CIESC Journal ›› 2020, Vol. 71 ›› Issue (9): 4058-4070.DOI: 10.11949/0438-1157.20200460
• Reviews and monographs • Previous Articles Next Articles
Hutao GAO( ),Xiaolin SHEN,Xinxiao SUN,Jia WANG(
),Xiaolin SHEN,Xinxiao SUN,Jia WANG( ),Qipeng YUAN(
),Qipeng YUAN( )
)
Received:2020-04-30
Revised:2020-05-21
Online:2020-09-05
Published:2020-09-05
Contact:
Jia WANG,Qipeng YUAN
通讯作者:
王佳,袁其朋
作者简介:高虎涛(1997—),男,硕士研究生,基金资助:CLC Number:
Hutao GAO, Xiaolin SHEN, Xinxiao SUN, Jia WANG, Qipeng YUAN. Metabolic engineering strategies in biosynthesis of amino acids and their derivatives[J]. CIESC Journal, 2020, 71(9): 4058-4070.
高虎涛, 申晓林, 孙新晓, 王佳, 袁其朋. 代谢工程调控策略在生物合成氨基酸及其衍生物中的应用[J]. 化工学报, 2020, 71(9): 4058-4070.
Add to citation manager EndNote|Ris|BibTeX

Fig.1 Application of carbon source efficient utilization strategy(a) partial biosynthetic pathway of L-lysine in Corynebacterium glutamicum; (b) biosynthetic pathway of L-3,4-dihydroxyphenylalanine in E. coli;(c) biosynthetic pathway of S-adenosylmethionine in B. amyloliquefaciens; (d) biosynthetic pathway of 4-hydroxyisoleucine in E. coli
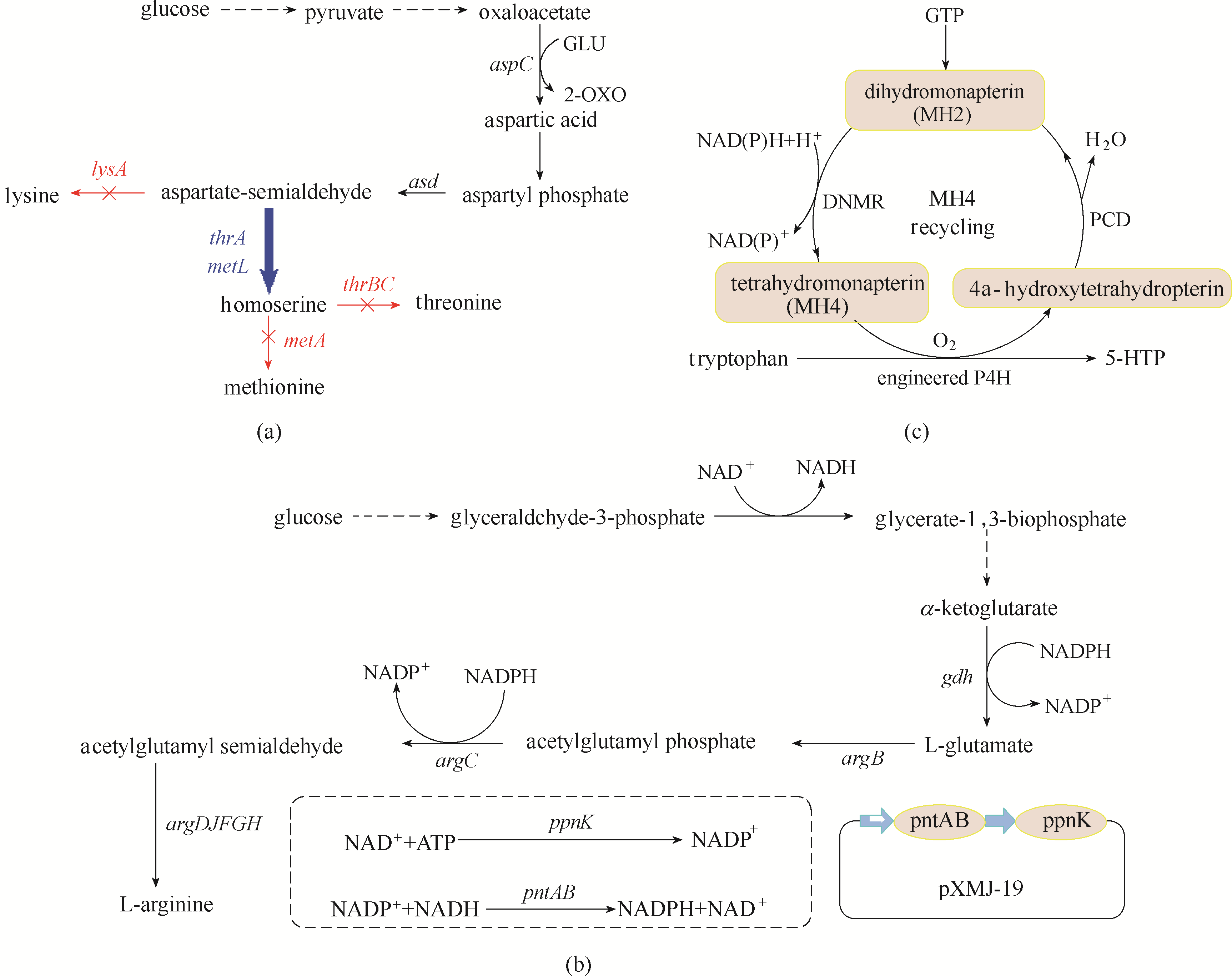
Fig.2 Application of speed-limiting step adjustment strategy(a) the metabolic pathway of homoserine production in E. coli; (b) the metabolic pathway of L-arginine in C. glutamicum; (c) the metabolic pathway of 5-hydroxytryptophan in E. coli

Fig.3 Application of carbon flux regulation strategy(a) the metabolic pathway of L-ornithine in C. glutamicum; (b) production and metabolism pathway of β-ornithine in E. coli
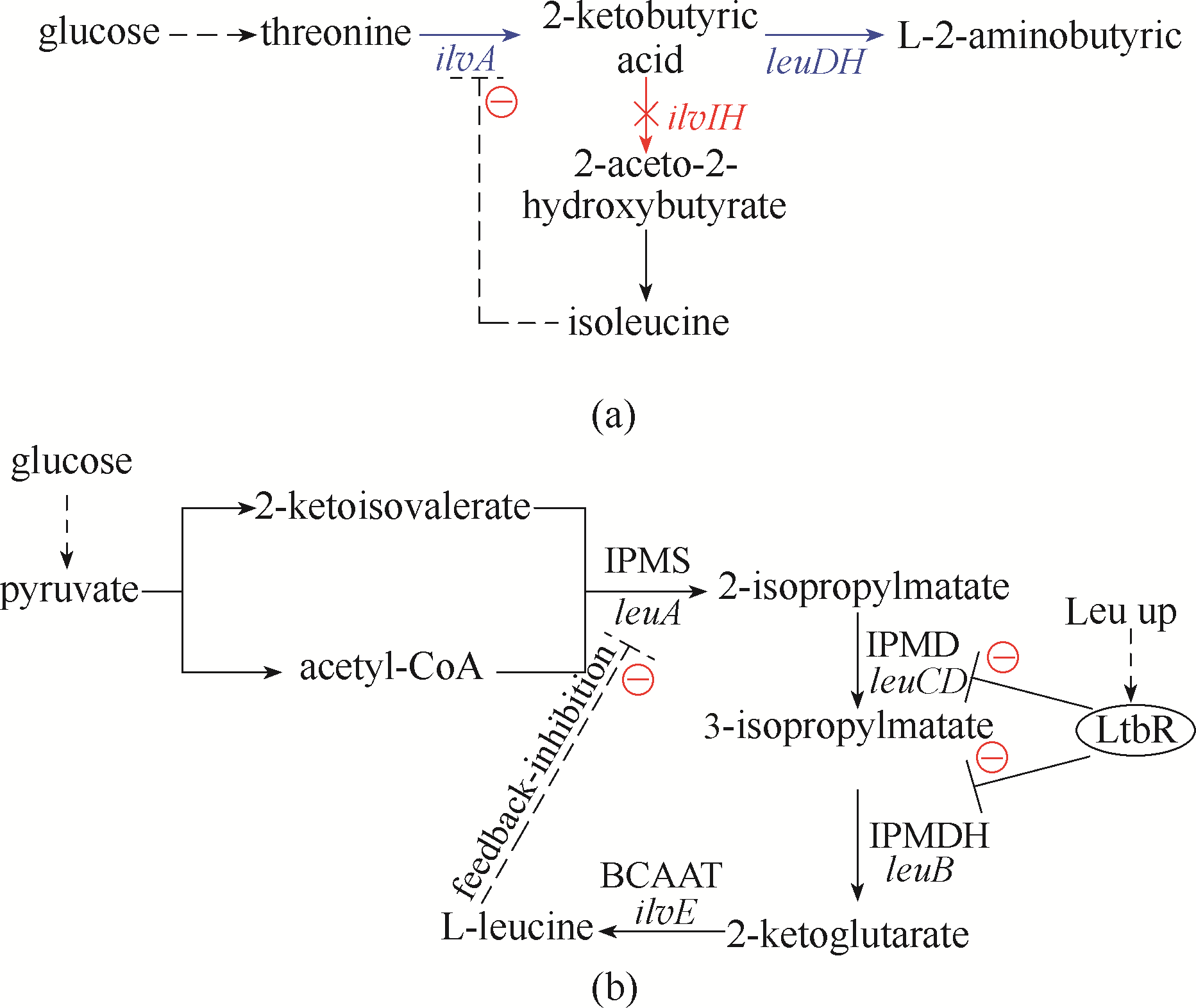
Fig.4 Application of transcription regulation and feedback inhibition regulation strategies(a) the metabolic pathway of 2-aminobutyric acid in E. coli; (b) L-leucine production and metabolic pathways in C. glutamicum
| 代谢工程策略 | 氨基酸及其衍生物 | 功能 | 工程菌 | 产量 | 文献 |
|---|---|---|---|---|---|
| 碳源的高效利用 | 左旋鸟氨酸 | 一种非蛋白质氨基酸,能够加速伤口愈合并能有效维持心脏健康 | 谷氨酸棒状杆菌S9114 | 43.6 g/L | [ |
| L-亮氨酸 | 在刺激肌肉蛋白质合成和葡萄糖稳态中具有重要作用 | 谷氨酸棒状杆菌 | 23.7 g/L | [ | |
| L-赖氨酸 | 促进人体的发育、增强免疫力,并有提高中枢神经功能的作用 | 谷氨酸棒状杆菌 ZL-19 | 201.6 g/L | [ | |
| L-3,4-二羟基苯丙氨酸 | 用于治疗帕金森氏疾病类药物的主要前体 | 大肠杆菌 | 1.51 g/L | [ | |
| 苯丙氨酸 | 用于生产人造甜味剂阿斯巴甜 | 大肠杆菌 | 1.4 g/L | [ | |
| S-腺苷甲硫氨酸 | 被用作预防和治疗骨关节炎的功能性营养品或药物 | 解淀粉芽孢杆菌 | 107.47mg/L | [ | |
| 4-羟基异亮氨酸 | 在治疗和预防2-型糖尿病中有广阔应用前景 | 大肠杆菌 | 24.1 g/L | [ | |
| 限速步骤的调节 | 高丝氨酸 | 诱导免疫系统,增强植物对疾病的抵抗力 | 大肠杆菌W3110 | 1.20 g/L | [ |
| L-高丙氨酸 | 抗癫痫药物左乙拉西坦和溴拉西坦的直接前体 | 大肠杆菌 | 5.4 g/L | [ | |
| L-精氨酸 | 具有舒张因子的作用,临床上用于舒张和扩张血管 | 谷氨酸棒状杆菌 | 61.13 g/L | [ | |
| L-赖氨酸 | 促进人体的发育、增强免疫力,并有提高中枢神经功能的作用 | 谷氨酸棒状杆菌 | 8.76 g/L | [ | |
| 5-羟基色氨酸 | 用于治疗和缓解抑郁症,有效治疗失眠和慢性头痛 | 大肠杆菌 | 1.11 g/L | [ | |
| 碳通量的调节 | L-鸟氨酸 | 用于治疗肝脏疾病和创伤,在肝脏保护中起着有效的作用 | 谷氨酸棒状杆菌 | 16 g/L | [ |
| 左旋肉碱 | 用于治疗功能障碍疾病 | 大肠杆菌 | 61.3 g/L | [ | |
| β-丙氨酸 | 生物体中泛酸生产的主要前体 | 大肠杆菌 | 43.12 g/L | [ | |
| 5-氨基戊酸酯 | 生产尼龙的结构单元 | 谷氨酸棒状杆菌 | 28 g/L | [ | |
| 5-氨基乙酰丙酸 | 用于多种癌症的光动力学诊断和治疗中 | 谷氨酸棒状杆菌 | 2.06 g/L | [ | |
| 转录调节与反馈抑制调节 | L-酪氨酸 | 食品和饲料添加剂 | 酿酒酵母 | 36.2 mg/L | [ |
| 2-氨基丁酸 | 抗结核性乙胺丁醇的重要前体 | 大肠杆菌 | 5.39 g/L | [ | |
| L-精氨酸 | 在食品和补充保健食品,制药和化妆品行业中有重要应用 | 谷氨酸棒状杆菌 | 61.9 g/L | [ | |
| L-亮氨酸 | 在刺激肌肉蛋白质合成和葡萄糖稳态中具有重要作用 | 谷氨酸棒状杆菌 | 5.7 g/L | [ | |
| L-苏氨酸 | 广泛用于食品、制药和化妆品行业 | 大肠杆菌 | 26.0 g/L | [ | |
| 转运系统的调节 | L-异亮氨酸 | 形成血红蛋白必需氨基酸,在调节血糖与能量方面起到关键作用 | 谷氨酸棒状杆菌 | 26.8 g/L | [ |
| 高丝氨酸 | 诱导免疫系统,增强植物对疾病的抵抗力 | 大肠杆菌W3110 | 39.54 g/L | [ | |
| L-半胱氨酸 | 在提高动物的免疫调节和促进动物生长发育方面起着重要作用 | 大肠杆菌 | 634.4 mg/L | [ | |
