碳酸化 压力/MPa | 循环次数 |
|---|---|---|---|---|
| 1 | 0 | 850 | 0.8 | 15 |
| 2 | 2 | 850 | 0.8 | 15 |
| 3 | 5 | 850 | 0.8 | 15 |
| 4 | 10 | 850 | 0.8 | 15 |
| 5 | 15 | 850 | 0.8 | 15 |
| 6 | 20 | 850 | 0.8 | 15 |
| 7 | 5 | 850 | 0.1 | 10 |
| 8 | 5 | 850 | 0.2 | 10 |
| 9 | 5 | 850 | 0.4 | 10 |
| 10 | 5 | 850 | 0.6 | 10 |
| 11 | 5 | 750 | 0.8 | 10 |
| 12 | 5 | 800 | 0.8 | 10 |
| 13 | 5 | 900 | 0.8 | 10 |
| 14 | 0 | 850 | 0.1 | 30 |
| 15 | 0 | 850 | 0.8 | 30 |
| 16 | 5 | 850 | 0.1 | 30 |
| 17 | 5 | 850 | 0.8 | 30 |
表1 实验设计方案
Table 1 Experimental design scheme
| 实验组 | TiO2掺杂量/% | 碳酸化 温度/℃ | 碳酸化 压力/MPa | 循环次数 |
|---|---|---|---|---|
| 1 | 0 | 850 | 0.8 | 15 |
| 2 | 2 | 850 | 0.8 | 15 |
| 3 | 5 | 850 | 0.8 | 15 |
| 4 | 10 | 850 | 0.8 | 15 |
| 5 | 15 | 850 | 0.8 | 15 |
| 6 | 20 | 850 | 0.8 | 15 |
| 7 | 5 | 850 | 0.1 | 10 |
| 8 | 5 | 850 | 0.2 | 10 |
| 9 | 5 | 850 | 0.4 | 10 |
| 10 | 5 | 850 | 0.6 | 10 |
| 11 | 5 | 750 | 0.8 | 10 |
| 12 | 5 | 800 | 0.8 | 10 |
| 13 | 5 | 900 | 0.8 | 10 |
| 14 | 0 | 850 | 0.1 | 30 |
| 15 | 0 | 850 | 0.8 | 30 |
| 16 | 5 | 850 | 0.1 | 30 |
| 17 | 5 | 850 | 0.8 | 30 |

图11 加压/常压碳酸化下煅烧CaCO3和煅烧CaCO3-5TiO2中CaO晶粒尺寸的变化
Fig.11 Variation of CaO grain size in calcined CaCO3 and calcined CaCO3-5TiO2 under pressurized/atmospheric carbonation

图12 加压/常压碳酸化下不同循环次数煅烧CaCO3和煅烧CaCO3-5TiO2的SEM图(a)煅烧CaCO3,未循环; (b)煅烧CaCO3,0.1MPa, 循环30次; (c)煅烧CaCO3,0.8 MPa, 循环30次; (d)煅烧CaCO3-5TiO2,未循环; (e)煅烧CaCO3-5TiO2,0.1 MPa, 循环30次; (f)煅烧CaCO3-5TiO2,0.8 MPa,循环30次;
Fig.12 SEM images of calcined CaCO3 and calcined CaCO3-5TiO2 at different cycles under pressurized/atmospheric carbonation(a) initial calcined CaCO3; (b) calcined CaCO3 cycled 30 times at 0.1 MPa; (c) calcined CaCO3 cycled 30 times at 0.8 MPa;(d) initial calcined CaCO3-5TiO2; (e) calcined CaCO3-5TiO2 cycled 30 times at 0.1 MPa; (f) calcined CaCO3-5TiO2 cycled 30 times at 0.8 MPa

