CIESC Journal ›› 2021, Vol. 72 ›› Issue (12): 6086-6092.DOI: 10.11949/0438-1157.20211409
• Reviews and monographs • Previous Articles Next Articles
Huan XIA1( ),Diannan LU2,Jun GE1,2(
),Diannan LU2,Jun GE1,2( ),Jianzhong WU3,Zheng LIU2(
),Jianzhong WU3,Zheng LIU2( )
)
Received:2021-10-08
Revised:2021-11-19
Online:2021-12-22
Published:2021-12-05
Contact:
Jun GE,Zheng LIU
通讯作者:
戈钧,刘铮
作者简介:夏欢(1989—),男,博士,基金资助:CLC Number:
Huan XIA, Diannan LU, Jun GE, Jianzhong WU, Zheng LIU. Advances in nanostructured enzyme catalysts[J]. CIESC Journal, 2021, 72(12): 6086-6092.
夏欢, 卢滇楠, 戈钧, 吴建中, 刘铮. 纳米结构酶催化剂研究进展[J]. 化工学报, 2021, 72(12): 6086-6092.
Add to citation manager EndNote|Ris|BibTeX
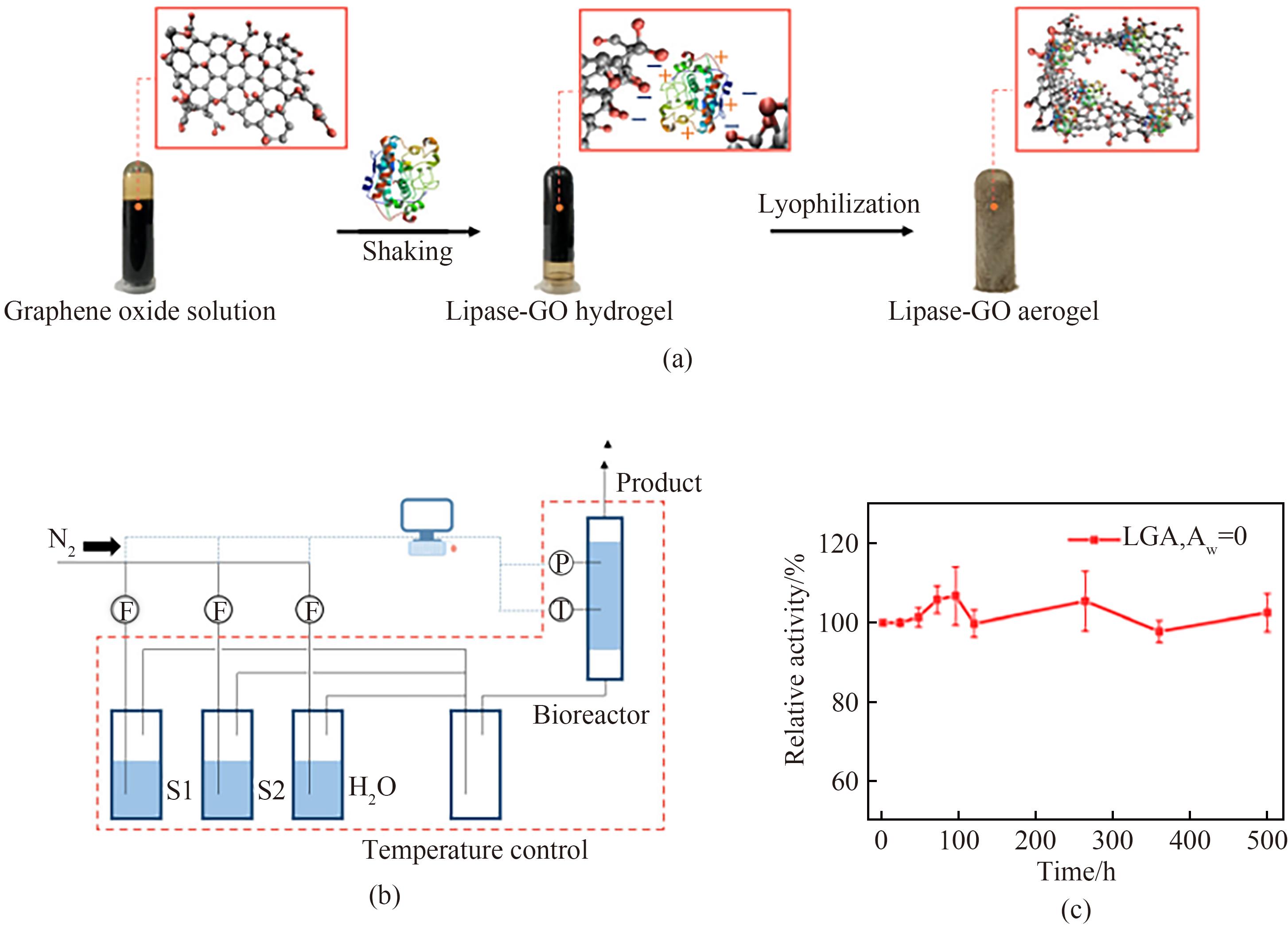
Fig.3 Preparation of procedures of lipase-GO aerogel (a), schematic diagram of experimental setup for gaseous enzymatic catalysis (b), the activity of lipase-GO aerogel during a continuous operation for 500 h (c)[50]
| 1 | Schmid A, Dordick J S, Hauer B, et al. Industrial biocatalysis today and tomorrow[J]. Nature, 2001, 409(6817): 258-268. |
| 2 | Hanefeld U, Cao L, Magner E. Enzyme immobilisation: fundamentals and application[J]. Chemical Society Reviews, 2013, 42(15): 6211-6212. |
| 3 | Luan P Q, Liu Y T, Li Y X, et al. Aqueous chemoenzymatic one-pot enantioselective synthesis of tertiary α-aryl cycloketones via Pd-catalyzed C―C formation and enzymatic C̿ C asymmetric hydrogenation[J]. Green Chemistry, 2021, 23(5): 1960-1964. |
| 4 | Cao Y F, Li X Y, Ge J. Enzyme catalyst engineering toward the integration of biocatalysis and chemocatalysis[J]. Trends in Biotechnology, 2021, 39(11): 1173-1183. |
| 5 | Hyun K H, Han S W, Koh W G, et al. Fabrication of biofuel cell containing enzyme catalyst immobilized by layer-by-layer method[J]. Journal of Power Sources, 2015, 286: 197-203. |
| 6 | Prodromidis M, Karayannis M. Enzyme based amperometric biosensors for food analysis[J]. Electroanalysis, 2002, 14(4): 241-261. |
| 7 | Ianniello R M, Yacynych A M. Immobilized enzyme chemically modified electrode as an amperometric sensor[J]. Analytical Chemistry, 1981, 53(13): 2090-2095. |
| 8 | Shoda S, Uyama H, Kadokawa J, et al. Enzymes as green catalysts for precision macromolecular synthesis[J]. Chemical Reviews, 2016, 116(4): 2307-2413. |
| 9 | Liu Q, Xun G H, Feng Y. The state-of-the-art strategies of protein engineering for enzyme stabilization[J]. Biotechnology Advances, 2019, 37(4): 530-537. |
| 10 | Singh R K, Tiwari M K, Singh R, et al. From protein engineering to immobilization: promising strategies for the upgrade of industrial enzymes[J]. International Journal of Molecular Sciences, 2013, 14(1): 1232-1277. |
| 11 | Santos J C S D, Barbosa O, Ortiz C, et al. Importance of the support properties for immobilization or purification of enzymes[J]. ChemCatChem, 2015, 7(16): 2413-2432. |