| L-蛋氨酸 | 用于脂肪肝,以及酒精等引起的肝损害 | 大肠杆菌 | 593.5 mg/L | [ | |
| L-瓜氨酸 | 对于保持肌肉和肝脏健康有重要作用 | 谷氨酸棒状杆菌 | 21 g/L | [ | |
| L-苏氨酸 | 促进人体发育和抗脂肪肝的药用效能 | 谷氨酸棒状杆菌 | 8.1 g/L | [ |
Table 1 Summary of amino acids and their derivatives involved in this article
| 代谢工程策略 | 氨基酸及其衍生物 | 功能 | 工程菌 | 产量 | 文献 |
|---|---|---|---|---|---|
| 碳源的高效利用 | 左旋鸟氨酸 | 一种非蛋白质氨基酸,能够加速伤口愈合并能有效维持心脏健康 | 谷氨酸棒状杆菌S9114 | 43.6 g/L | [ |
| L-亮氨酸 | 在刺激肌肉蛋白质合成和葡萄糖稳态中具有重要作用 | 谷氨酸棒状杆菌 | 23.7 g/L | [ | |
| L-赖氨酸 | 促进人体的发育、增强免疫力,并有提高中枢神经功能的作用 | 谷氨酸棒状杆菌 ZL-19 | 201.6 g/L | [ | |
| L-3,4-二羟基苯丙氨酸 | 用于治疗帕金森氏疾病类药物的主要前体 | 大肠杆菌 | 1.51 g/L | [ | |
| 苯丙氨酸 | 用于生产人造甜味剂阿斯巴甜 | 大肠杆菌 | 1.4 g/L | [ | |
| S-腺苷甲硫氨酸 | 被用作预防和治疗骨关节炎的功能性营养品或药物 | 解淀粉芽孢杆菌 | 107.47mg/L | [ | |
| 4-羟基异亮氨酸 | 在治疗和预防2-型糖尿病中有广阔应用前景 | 大肠杆菌 | 24.1 g/L | [ | |
| 限速步骤的调节 | 高丝氨酸 | 诱导免疫系统,增强植物对疾病的抵抗力 | 大肠杆菌W3110 | 1.20 g/L | [ |
| L-高丙氨酸 | 抗癫痫药物左乙拉西坦和溴拉西坦的直接前体 | 大肠杆菌 | 5.4 g/L | [ | |
| L-精氨酸 | 具有舒张因子的作用,临床上用于舒张和扩张血管 | 谷氨酸棒状杆菌 | 61.13 g/L | [ | |
| L-赖氨酸 | 促进人体的发育、增强免疫力,并有提高中枢神经功能的作用 | 谷氨酸棒状杆菌 | 8.76 g/L | [ | |
| 5-羟基色氨酸 | 用于治疗和缓解抑郁症,有效治疗失眠和慢性头痛 | 大肠杆菌 | 1.11 g/L | [ | |
| 碳通量的调节 | L-鸟氨酸 | 用于治疗肝脏疾病和创伤,在肝脏保护中起着有效的作用 | 谷氨酸棒状杆菌 | 16 g/L | [ |
| 左旋肉碱 | 用于治疗功能障碍疾病 | 大肠杆菌 | 61.3 g/L | [ | |
| β-丙氨酸 | 生物体中泛酸生产的主要前体 | 大肠杆菌 | 43.12 g/L | [ | |
| 5-氨基戊酸酯 | 生产尼龙的结构单元 | 谷氨酸棒状杆菌 | 28 g/L | [ | |
| 5-氨基乙酰丙酸 | 用于多种癌症的光动力学诊断和治疗中 | 谷氨酸棒状杆菌 | 2.06 g/L | [ | |
| 转录调节与反馈抑制调节 | L-酪氨酸 | 食品和饲料添加剂 | 酿酒酵母 | 36.2 mg/L | [ |
| 2-氨基丁酸 | 抗结核性乙胺丁醇的重要前体 | 大肠杆菌 | 5.39 g/L | [ | |
| L-精氨酸 | 在食品和补充保健食品,制药和化妆品行业中有重要应用 | 谷氨酸棒状杆菌 | 61.9 g/L | [ | |
| L-亮氨酸 | 在刺激肌肉蛋白质合成和葡萄糖稳态中具有重要作用 | 谷氨酸棒状杆菌 | 5.7 g/L | [ | |
| L-苏氨酸 | 广泛用于食品、制药和化妆品行业 | 大肠杆菌 | 26.0 g/L | [ | |
| 转运系统的调节 | L-异亮氨酸 | 形成血红蛋白必需氨基酸,在调节血糖与能量方面起到关键作用 | 谷氨酸棒状杆菌 | 26.8 g/L | [ |
| 高丝氨酸 | 诱导免疫系统,增强植物对疾病的抵抗力 | 大肠杆菌W3110 | 39.54 g/L | [ | |
| L-半胱氨酸 | 在提高动物的免疫调节和促进动物生长发育方面起着重要作用 | 大肠杆菌 | 634.4 mg/L | [ | |
| L-蛋氨酸 | 用于脂肪肝,以及酒精等引起的肝损害 | 大肠杆菌 | 593.5 mg/L | [ | |
| L-瓜氨酸 | 对于保持肌肉和肝脏健康有重要作用 | 谷氨酸棒状杆菌 | 21 g/L | [ | |
| L-苏氨酸 | 促进人体发育和抗脂肪肝的药用效能 | 谷氨酸棒状杆菌 | 8.1 g/L | [ |
| 1 | Choi S, Song C W, Shin J H, et al. Biorefineries for the production of top building block chemicals and their derivatives[J]. Metabolic Engineering, 2015, 28: 223-239. |
| 2 | Piao X, Wang L, Lin B, et al. Metabolic engineering of Escherichia coli for production of L-aspartate and its derivative β-alanine with high stoichiometric yield[J]. Metabolic Engineering, 2019, 54: 244-254. |
| 3 | Mu W, Zhang T, Jiang B. An overview of biological production of L-theanine[J]. Biotechnology Advances, 2015, 33(3): 335-342. |
| 4 | Wang H, Liu W, Shi F, et al. Metabolic pathway engineering for high-level production of 5-hydroxytryptophan in Escherichia coli[J]. Metabolic Engineering, 2018, 48: 279-287. |
| 5 | Song Y, Li J, Shin H D, et al. Biotechnological production of alpha-keto acids: current status and perspectives[J]. Bioresource Technology, 2016, 219: 716-724. |
| 6 | Wendisch V F.Metabolic engineering advances and prospects for amino acid production[J].Metabolic Engineering, 2020, 58: 17-34. |
| 7 | D􀆳este M, Alvarado-Morales M, Angelidaki I. Amino acids production focusing on fermentation technologies — a review[J]. Biotechnology Advances,2018, 36(1): 14-25. |
| 8 | Farmer W R, Liao J C. Progress in metabolic engineering[J]. Current Opinion in Biotechnology, 1996, 7(2): 198-204. |
| 9 | Kusumoto I. Industrial production of L-glutamine[J]. The Journal of Nutrition, 2001, 131(9): 2552S-2555S. |