图13 加压/常压碳酸化下不同循环次数煅烧CaCO3和煅烧CaCO3-5TiO2的TEM图(a)煅烧CaCO3,未循环; (b)煅烧CaCO3,0.1 MPa, 循环30次; (c)煅烧CaCO3,0.8 MPa, 循环30次; (d)煅烧CaCO3-5TiO2,未循环; (e)煅烧CaCO3-5TiO2,0.1 MPa, 循环30次; (f)煅烧CaCO3-5TiO2,0.8 MPa, 循环30次;
Fig.13 TEM images of calcined CaCO3 and calcined CaCO3-5TiO2 at different cycles under pressurized/atmospheric carbonation(a) initial calcined CaCO3; (b) calcined CaCO3 cycled 30 times at 0.1 MPa; (c) calcined CaCO3 cycled 30 times at 0.8 MPa;(d) initial calcined CaCO3-5TiO2; (e) calcined CaCO3-5TiO2 cycled 30 times at 0.1 MPa; (f) calcined CaCO3-5TiO2 cycled 30 times at 0.8 MPa
| 样品 | 循环次数 | 碳酸化压力/MPa | 比表面积/(m²/g) | 比孔容/(cm³/g) |
|---|---|---|---|---|
| 煅烧纯CaCO3 | 0 | — | 19.582 | 0.133 |
| 30 | 0.1 | 3.248 | 0.028 | |
| 30 | 0.8 | 4.401 | 0.052 | |
| 煅烧CaCO3-5TiO2 | 0 | — | 11.768 | 0.117 |
| 30 | 0.1 | 7.414 | 0.069 | |
| 30 | 0.8 | 8.918 | 0.075 |
表2 煅烧CaCO3和煅烧CaCO3-5TiO2的比表面积和比孔容
Table 2 Specific surface area and pore volume of calcined CaCO3 and calcined CaCO3-5TiO2
| 样品 | 循环次数 | 碳酸化压力/MPa | 比表面积/(m²/g) | 比孔容/(cm³/g) |
|---|---|---|---|---|
| 煅烧纯CaCO3 | 0 | — | 19.582 | 0.133 |
| 30 | 0.1 | 3.248 | 0.028 | |
| 30 | 0.8 | 4.401 | 0.052 | |
| 煅烧CaCO3-5TiO2 | 0 | — | 11.768 | 0.117 |
| 30 | 0.1 | 7.414 | 0.069 | |
| 30 | 0.8 | 8.918 | 0.075 |
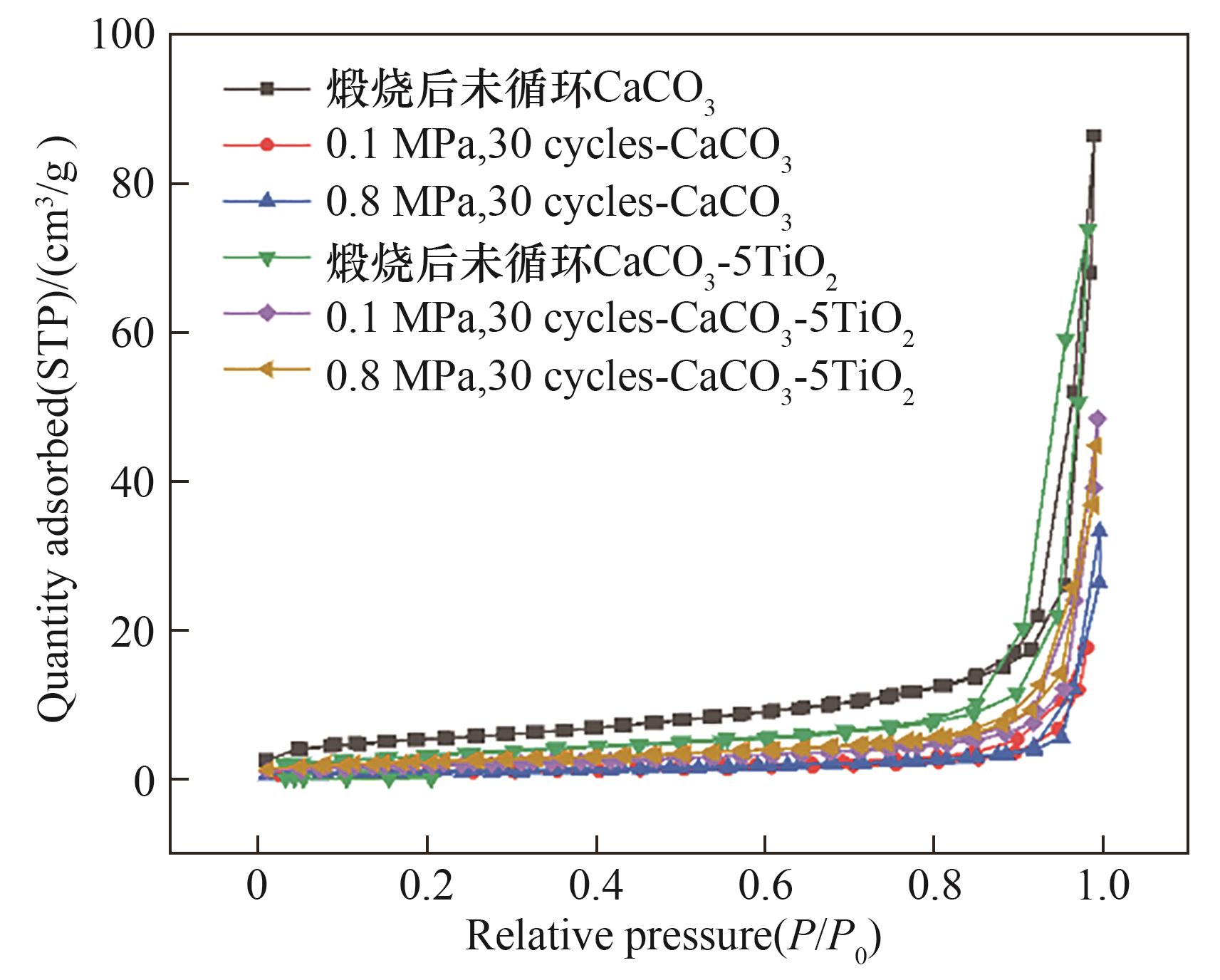
图14 加压/常压碳酸化循环储热中煅烧CaCO3和煅烧CaCO3-5TiO2的N2吸-脱附曲线
Fig.14 N2 absorption-desorption curves of calcined CaCO3 and calcined CaCO3-5TiO2 in pressurized/atmospheric carbonated heat storage cycles
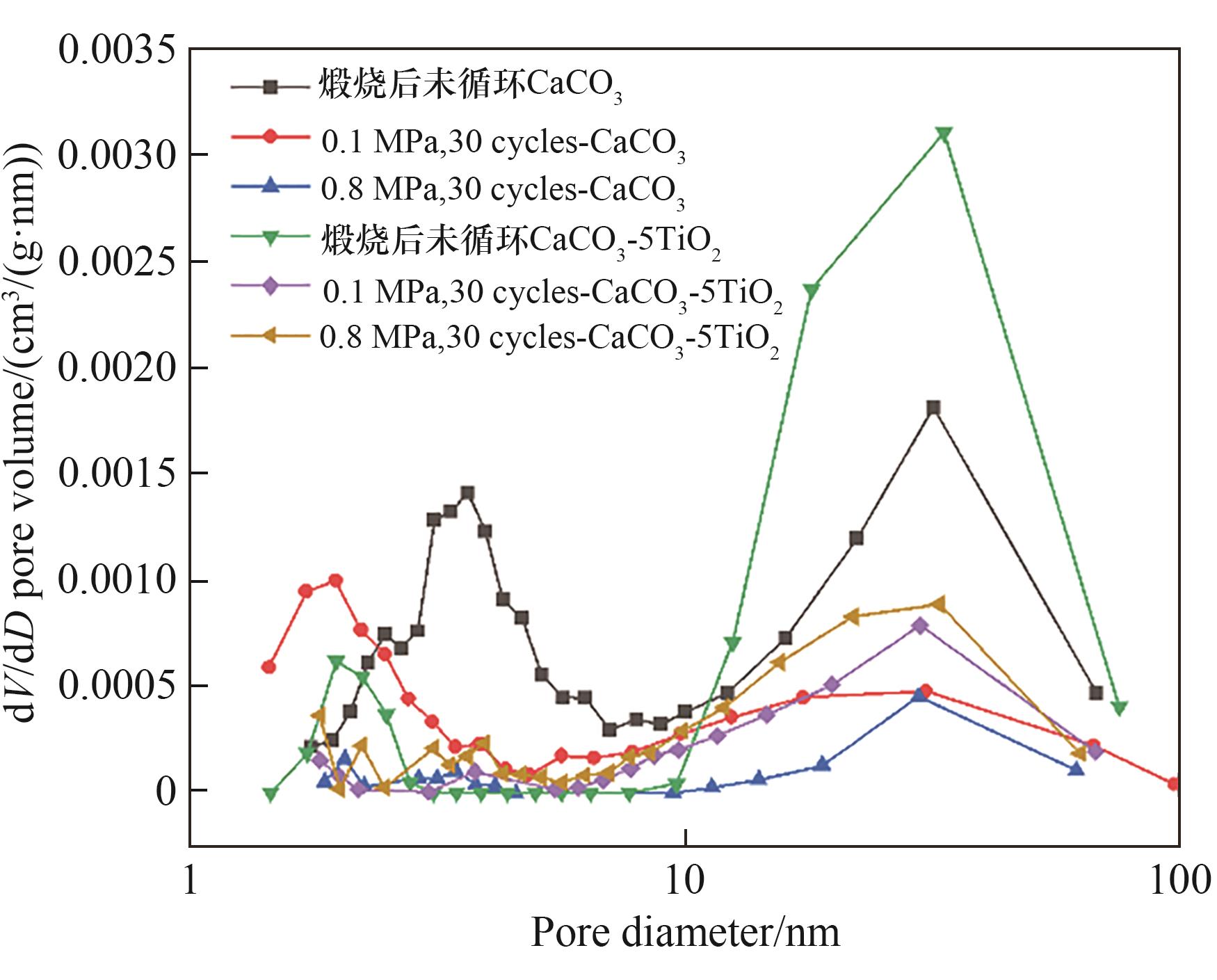
图15 加压/常压碳酸化循环储热中煅烧CaCO3和煅烧CaCO3-5TiO2的孔径分布
Fig.15 Pore size distribution of calcined CaCO3 and calcined CaCO3-5TiO2 in pressurized/atmospheric carbonated heat storage cycles