| 12 | Rodrigues R C, Berenguer-Murcia Á, Fernandez-Lafuente R. Coupling chemical modification and immobilization to improve the catalytic performance of enzymes[J]. Advanced Synthesis & Catalysis, 2011, 353(13): 2216-2238. |
| 13 | Rueda N, dos Santos J C S, Ortiz C, et al. Chemical modification in the design of immobilized enzyme biocatalysts: drawbacks and opportunities[J]. The Chemical Record, 2016, 16(3): 1436-1455. |
| 14 | Mamun A A, Bledzki A K. Micro fibre reinforced PLA and PP composites: enzyme modification, mechanical and thermal properties[J]. Composites Science and Technology, 2013, 78: 10-17. |
| 15 | Sartore L, Caliceti P, Schiavon O, et al. Enzyme modification by MPEG with an amino acid or peptide as spacer arms[J]. Applied Biochemistry and Biotechnology, 1991, 27(1): 45-54. |
| 16 | Goldstein L. Kinetic behavior of immobilized enzyme systems[J]. Methods in Enzymology, 1976, 44: 397-443. |
| 17 | Boudrant J, Woodley J M, Fernandez-Lafuente R. Parameters necessary to define an immobilized enzyme preparation[J]. Process Biochemistry, 2020, 90: 66-80. |
| 18 | Nisha S, Karthick A, Gobi N. A review on methods, application and properties of immobilized enzyme[J]. Chemical Science Review and Letters, 2012, 1: 148-155. |
| 60 | Lian X, Fang Y, Joseph E, et al. Enzyme-MOF (metal-organic framework) composites[J]. Chemical Society Reviews, 2017, 46(11): 3386-3401. |
| 61 | Xia H, Li Z X, Zhong X, et al. HKUST-1 catalyzed efficient in situ regeneration of NAD+ for dehydrogenase mediated oxidation[J]. Chemical Engineering Science, 2019, 203: 43-53. |
| 62 | Liang K, Coghlan C J, Bell S G, et al. Enzyme encapsulation in zeolitic imidazolate frameworks: a comparison between controlled co-precipitation and biomimetic mineralisation[J]. Chemical Communications, 2016, 52(3): 473-476. |
| 63 | Tocco D, Carucci C, Todde D, et al. Enzyme immobilization on metal organic frameworks: laccase from Aspergillus sp. is better adapted to ZIF-zni rather than Fe-BTC[J]. Colloids and Surfaces B: Biointerfaces, 2021, 208: 112147. |
| 19 | Liang S, Wu X L, Xiong J, et al. Metal-organic frameworks as novel matrices for efficient enzyme immobilization: an update review[J]. Coordination Chemistry Reviews, 2020, 406: 213149. |
| 20 | Wang L B, Wang Y C, He R, et al. A new nanobiocatalytic system based on allosteric effect with dramatically enhanced enzymatic performance[J]. Journal of the American Chemical Society, 2013, 135(4): 1272-1275. |
| 21 | Borzouee F, Varshosaz J, Cohan R A, et al. A comparative analysis of different enzyme immobilization nanomaterials: progress, constraints and recent trends[J]. Current Medicinal Chemistry, 2021, 28(20): 3980-4003. |