| 10 | Park J H, Lee S Y. Towards systems metabolic engineering of microorganisms for amino acid production[J]. Current Opinion in Biotechnology, 2008, 19(5): 454-460. |
| 11 | Chae T U, Choi S Y, Kim J W, et al. Recent advances in systems metabolic engineering tools and strategies[J]. Current Opinion in Biotechnology, 2017, 47: 67-82. |
| 12 | Kohlstedt M, Becker J, Wittmann C. Metabolic fluxes and beyond-systems biology understanding and engineering of microbial metabolism[J]. Applied Microbiology and Biotechnology, 2010, 88(5): 1065-1075. |
| 13 | Lee J W, Na D, Park J M, et al. Systems metabolic engineering of microorganisms for natural and non-natural chemicals[J]. Nature Chemical Biology, 2012, 8(6): 536-546. |
| 14 | Chen Z, Huang J, Wu Y, et al. Metabolic engineering of Corynebacterium glutamicum for the production of 3-hydroxypropionic acid from glucose and xylose[J]. Metabolic Engineering, 2017, 39: 151-158. |
| 15 | Kogure T, Kubota T, Suda M, et al. Metabolic engineering of Corynebacterium glutamicum for shikimate overproduction by growth-arrested cell reaction[J]. Metabolic Engineering, 2016, 38: 204-216. |
| 16 | Yao R, Shimizu K. Recent progress in metabolic engineering for the production of biofuels and biochemicals from renewable sources with particular emphasis on catabolite regulation and its modulation[J]. Process Biochemistry, 2013, 48(9): 1409-1417. |
| 17 | Xu J Z, Yu H B, Han M, et al. Metabolic engineering of glucose uptake systems in Corynebacterium glutamicum for improving the efficiency of L-lysine production[J]. Journal of Industrial Microbiology & Biotechnology, 2019, 46(7): 937-949. |
| 18 | Lindner S N, Knebel S, Pallerla S R, et al. Cg2091 encodes a polyphosphate/ATP-dependent glucokinase of Corynebacterium glutamicum[J]. Applied Microbiology and Biotechnology, 2010, 87(2): 703-713. |
| 19 | Lindner S N, Seibold G M, Henrich A, et al. Phosphotransferase system-independent glucose utilization in Corynebacterium glutamicum by inositol permeases and glucokinases[J]. Applied and Environmental Microbiology, 2011, 77(11): 3571-3581. |
| 20 | Zhang B, Gao G, Chu X H, et al. Metabolic engineering of Corynebacterium glutamicum S9114 to enhance the production of L-ornithine driven by glucose and xylose[J]. Bioresource Technology, 2019, 284: 204-213. |
| 21 | Klaffl S, Brocker M, Kalinowski J, et al. Complex regulation of the phosphoenolpyruvate carboxykinase gene pck and characterization of its GntR-type regulator IolR as a repressor of myo-inositol utilization genes in Corynebacterium glutamicum[J]. Journal of Bacteriology, 2013, 195(18): 4283-4296. |
| 22 | Vogt M, Haas S, Klaffl S, et al. Pushing product formation to its limit: metabolic engineering of Corynebacterium glutamicum for L-leucine overproduction[J]. Metabolic Engineering, 2014, 22: 40-52. |
| 23 | Fordjour E, Adipah F K, Zhou S, et al. Metabolic engineering of Escherichia coli BL21 (DE3) for de novo production of L-DOPA from D-glucose[J]. Microbial Cell Factories, 2019, 18(1): 74. |
| 24 | Patnaik R, Liao J C. Engineering of Escherichia coli central metabolism for aromatic metabolite production with near theoretical yield[J]. Applied and Environmental Microbiology, 1994, 60(11): 3903. |