| 1 | 潘怡, 刘敦禹, 金晶. CaO/CaCO3储能系统材料设计研究进展[J]. 动力工程学报, 2024, 44(5): 770-781. |
| Pan Y, Liu D Y, Jin J. Research progress on material design of CaO/CaCO3 energy storage system[J]. Journal of Chinese Society of Power Engineering, 2024, 44 (5): 770-781. | |
| 2 | Han R, Xing S, Wu X Q, et al. Relevant influence of alkali carbonate doping on the thermochemical energy storage of Ca-based natural minerals during CaO/CaCO3 cycles[J]. Renewable Energy, 2022, 181: 267-277. |
| 3 | Liu Y N, Deng S, Zhao R K, et al. Energy-saving pathway exploration of CCS integrated with solar energy: a review of innovative concepts[J]. Renewable and Sustainable Energy Reviews, 2017, 77: 652-669. |
| 4 | Wang X R, Liu X L, Zheng H B, et al. Hierarchically doping calcium carbonate pellets for directly solar-driven high-temperature thermochemical energy storage[J]. Solar Energy, 2023, 251: 197-207. |
| 5 | Cheng K L, Li J H, Yu J C, et al. Novel thermoelectric generator enhanced supercritical carbon dioxide closed-brayton-cycle power generation systems: performance comparison and configuration optimization[J]. Energy, 2023, 284: 129368. |
| 6 | Ortiz C, Romano M C, Valverde J M, et al. Process integration of Calcium-looping thermochemical energy storage system in concentrating solar power plants[J]. Energy, 2018, 155: 535-551. |
| 7 | 郑玉圆, 葛志伟, 韩翔宇, 等. 中高温钙基材料热化学储热的研究进展与展望[J]. 化工学报, 2023, 74(8): 3171-3192. |
| Zheng Y Y, Ge Z W, Han X Y, et al. Progress and prospect of medium and high temperature thermochemical energy storage of calcium-based materials[J]. CIESC Journal, 2023, 74(8): 3171-3192. | |
| 8 | 孙健, 柏生斌, 周子健, 等. CaCO3/CaO复合材料热化学储能特性研究进展[J]. 华中科技大学学报(自然科学版), 2023, 51(1): 123-132. |