| 22 | Sharifi M, Sohrabi M J, Hosseinali S H, et al. Enzyme immobilization onto the nanomaterials: application in enzyme stability and prodrug-activated cancer therapy[J]. International Journal of Biological Macromolecules, 2020, 143: 665-676. |
| 23 | Liu W S, Wang L, Jiang R R. Specific enzyme immobilization approaches and their application with nanomaterials[J]. Topics in Catalysis, 2012, 55: 1146-1156. |
| 64 | Tuninetti J S, Serrano M P, Thomas A H, et al. Shelter for biologically relevant molecules: photoprotection and enhanced thermal stability of folic acid loaded in a ZIF-8 MOF porous host[J]. Industrial & Engineering Chemistry Research, 2020, 59: 22155-22162. |
| 65 | Badoei-dalfard A, Khankari S, Karami Z. One-pot synthesis and biochemical characterization of protease metal organic framework (protease@MOF) and its application on the hydrolysis of fish protein-waste[J]. Colloids and Surfaces B: Biointerfaces, 2020, 196: 111318. |
| 66 | Hu C, Bai Y, Hou M, et al. Defect-induced activity enhancement of enzyme-encapsulated metal-organic frameworks revealed in microfluidic gradient mixing synthesis[J]. Science Advances, 2020, 6(5): 5785. |
| 67 | Xia H, Li N, Huang W Q, et al. Enzymatic cascade reactions mediated by highly efficient biomimetic quasi metal-organic frameworks[J]. ACS Applied Materials & Interfaces, 2021, 13(19): 22240-22253. |
| 68 | Lykourinou V, Chen Y, Wang X S, et al. Immobilization of MP-11 into a mesoporous metal–organic framework, MP-11@mesoMOF: a new platform for enzymatic catalysis[J]. Journal of the American Chemical Society, 2011, 133(27): 10382-10385. |
| 69 | Li P, Moon S Y, Guelta M A, et al. Nanosizing a metal–organic framework enzyme carrier for accelerating nerve agent hydrolysis[J]. ACS Nano, 2016, 10(10): 9174-9182. |
| 70 | Deng H X, Grunder S, Cordova K E, et al. Large-pore apertures in a series of metal-organic frameworks[J]. Science, 2012, 336(6084): 1018-1023. |
| 24 | Husain Q. Nanomaterials as novel supports for the immobilization of amylolytic enzymes and their applications: a review[J]. Biocatalysis, 2017, 3(1): 37-53. |
| 25 | Hong T T, Liu W F, Li M, et al. Recent advances in the fabrication and application of nanomaterial-based enzymatic microsystems in chemical and biological sciences[J]. Analytica Chimica Acta, 2019, 1067: 31-47. |
| 26 | Rani M, Shanker U, Chaurasia A K. Catalytic potential of laccase immobilized on transition metal oxides nanomaterials: degradation of alizarin red S dye[J]. Journal of Environmental Chemical Engineering, 2017, 5(3): 2730-2739. |
| 71 | Kimura K, Suzuki A, Inokuchi H, et al. Hydrogenase activity in the dry state: isotope exchange and reversible oxidoreduction of cytochrome c3[J]. Biochimica et Biophysica Acta, 1979, 567(1): 96-105. |