| 25 | Báez J L, Bolívar F, Gosset G. Determination of 3-deoxy-D-arabino-heptulosonate 7-phosphate productivity and yield from glucose in Escherichia coli devoid of the glucose phosphotransferase transport system[J]. Biotechnology and Bioengineering, 2001, 73(6): 530-535. |
| 26 | Muñoz A J, Hernández-Chávez G, De Anda R, et al. Metabolic engineering of Escherichia coli for improving L-3,4-dihydroxyphenylalanine (L-DOPA) synthesis from glucose[J]. Journal of Industrial Microbiology & Biotechnology, 2011, 38(11): 1845. |
| 27 | Zou X, Guo L, Huang L, et al. Pathway construction and metabolic engineering for fermentative production of β-alanine in Escherichia coli[J]. Applied Microbiology and Biotechnology, 2020, 104(6): 2545-2559. |
| 28 | Glick B R. Metabolic load and heterologous gene expression[J]. Biotechnology Advances, 1995, 13(2): 247-261. |
| 29 | Koma D, Kishida T, Yamanaka H, et al. Escherichia coli chromosome-based T7-dependent constitutive overexpression system and its application to generating a phenylalanine producing strain[J]. Journal of Bioscience and Bioengineering, 2018, 126(5): 586-595. |
| 30 | Li Z J, Hong P H, Da Y Y, et al. Metabolic engineering of Escherichia coli for the production of L-malate from xylose[J]. Metabolic Engineering, 2018, 48: 25-32. |
| 31 | Ruan L, Li L, Zou D, et al. Metabolic engineering of Bacillus amyloliquefaciens for enhanced production of S-adenosylmethionine by coupling of an engineered S-adenosylmethionine pathway and the tricarboxylic acid cycle[J]. Biotechnology for Biofuels, 2019, 12(1): 211. |
| 32 | Smirnov S V, Kodera T, Samsonova N N, et al. Metabolic engineering of Escherichia coli to produce (2S, 3R, 4S)-4-hydroxyisoleucine[J]. Applied Microbiology and Biotechnology, 2010, 88(3): 719-726. |
| 33 | Frost J W. Altered glucose transport and shikimate pathway product yields in E.coli[J]. Biotechnology Progress, 2003, 19(5): 1450-1459. |
| 34 | Berry A. Improving production of aromatic compounds in Escherichia coli by metabolic engineering[J]. Trends in Biotechnology, 1996, 14(7): 250-256. |
| 35 | Marienhagen J. Metabolic engineering of Corynebacterium glutamicum for the metabolization of methanol[J]. Appl. Environ. Microbiol., 2015, 81(6): 2215-2225. |
| 36 | Liu H, Marsafari M, Wang F, et al. Engineering acetyl-CoA metabolic shortcut for eco-friendly production of polyketides triacetic acid lactone in Yarrowia lipolytica[J]. Metabolic Engineering, 2019, 56: 60-68. |
| 37 | Jansson C. Metabolic engineering of Cyanobacteria for direct conversion of CO2 to hydrocarbon biofuels[M]. Lüttge U, Beyschlag W, Büdel B, et al. Progress in Botany 73. Berlin, Heidelberg: Springer, 2012: 81-93. |
| 38 | Nguyen D T N, Lee O K, Lim C, et al. Metabolic engineering of type Ⅱ methanotroph, Methylosinus trichosporium OB3b, for production of 3-hydroxypropionic acid from methane via a malonyl-CoA reductase-dependent pathway[J]. Metabolic Engineering, 2020, 59: 142-150. |
| 39 | Gleizer S, Ben Nissan R, Bar On Y M, et al. Conversion of Escherichia coli to generate all biomass carbon from CO2[J]. Cell, 2019, 179(6): 1255-1263. |
| 40 | Wu G, Yan Q, Jones J A, et al. Metabolic burden: cornerstones in synthetic biology and metabolic engineering applications[J]. Trends in Biotechnology, 2016, 34(8): 652-664. |
| 41 | Li H, Wang B, Zhu L, et al. Metabolic engineering of Escherichia coli W3110 for L-homoserine production[J]. Process Biochemistry, 2016, 51(12): 1973-1983. |