| Sun J, Bai S B, Zhou Z J, et al. Research progress on thermochemical energy storage properties of CaCO3/CaO compound composites[J]. Journal of Huazhong University of Science and Technology (Natural Science Edition), 2023, 51(1): 123-132. | |
| 9 | Cannone S F, Stendardo S, Lanzini A. Solar-powered Rankine cycle assisted by an innovative calcium looping process as an energy storage system[J]. Industrial & Engineering Chemistry Research, 2020, 59(15): 6977-6993. |
| 10 | Edwards S E B, Materić V. Calcium looping in solar power generation plants[J]. Solar Energy, 2012, 86(9): 2494-2503. |
| 11 | Ortiz D C, Valverde M J M, Chacartegui R, et al. Carbonation of limestone derived CaO for thermochemical energy storage: from kinetics to process integration in concentrating solar plants[J]. ACS Sustainable Chemistry and Engineering, 2018, 6(5): 6404-6417. |
| 12 | Chen X Y, Jin X G, Ling X, et al. Indirect integration of thermochemical energy storage with the recompression supercritical CO2 Brayton cycle[J]. Energy, 2020, 209: 118452. |
| 13 | Rodrigues D, Pinheiro C I C, Filipe R M, et al. Optimization of an improved calcium-looping process for thermochemical energy storage in concentrating solar power plants[J]. Journal of Energy Storage, 2023, 72:108199. |
| 14 | Nie F L, Ma T Z, Zhang Q Q, et al. Heat storage and release characteristics of a prototype CaCO3/CaO thermochemical energy storage system based on a novel fluidized bed solar reactor[J]. Journal of Cleaner Production, 2024, 450: 142003. |
| 15 | Li C L, Li Y J, Zhang C X, et al. Ca3B2O6-modified papermaking white mud for CaCO3/CaO thermochemical energy storage[J]. Chemical Engineering Journal, 2023, 461: 142096. |
| 16 | Liu X L, Yuan C J, Zheng H B, et al. Synergy of Li2CO3 promoters and Al-Mn-Fe stabilizers in CaCO3 pellets enables efficient direct solar-driven thermochemical energy storage[J]. Materials Today Energy, 2022, 30: 101174. |