| 72 | Fu Z, Xu W, Chen G, et al. Molecular dynamics simulations reveal how graphene oxide stabilizes and activates lipase in an anhydrous gas[J]. Physical Chemistry Chemical Physics, 2019, 21(45): 25425-25430. |
| 27 | Barbosa C G, Caseli L, Péres L O. Conjugated polymers nanostructured as smart interfaces for controlling the catalytic properties of enzymes[J]. Journal of Colloid and Interface Science, 2016, 476: 206-213. |
| 28 | Xue Q N, Li Z Y, Wang Q K, et al. Nanostrip flexible microwave enzymatic biosensor for noninvasive epidermal glucose sensing[J]. Nanoscale Horizons, 2020, 5(6): 934-943. |
| 29 | Wu X L, Ou G, Yang C, et al. Enhanced enzymatic reactions by solar-to-thermal conversion nanoparticles[J]. Chemical Communications, 2017, 53(36): 5048-5051. |
| 30 | Ji Y, Wang Y, Zeng W, et al. A heparin derivatives library constructed by chemical modification and enzymatic depolymerization for exploitation of non-anticoagulant functions[J]. Carbohydrate Polymers, 2020, 249: 116824. |
| 31 | Yong Y, Su R, Liu X R, et al. Lectin corona enhances enzymatic catalysis on the surface of magnetic nanoparticles[J]. Biochemical Engineering Journal, 2018, 129: 26-32. |
| 32 | Yan M, Ge J, Liu Z, et al. Encapsulation of single enzyme in nanogel with enhanced biocatalytic activity and stability[J]. Journal of the American Chemical Society, 2006, 128(34): 11008-11009. |
| 33 | Ge J, Lu D N, Wang J, et al. Molecular fundamentals of enzyme nanogels[J]. Journal of Physical Chemistry B, 2008, 112(45): 14319-14324. |
| 34 | Zhang Y, Chen Q, Ge J, et al. Controlled display of enzyme activity with a stretchable hydrogel[J]. Chemical Communications, 2013, 49(84): 9815-9817. |
| 35 | Wang R, Zhang Y F, Ge J, et al. Activation of enzyme nanogel in organic solvents by PEG–substrate joint imprinting[J]. RSC Advances, 2014, 4(76): 40301. |
| 36 | Ge J, Lu D, Wang J, et al. Lipase nanogel catalyzed transesterification in anhydrous dimethyl sulfoxide[J]. Biomacromolecules, 2009, 10(6): 1612-1618. |
| 37 | Zhu J Y, Zhang Y F, Lu D N, et al. Temperature-responsive enzyme–polymer nanoconjugates with enhanced catalytic activities in organic media[J]. Chemical Communications, 2013, 49(54): 6090. |
| 38 | Ge J, Lu D N, Yang C, et al. A lipase-responsive vehicle using amphipathic polymer synthesized with the lipase as catalyst[J]. Macromolecular Rapid Communications, 2011, 32(6): 546-550. |
| 39 | Zhang Y F, Dai Y, Hou M, et al. Chemo-enzymatic synthesis of valrubicin using Pluronic conjugated lipase with temperature responsiveness in organic media[J]. RSC Advances, 2013, 3(45): 22963. |
| 40 | Wu X L, Ge J, Zhu J Y, et al. A general method for synthesizing enzyme–polymer conjugates in reverse emulsions using Pluronic as a reactive surfactant[J]. Chemical Communications, 2015, 51(47): 9674-9677. |