| 42 | Zhang K, Li H, Cho K M, et al. Expanding metabolism for total biosynthesis of the nonnatural amino acid L-homoalanine[J]. Proceedings of the National Academy of Sciences, 2010, 107(14): 6234. |
| 43 | Ginesy M, Belotserkovsky J, Enman J, et al. Metabolic engineering of Escherichia coli for enhanced arginine biosynthesis[J]. Microbial Cell Factories, 2015, 14(1): 29. |
| 44 | Man Z, Xu M, Rao Z, et al. Systems pathway engineering of Corynebacterium crenatum for improved L-arginine production[J]. Scientific Reports, 2016, 6(1): 28629. |
| 45 | Zhan M, Kan B, Dong J, et al. Metabolic engineering of Corynebacterium glutamicum for improved L-arginine synthesis by enhancing NADPH supply[J]. Journal of Industrial Microbiology & Biotechnology, 2019, 46(1): 45-54. |
| 46 | Bommareddy R R, Chen Z, Rappert S, et al. A de novo NADPH generation pathway for improving lysine production of Corynebacterium glutamicum by rational design of the coenzyme specificity of glyceraldehyde 3-phosphate dehydrogenase[J]. Metabolic Engineering, 2014, 25: 30-37. |
| 47 | Lin Y, Sun X, Yuan Q, et al. Engineering bacterial phenylalanine 4-hydroxylase for microbial synthesis of human neurotransmitter precursor 5-hydroxytryptophan[J]. ACS Synthetic Biology, 2014, 3(7): 497-505. |
| 48 | Pribat A, Blaby I K, Lara Núñez A, et al. FolX and FolM are essential for tetrahydromonapterin synthesis in Escherichia coli and Pseudomonas aeruginosa[J]. J. Bacteriol., 2010, 192(2): 475-482. |
| 49 | Gao W, Sun H X, Xiao H, et al. Combining metabolomics and transcriptomics to characterize tanshinone biosynthesis in Salvia miltiorrhiza[J]. BMC Genomics, 2014, 15(1): 73. |
| 50 | Thiele I, Palsson B. A protocol for generating a high-quality genome-scale metabolic reconstruction[J]. Nature Protocols, 2010, 5(1): 93-121. |
| 51 | Amador Noguez D, Feng X J, Fan J, et al. Systems-level metabolic flux profiling elucidates a complete, bifurcated tricarboxylic acid cycle in Clostridium acetobutylicum[J]. Journal of Bacteriology, 2011, 193(23): 6805. |
| 52 | Venkataramanan K P, Min L, Hou S, et al. Complex and extensive post-transcriptional regulation revealed by integrative proteomic and transcriptomic analysis of metabolite stress response in Clostridium acetobutylicum[J]. Biotechnology for Biofuels, 2015, 8(1): 81. |
| 53 | Xu J Y, Xu Y, Chu X, et al. Protein acylation affects the artificial biosynthetic pathway for pinosylvin production in engineered E. coli[J]. ACS Chemical Biology, 2018, 13(5): 1200-1208. |
| 54 | Yoo M, Nguyen N P T, Soucaille P. Trends in systems biology for the analysis and engineering of Clostridium acetobutylicum metabolism[J]. Trends in Microbiology, 2020, 28(2): 118-140. |
| 55 | Cheah Y E, Xu Y, Sacco S A, et al. Systematic identification and elimination of flux bottlenecks in the aldehyde production pathway of Synechococcus elongatus PCC 7942[J]. Metabolic Engineering, 2020, 60: 56-65. |
| 56 | Maiti M K. Functional characterization of two structurally novel diacylglycerol acyltransferase 2 isozymes responsible for the enhanced production of stearate-rich storage lipid in Candida tropicalis SY005[J]. Plos One, 2014, 9(4): e94472. |
| 57 | Yuzawa S, Mirsiaghi M, Jocic R, et al. Short-chain ketone production by engineered polyketide synthases in Streptomyces albus[J]. Nature Communications, 2018, 9(1): 4569. |