| 17 | Huang X K, Ma X T, Li J, et al. Enhancement effects of hydrolysable/soluble Al-type dopants on the efficiency of CaO/CaCO3 thermochemical energy storage[J]. Chemical Engineering Journal, 2024, 490: 151555. |
| 18 | Sun H, Li Y J, Bian Z G, et al. Thermochemical energy storage performances of Ca-based natural and waste materials under high pressure during CaO/CaCO3 cycles[J].Energy Conversion and Management, 2019, 197: 111885. |
| 19 | Li B Y, Li Y J, Sun H, et al. Thermochemical heat storage performance of CaO pellets fabricated by extrusion-spheronization under harsh calcination conditions[J]. Energy & Fuels, 2020, 34(5): 6462-6473. |
| 20 | Xu Y F, Li Y J, Zhang C X, et al. High-temperature thermochemical heat storage performance of CaO honeycombs during CaO/CaCO3 cycles[J]. Energy & Fuels, 2021, 35(20): 16882-16893. |
| 21 | Pang H, Xu H R, Sun A W, et al. Characteristics of MgO-based sorbents for CO2 capture at elevated temperature and pressure[J]. Applied Surface Science, 2022, 598: 153852. |
| 22 | Khosa A A, Xu T X, Xia B Q, et al. Technological challenges and industrial applications of CaCO3/CaO based thermal energy storage system—a review[J]. Solar Energy, 2019, 193:618-636. |
| 23 | Tian X K, Lin S C, Yan J, et al. Sintering mechanism of calcium oxide/calcium carbonate during thermochemical heat storage process[J]. Chemical Engineering Journal, 2022, 428: 131229. |
| 24 | Khosa A A, Zhao C Y. Heat storage and release performance analysis of CaCO3/CaO thermal energy storage system after doping nano silica[J]. Solar Energy, 2019, 188: 619-630. |
| 25 | Wang K, Gu F, Clough P T, et al. Porous MgO-stabilized CaO-based powders/pellets via a citric acid-based carbon template for thermochemical energy storage in concentrated solar power plants[J]. Chemical Engineering Journal, 2020, 390: 124163. |