| 41 | Hou M, Wang R, Wu X L, et al. Synthesis of lutein esters by using a reusable lipase-pluronic conjugate as the catalyst[J]. Catalysis Letters, 2015, 145(10): 1825-1829. |
| 42 | Ge J, Lei J D, Zare R N. Protein–inorganic hybrid nanoflowers[J]. Nature Nanotechnology, 2012, 7(7): 428-432. |
| 43 | Zhu L, Gong L, Zhang Y F, et al. Rapid detection of phenol using a membrane containing laccase nanoflowers[J]. Chemistry - an Asian Journal, 2013, 8(10): 2358-2360. |
| 44 | Li Z, Ding Y, Li S, et al. Highly active, stable and self-antimicrobial enzyme catalysts prepared by biomimetic mineralization of copper hydroxysulfate[J]. Nanoscale, 2016, 8(40): 17440-17445. |
| 45 | Li Z X, Zhang Y F, Su Y C, et al. Spatial co-localization of multi-enzymes by inorganic nanocrystal–protein complexes[J]. Chemical Communications, 2014, 50(83): 12465-12468. |
| 46 | Lyu F J, Zhang Y F, Zare R N, et al. One-pot synthesis of protein-embedded metal-organic frameworks with enhanced biological activities[J]. Nano Letters, 2014, 14(10): 5761-5765. |
| 47 | Wu X L, Ge J, Yang C, et al. Facile synthesis of multiple enzyme-containing metal–organic frameworks in a biomolecule-friendly environment[J]. Chemical Communications, 2015, 51(69): 13408-13411. |
| 48 | Zhang C, Wang X R, Hou M, et al. Immobilization on metal–organic framework engenders high sensitivity for enzymatic electrochemical detection[J]. ACS Applied Materials & Interfaces, 2017, 9(16): 13831-13836. |
| 49 | Wu X L, Yue H, Zhang Y, et al. Packaging and delivering enzymes by amorphous metal-organic frameworks[J]. Nature Communications, 2019, 10: 5165. |
| 50 | Xu W N, Fu Z W, Chen G, et al. Graphene oxide enabled long-term enzymatic transesterification in an anhydrous gas flux[J]. Nature Communications, 2019, 10: 2684. |
| 51 | López-Gallego F, Yate L. Selective biomineralization of Co3(PO4)2-sponges triggered by His-tagged proteins: efficient heterogeneous biocatalysts for redox processes[J]. Chemical Communications, 2015, 51(42): 8753-8756. |
| 52 | Silva-Torres O, Bojorquez-Vazquez L, Simakov A, et al. Enhanced laccase activity of biocatalytic hybrid copper hydroxide nanocages[J]. Enzyme and Microbial Technology, 2019, 128: 59-66. |
| 53 | Zhang B L, Li P T, Zhang H P, et al. Papain/Zn3(PO4)2 hybrid nanoflower: preparation, characterization and its enhanced catalytic activity as an immobilized enzyme[J]. RSC Advances, 2016, 6(52): 46702-46710. |
| 54 | Salvi H M, Yadav G D. Organic-inorganic epoxide hydrolase hybrid nanoflowers with enhanced catalytic activity: hydrolysis of styrene oxide to 1-phenyl-1,2-ethanediol[J]. Journal of Biotechnology, 2021, 341: 113-120. |