| 58 | Bali A P, Lennox Hvenekilde D, Myling Petersen N, et al. Improved biotin, thiamine, and lipoic acid biosynthesis by engineering the global regulator IscR[J]. Metabolic Engineering, 2020, 60: 97-109. |
| 59 | Mundhada H, Schneider K, Christensen H B, et al. Engineering of high yield production of L-serine in Escherichia coli[J]. Biotechnology and Bioengineering, 2016, 113(4): 807-816. |
| 60 | Zhang B, Yu M, Zhou Y, et al. Systematic pathway engineering of Corynebacterium glutamicum S9114 for L-ornithine production[J]. Microbial Cell Factories, 2017, 16(1): 158. |
| 61 | Arense P, Bernal V, Charlier D, et al. Metabolic engineering for high yielding L(-)-carnitine production in Escherichia coli[J]. Microbial Cell Factories, 2013, 12(1): 56. |
| 62 | Rohles C M, Gießelmann G, Kohlstedt M, et al. Systems metabolic engineering of Corynebacterium glutamicum for the production of the carbon-5 platform chemicals 5-aminovalerate and glutarate[J]. Microbial Cell Factories, 2016, 15(1): 154. |
| 63 | Feng L, Zhang Y, Fu J, et al. Metabolic engineering of Corynebacterium glutamicum for efficient production of 5-aminolevulinic acid[J]. Biotechnology and Bioengineering, 2016, 113(6): 1284-1293. |
| 64 | Miscevic D, Srirangan K, Kefale T, et al. Heterologous production of 3-hydroxyvalerate in engineered Escherichia coli[J]. Metabolic Engineering, 2020, 61: 141-151. |
| 65 | Park J H, Lee K H, Kim T Y, et al. Metabolic engineering of Escherichia coli for the production of L-valine based on transcriptome analysis and in silico gene knockout simulation[J]. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(19): 7797-7802. |
| 66 | Lee K H, Park J H, Kim T Y, et al. Systems metabolic engineering of Escherichia coli for L-threonine production[J]. Molecular Systems Biology, 2007, 3(1): 149. |
| 67 | Fisher M A, Boyarskiy S, Yamada M R, et al. Enhancing tolerance to short-chain alcohols by engineering the Escherichia coli AcrB efflux pump to secrete the non-native substrate n-butanol[J]. ACS Synthetic Biology, 2014, 3(1): 30-40. |
| 68 | Alves T C, Pongratz R L, Zhao X, et al. Integrated, step-wise, mass-isotopomeric flux analysis of the TCA cycle[J]. Cell Metabolism, 2015, 22(5): 936-947. |
| 69 | Quek L E, Krycer J R, Ohno S, et al. Dynamic 13C flux analysis captures the reorganization of adipocyte glucose metabolism in response to insulin[J]. iScience, 2020, 23(2): 100855. |
| 70 | Farmer W R, Liao J C. Precursor balancing for metabolic engineering of lycopene production in Escherichia coli[J]. Biotechnology Progress, 2001, 17(1): 57-61. |
| 71 | Künzler M, Paravicini G, Egli C M, et al. Cloning, primary structure and regulation of the ARO4 gene, encoding the tyrosine-inhibited 3-deoxy-D-arabino-heptulosonate-7-phosphate synthase from Saccharomyces cerevisiae[J]. Gene, 1992, 113(1): 67-74. |
| 72 | Brown J F, Dawes I W. Regulation of chorismate mutase in Saccharomyces cerevisiae[J]. Molecular and General Genetics MGG, 1990, 220(2): 283-288. |
| 73 | Luttik M A H, Vuralhan Z, Suir E, et al. Alleviation of feedback inhibition in Saccharomyces cerevisiae aromatic amino acid biosynthesis: quantification of metabolic impact[J]. Metabolic Engineering, 2008, 10(3): 141-153. |
| 74 | Xu J M, Li J Q, Zhang B, et al. Fermentative production of the unnatural amino acid L-2-aminobutyric acid based on metabolic engineering[J]. Microbial Cell Factories, 2019, 18(1): 43. |