| 26 | Khosa A A, Yan J, Zhao C Y. Investigating the effects of ZnO dopant on the thermodynamic and kinetic properties of CaCO3/CaO TCES system[J]. Energy, 2021, 215: 119132. |
| 27 | Han R, Gao J H, Wei S Y, et al. High-performance CaO-based composites synthesized using a space-confined chemical vapor deposition strategy for thermochemical energy storage[J]. Solar Energy Materials and Solar Cells, 2020, 206: 110346. |
| 28 | Xu T X, Tian X K, Khosa A A, et al. Reaction performance of CaCO3/CaO thermochemical energy storage with TiO2 dopant and experimental study in a fixed-bed reactor[J]. Energy, 2021, 236: 121451. |
| 29 | Hao Y J, Tian L G, Duan E H, et al. Low-temperature methane oxidation triggered by peroxide radicals over noble-metal-free MgO catalyst[J]. ACS Applied Materials & Interfaces, 2020, 12(19): 21761-21771. |
| 30 | Liu H, Pan F F, Wu S F. The grain growth mechanism of nano-CaO regenerated by nano-CaCO3 in calcium looping [J]. RSC Advances, 2019, 9(46): 26949-26955. |
| 31 | Li Y J, Zhao C S, Chen H C, et al. Modified CaO-based sorbent looping cycle for CO2 mitigation[J]. Fuel, 2009, 88(4): 697-704. |
| 32 | Hu Y, Liu W, Chen H, et al. Screening of inert solid supports for CaO-based sorbents for high temperature CO2 capture[J]. Fuel, 2016, 181: 199-206. |
| 33 | Bai S B, Sun J, Zhou Z J, et al. Structurally improved, TiO2-incorporated, CaO-based pellets for thermochemical energy storage in concentrated solar power plants[J]. Solar Energy Materials and Solar Cells, 2021, 226: 111076. |
| 34 | 刘昊, 吴素芳. 固相反应对纳米钙基CO2吸附剂自激活的影响[J]. 高校化学工程学报, 2021, 35(1): 57-64. |