| 73 | Wu X L, Wang R, Zhang Y F, et al. Enantioselective ammonolysis of phenylglycine methyl ester with lipase-pluronic nanoconjugate in tertiary butanol[J]. Catalysis Letters, 2014, 144(8): 1407-1410. |
| 74 | Yuki O, Zhang Y F, Ge J, et al. Epoxidation of fatty acids by pluronic-conjugated lipase in organic media[J]. Catalysis Letters, 2016, 146(6): 1073-1078. |
| 75 | Cheng H, Zhao Y L, Luo X J, et al. Cross-linked enzyme-polymer conjugates with excellent stability and detergent-enhanced activity for efficient organophosphate degradation[J]. Bioresources and Bioprocessing, 2018, 5(1): 1-9. |
| 76 | Cao Y, Li X, Xiong J, et al. Investigating the origin of high efficiency in confined multienzyme catalysis[J]. Nanoscale, 2019, 11(45): 22108-22117. |
| 55 | Gul O T, Ocsoy I. Co-enzymes based nanoflowers incorporated-magnetic carbon nanotubes: a new generation nanocatalyst for superior removal of cationic and anionic dyes with great repeated use[J]. Environmental Technology & Innovation, 2021, 24: 101992. |
| 56 | Dube S, Rawtani D. Understanding intricacies of bioinspired organic-inorganic hybrid nanoflowers: a quest to achieve enhanced biomolecules immobilization for biocatalytic, biosensing and bioremediation applications[J]. Advances in Colloid and Interface Science, 2021, 295: 102484. |
| 57 | Gkaniatsou E, Sicard C, Ricoux R, et al. Metal–organic frameworks: a novel host platform for enzymatic catalysis and detection[J]. Materials Horizons, 2017, 4(1): 55-63. |
| 58 | Doonan C, Riccò R, Liang K, et al. Metal-organic frameworks at the biointerface: synthetic strategies and applications[J]. Accounts of Chemical Research, 2017, 50(6): 1423-1432. |
| 59 | Xia H, Zhong X, Li Z X, et al. Palladium-mediated hybrid biocatalysts with enhanced enzymatic catalytic performance via allosteric effects[J]. Journal of Colloid and Interface Science, 2019, 533: 1-8. |
| 77 | Chen G, Kong X, Lu D N, et al. Kinetics of CO2 diffusion in human carbonic anhydrase: a study using molecular dynamics simulations and the Markov-state model[J]. Physical Chemistry Chemical Physics, 2017, 19(18): 11690-11697. |
| 78 | Chen G, Lu D N, Wu J Z, et al. Detachment of HCO3- from the active site of carbonic anhydrase: molecular dynamics simulation and machine learning[J]. The Journal of Physical Chemistry C, 2018, 122(35): 20539-20549. |
| [1] | Yepin CHENG, Daqing HU, Yisha XU, Huayan LIU, Hanfeng LU, Guokai CUI. Application of ionic liquid-based deep eutectic solvents for CO2 conversion [J]. CIESC Journal, 2023, 74(9): 3640-3653. |
| [2] | Yuanchao LIU, Bin GUAN, Jianbin ZHONG, Yifan XU, Xuhao JIANG, Duan LI. Investigation of thermoelectric transport properties of single-layer XSe2 (X=Zr/Hf) [J]. CIESC Journal, 2023, 74(9): 3968-3978. |