| 75 | Park S H, Kim H U, Kim T Y, et al. Metabolic engineering of Corynebacterium glutamicum for L-arginine production[J]. Nature Communications, 2014, 5(1): 4618. |
| 76 | Xu M, Rao Z, Dou W, et al. The role of ARGR repressor regulation on L-arginine production in Corynebacterium crenatum[J]. Applied Biochemistry and Biotechnology, 2013, 170(3): 587-597. |
| 77 | Ding Z, Fang Y, Zhu L, et al. Deletion of arcA, iclR, and tdcC in Escherichia coli to improve L-threonine production[J]. Biotechnology and Applied Biochemistry, 2019, 66(5): 794-807. |
| 78 | Huang J F, Liu Z Q, Jin L Q, et al. Metabolic engineering of Escherichia coli for microbial production of L-methionine[J]. Biotechnology and Bioengineering, 2017, 114(4): 843-851. |
| 79 | Grove A. Regulation of metabolic pathways by MarR family transcription factors[J]. Computational and Structural Biotechnology Journal, 2017, 15: 366-371. |
| 80 | Swint-Kruse L, Matthews K S. Allostery in the LacI/GalR family: variations on a theme[J]. Current Opinion in Microbiology, 2009, 12(2): 129-137. |
| 81 | Subrata B, Susobhan C, Dilip S, et al. Dynamical perspective of protein-DNA interaction[J]. Biomolecular Concepts, 2014, 5(1): 21-43. |
| 82 | Wray L V, Ferson A E, Fisher S H. Expression of the Bacillus subtilis ureABC operon is controlled by multiple regulatory factors including CodY, GlnR, TnrA, and Spo0H[J]. Journal of Bacteriology, 1997, 179(17): 5494. |
| 83 | Loper J C. Multiple regulatory elements control expression of the gene encoding the Saccharomyces cerevisiae cytochrome P450, lanosterol 14 alpha-demethylase (ERG11)[J]. Journal of Biological Chemistry, 1992, 267(3): 2046. |
| 84 | Yin L, Shi F, Hu X, et al. Increasing L-isoleucine production in Corynebacterium glutamicum by overexpressing global regulator Lrp and two-component export system BrnFE[J]. Journal of Applied Microbiology, 2013, 114(5): 1369-1377. |
| 85 | Rendić M. Amino acids in animal nutrition[J]. Amino Acids in Animal Nutrition, 2003, 64(1): 488. |
| 86 | Park S, Imlay J A. High levels of intracellular cysteine promote oxidative DNA damage by driving the fenton reaction[J]. Journal of Bacteriology, 2003, 185(6): 1942. |
| 87 | Wei L, Wang H, Xu N, et al. Metabolic engineering of Corynebacterium glutamicum for L-cysteine production[J]. Applied Microbiology and Biotechnology, 2019, 103(3): 1325-1338. |
| 88 | Lubitz D, Jorge J M P, Pérez-García F, et al. Roles of export genes cgmA and lysE for the production of L-arginine and L-citrulline by Corynebacterium glutamicum[J]. Applied Microbiology and Biotechnology, 2016, 100(19): 8465-8474. |
| 89 | Simic P, Willuhn J, Sahm H, et al. Identification of glyA (encoding serine hydroxymethyltransferase) and its use together with the exporter ThrE to increase L-threonine accumulation by Corynebacterium glutamicum[J]. Appl. Environ. Microbiol., 2002, 68(7): 3321-3327. |
| 90 | Matano C, Uhde A, Youn J W, et al. Engineering of Corynebacterium glutamicum for growth and L-lysine and lycopene production from N-acetyl-glucosamine[J]. Applied Microbiology and Biotechnology, 2014, 98(12): 5633-5643. |
| 91 | Qin J, Zhou Y J, Krivoruchko A, et al. Modular pathway rewiring of Saccharomyces cerevisiae enables high-level production of L-ornithine[J]. Nature Communications, 2015, 6(1): 8224. |
| 92 | Smolke C D. Building outside of the box: iGEM and the biobricks foundation[J]. Nature Biotechnology, 2009, 27(12): 1099-1102. |