| Liu H, Wu S F. Effect of solid-phase reaction on self-reactivation of nano-calcium-based CO2 adsorbents[J]. Journal of Chemical Engineering of Chinese Universities, 2021, 35(1): 57-64. | |
| 35 | Benitez-Guerrero M, Valverde J M, Sanchez-Jimenez P E, et al. Calcium-looping performance of mechanically modified Al2O3-CaO composites for energy storage and CO2 capture[J]. Chemical Engineering Journal, 2018, 334: 2343-2355. |
| [1] | 王三龙, 王跃霖, 曹宇. 基于相异质结的高效无机钙钛矿太阳能电池的性能研究[J]. 化工学报, 2025, 76(3): 1346-1352. |
| [2] | 张履胜, 王治红, 柳青, 李雪雯, 谭仁敏. 液-液相变吸收剂捕集二氧化碳研究进展[J]. 化工学报, 2025, 76(3): 933-950. |
| [3] | 杨晋宁, 王卫凡, 徐冬, 刘毅, 翁小涵, 原野, 王志. 工业烟道气碳捕集膜技术放大研究进展[J]. 化工学报, 2025, 76(2): 504-518. |
| [4] | 姚佳逸, 张东辉, 唐忠利, 李文彬. 基于二级双回流的变压吸附捕碳工艺研究[J]. 化工学报, 2025, 76(2): 744-754. |
| [5] | 宫政, 高秀鲁, 赵玲, 胡冬冬. 超临界CO2发泡PBAT/PLA复合材料及其形状记忆性能[J]. 化工学报, 2025, 76(2): 888-896. |
| [6] | 贾晶宇, 孔德齐, 沈圆辉, 张东辉, 李文彬, 唐忠利. 合成氨反应器尾气变压吸附氨分离工艺的模拟与分析[J]. 化工学报, 2025, 76(2): 718-730. |
| [7] | 王佳欣, 韦艳红, 农顺洋, 熊艳舒, 李楣, 李文. 多重量子化学理论计算解析多胺修饰壳聚糖气凝胶吸附美拉德色素分子机制[J]. 化工学报, 2025, 76(1): 107-119. |
| [8] | 董新宇, 边龙飞, 杨怡怡, 张宇轩, 刘璐, 王腾. 冷却条件下倾斜上升管S-CO2流动与传热特性研究[J]. 化工学报, 2024, 75(S1): 195-205. |
| [9] | 陈彦霖, 周爱国, 郑家乐, 杨川箬, 葛天舒. 载体对于胺浸渍类DAC吸附剂性能的影响[J]. 化工学报, 2024, 75(S1): 217-222. |
| [10] | 王新月, 徐小虎, 张海洋, 尹春华. 维生素A醋酸酯/环糊精包合及性质研究[J]. 化工学报, 2024, 75(S1): 321-328. |
| [11] | 赵焕娟, 包颖昕, 于康, 刘婧, 钱新明. 多元组分爆轰不稳定性定量实验研究[J]. 化工学报, 2024, 75(S1): 339-348. |
| [12] | 任冠宇, 张义飞, 李新泽, 杜文静. 翼型印刷电路板式换热器流动传热特性数值研究[J]. 化工学报, 2024, 75(S1): 108-117. |
| [13] | 杨勇, 祖子轩, 李煜坤, 王东亮, 范宗良, 周怀荣. T型圆柱形微通道内CO2碱液吸收数值模拟[J]. 化工学报, 2024, 75(S1): 135-142. |
| [14] | 裴蓓, 郝治斌, 徐天祥, 钟子琪, 李瑞, 贾冲, 段玉龙. 表面活性剂对含盐双流体细水雾灭火效能的影响[J]. 化工学报, 2024, 75(9): 3369-3378. |
| [15] | 唐宇昊, 张迎迎, 赵智伟, 鲁梦悦, 张飞飞, 王小青, 杨江峰. 弱极性超微孔Sc/In-CPM-66A用于CH4/N2吸附分离性能[J]. 化工学报, 2024, 75(9): 3210-3220. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号