| [3] | Jiaqi CHEN, Wanyu ZHAO, Ruichong YAO, Daolin HOU, Sheying DONG. Synthesis of pistachio shell-based carbon dots and their corrosion inhibition behavior on Q235 carbon steel [J]. CIESC Journal, 2023, 74(8): 3446-3456. |
| [4] | Yaxin CHEN, Hang YUAN, Guanzhang LIU, Lei MAO, Chun YANG, Ruifang ZHANG, Guangya ZHANG. Advances in enzyme self-immobilization mediated by protein nanocages [J]. CIESC Journal, 2023, 74(7): 2773-2782. |
| [5] | Meibo XING, Zhongtian ZHANG, Dongliang JING, Hongfa ZHANG. Enhanced phase change energy storage/release properties by combining porous materials and water-based carbon nanotube under magnetic regulation [J]. CIESC Journal, 2023, 74(7): 3093-3102. |
| [6] | Xiaoling TANG, Jiarui WANG, Xuanye ZHU, Renchao ZHENG. Biosynthesis of chiral epichlorohydrin by halohydrin dehalogenase based on Pickering emulsion system [J]. CIESC Journal, 2023, 74(7): 2926-2934. |
| [7] | Jiali GE, Tuxiang GUAN, Xinmin QIU, Jian WU, Liming SHEN, Ningzhong BAO. Synthesis of FeF3 nanoparticles covered by vertical porous carbon for high performance Li-ion battery cathode [J]. CIESC Journal, 2023, 74(7): 3058-3067. |
| [8] | Qin YANG, Chuanjian QIN, Mingzi LI, Wenjing YANG, Weijie ZHAO, Hu LIU. Fabrication and properties of dual shape memory MXene based hydrogels for flexible sensor [J]. CIESC Journal, 2023, 74(6): 2699-2707. |
| [9] | Yuanchao LIU, Xuhao JIANG, Ke SHAO, Yifan XU, Jianbin ZHONG, Zhuan LI. Influence of geometrical dimensions and defects on the thermal transport properties of graphyne nanoribbons [J]. CIESC Journal, 2023, 74(6): 2708-2716. |
| [10] | Lei MAO, Guanzhang LIU, Hang YUAN, Guangya ZHANG. Efficient preparation of carbon anhydrase nanoparticles capable of capturing CO2 and their characteristics [J]. CIESC Journal, 2023, 74(6): 2589-2598. |
| [11] | Yang HU, Yan SUN. Self-propulsion of enzyme and enzyme-induced micro-/nanomotor [J]. CIESC Journal, 2023, 74(1): 116-132. |
| [12] | Xin LIU, Jun GE, Chun LI. Light-driven microbial hybrid systems improve level of biomanufacturing [J]. CIESC Journal, 2023, 74(1): 330-341. |
| [13] | Jing ZHANG, Tao LIU, Wei ZHANG, Zhenyu CHU, Wanqin JIN. Preparation of a novel separation-sensing membrane and its dynamic monitoring of blood glucose [J]. CIESC Journal, 2023, 74(1): 459-468. |
| [14] | Zhuotao TAN, Siyu QI, Mengjiao XU, Jie DAI, Chenjie ZHU, Hanjie YING. Application of the redox cascade systems with coenzyme self-cycling in biocatalytic processes: opportunities and challenges [J]. CIESC Journal, 2023, 74(1): 45-59. |
| [15] | Guojuan QU, Tao JIANG, Tao LIU, Xiang MA. Modulating luminescent behaviors of Au nanoclusters via supramolecular strategies [J]. CIESC Journal, 2023, 74(1): 397-407. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||