| 93 | Zhou L B, Zeng A P. Exploring lysine riboswitch for metabolic flux control and improvement of L-lysine synthesis in Corynebacterium glutamicum[J]. ACS Synthetic Biology, 2015, 4(6): 729-734. |
| 94 | Lee J H, Wendisch V F. Production of amino acids — genetic and metabolic engineering approaches[J]. Bioresource Technology, 2017, 245: 1575-1587. |
| 95 | Becker J, Wittmann C. Systems and synthetic metabolic engineering for amino acid production — the heartbeat of industrial strain development[J]. Current Opinion in Biotechnology, 2012, 23(5): 718-726. |
| [1] | Xin LIU, Jun GE, Chun LI. Light-driven microbial hybrid systems improve level of biomanufacturing [J]. CIESC Journal, 2023, 74(1): 330-341. |
| [2] | Haoran BI, Yang ZHANG, Kai WANG, Chenchen XU, Yiying HUO, Biqiang CHEN, Tianwei TAN. Progress for green chemicals production by microbial manufacturing [J]. CIESC Journal, 2023, 74(1): 1-13. |
| [3] | Xue LIU, Lijuan ZHANG, Guangrong ZHAO. Commensalistic Escherichia coli coculture for biosynthesis of daidzein [J]. CIESC Journal, 2022, 73(9): 4015-4024. |
| [4] | Jingnan WANG, Jian PANG, Lei QIN, Chao GUO, Bo LYU, Chun LI, Chao WANG. Breeding and modification strategies of butenyl-spinosyn high-yield strains [J]. CIESC Journal, 2022, 73(2): 566-576. |
| [5] | Yi SUN, Teng ZHANG, Bo LYU, Chun LI. Improvement for fine regulation of microbial cell factory by intracellular biosensors [J]. CIESC Journal, 2022, 73(2): 521-534. |
| [6] | Xinhui WANG, Ying WANG, Mingdong YAO, Wenhai XIAO. Research progress of vitamin A biosynthesis [J]. CIESC Journal, 2022, 73(10): 4311-4323. |
| [7] | Wulin ZHOU, Huifang GAO, Yuling WU, Xian ZHANG, Meijuan XU, Taowei YANG, Minglong SHAO, Zhiming RAO. Engineering of Saccharomyces cerevisiae for biosynthesis of campesterol [J]. CIESC Journal, 2021, 72(8): 4314-4324. |
| [8] | GAO Zixi, GUO Shuqi, FEI Qiang. Recent progress in microbial bioconversion of greenhouse gases into single cell protein [J]. CIESC Journal, 2021, 72(6): 3202-3214. |
| [9] | WANG Xin, ZHAO Peng, LI Qingyang, TIAN Pingfang. Research advances in semiconductor synthetic biology [J]. CIESC Journal, 2021, 72(5): 2426-2435. |
| [10] | MAO Jinzhu, XIAO Shuling, YANG Zhichun, WANG Xiaoyu, ZHANG Shi, CHEN Junhong, XIE Jisheng, CHEN Fude, HUANG Zinuo, FENG Tianyu, ZHANG Aihui, FANG Baishan. Application of synthetic biology in pesticides residues detection [J]. CIESC Journal, 2021, 72(5): 2413-2425. |
| [11] | ZHAO Zhenyao, ZHANG Baocai, LI Feng, SONG Hao. Design and construction of exoelectrogens by synthetic biology [J]. CIESC Journal, 2021, 72(1): 468-482. |
| [12] | WANG Lian, WU Di, ZHOU Jingwen. Research progress of lignans biosynthesis and their microbial production [J]. CIESC Journal, 2021, 72(1): 320-333. |
| [13] | WANG Kaifeng, WANG Jinpeng, WEI Ping, JI Xiaojun. Metabolic engineering of Yarrowia lipolytica to produce fatty acids and their derivatives [J]. CIESC Journal, 2021, 72(1): 351-365. |
| [14] | Jing XU, Zixuan YOU, Junqi ZHANG, Zheng CHEN, Deguang WU, Feng LI, Hao SONG. Advances in engineering electroactive biofilms by synthetic biology approaches [J]. CIESC Journal, 2020, 71(9): 3950-3962. |
| [15] | Lei QIN, Jie YU, Xiaoyu NING, Wentao SUN, Chun LI. Synthetic biological system construction and green intelligent biological manufacturing [J]. CIESC Journal, 2020, 71(9): 3979-3994. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||