CIESC Journal ›› 2022, Vol. 73 ›› Issue (8): 3758-3767.DOI: 10.11949/0438-1157.20220199
• Material science and engineering, nanotechnology • Previous Articles Next Articles
Shuang HAN( ), Nan ZHANG, Hui WANG, Xuan ZHANG, Jinluan YANG, Manlin ZHANG, Zhichao ZHANG(
), Nan ZHANG, Hui WANG, Xuan ZHANG, Jinluan YANG, Manlin ZHANG, Zhichao ZHANG( )
)
Received:2022-02-11
Revised:2022-04-23
Online:2022-09-06
Published:2022-08-05
Contact:
Zhichao ZHANG
韩双( ), 张楠, 王慧, 张璇, 杨金栾, 张蔓琳, 张志超(
), 张楠, 王慧, 张璇, 杨金栾, 张蔓琳, 张志超( )
)
通讯作者:
张志超
作者简介:韩双(1983—),女,博士,副教授,unihanshuang@syuct.edu.cn
基金资助:CLC Number:
Shuang HAN, Nan ZHANG, Hui WANG, Xuan ZHANG, Jinluan YANG, Manlin ZHANG, Zhichao ZHANG. Preparation and application of chlortetracycline electrochemical sensor based on molecularly imprinting technique[J]. CIESC Journal, 2022, 73(8): 3758-3767.
韩双, 张楠, 王慧, 张璇, 杨金栾, 张蔓琳, 张志超. 金霉素分子印迹电化学传感器的制备与应用[J]. 化工学报, 2022, 73(8): 3758-3767.
Add to citation manager EndNote|Ris|BibTeX
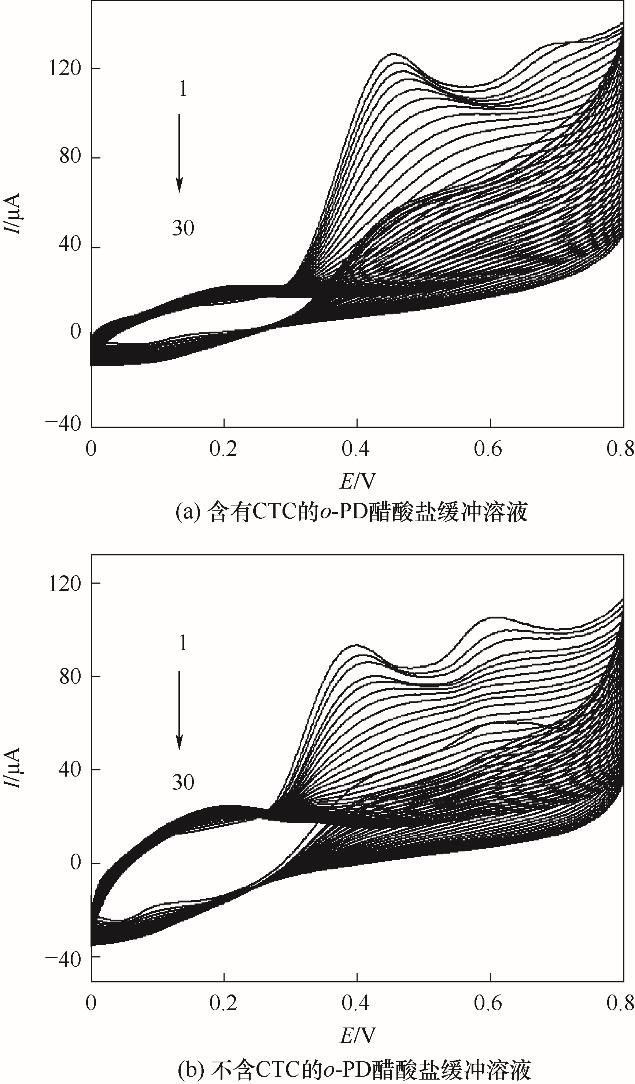
Fig.5 Cyclic voltammograms for the electropolymerization of 5.0 mmol/L o-PD on GCE/RGO-PEI in the presence (a) and in the absence (b) of CTC in pH4.8 acetate buffer (scan rate: 0.050 V/s)

Fig.6 Cyclic voltammograms of 5.0 mmol/L K3[Fe(CN)6]/0.1 mol/L KCl on the bare GCE (a), GCE/RGO-PEI (b), GCE/RGO-PEI after electropolymerization (c), MIP GCE/RGO-PEI/PPD after the removal of CTC (d), MIP GCE/RGO-PEI/PPD after incubation of CTC (e)
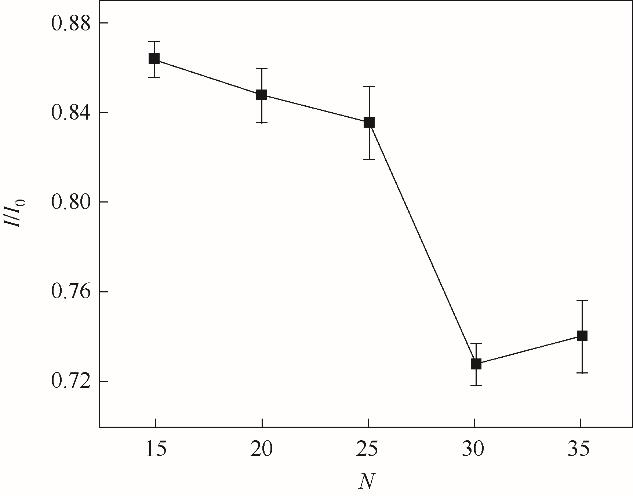
Fig.9 Effect of the electropolymerization cycles on the peak current of the CV response in the presence of 5.0 mmol/L K3[Fe(CN)6]/ 0.1 mol/L KCl (I0 is the current of the MIP GCE/RGO-PEI/PPD after elution; I is the current of the MIP GCE/RGO-PEI/PPD after incubation of CTC)
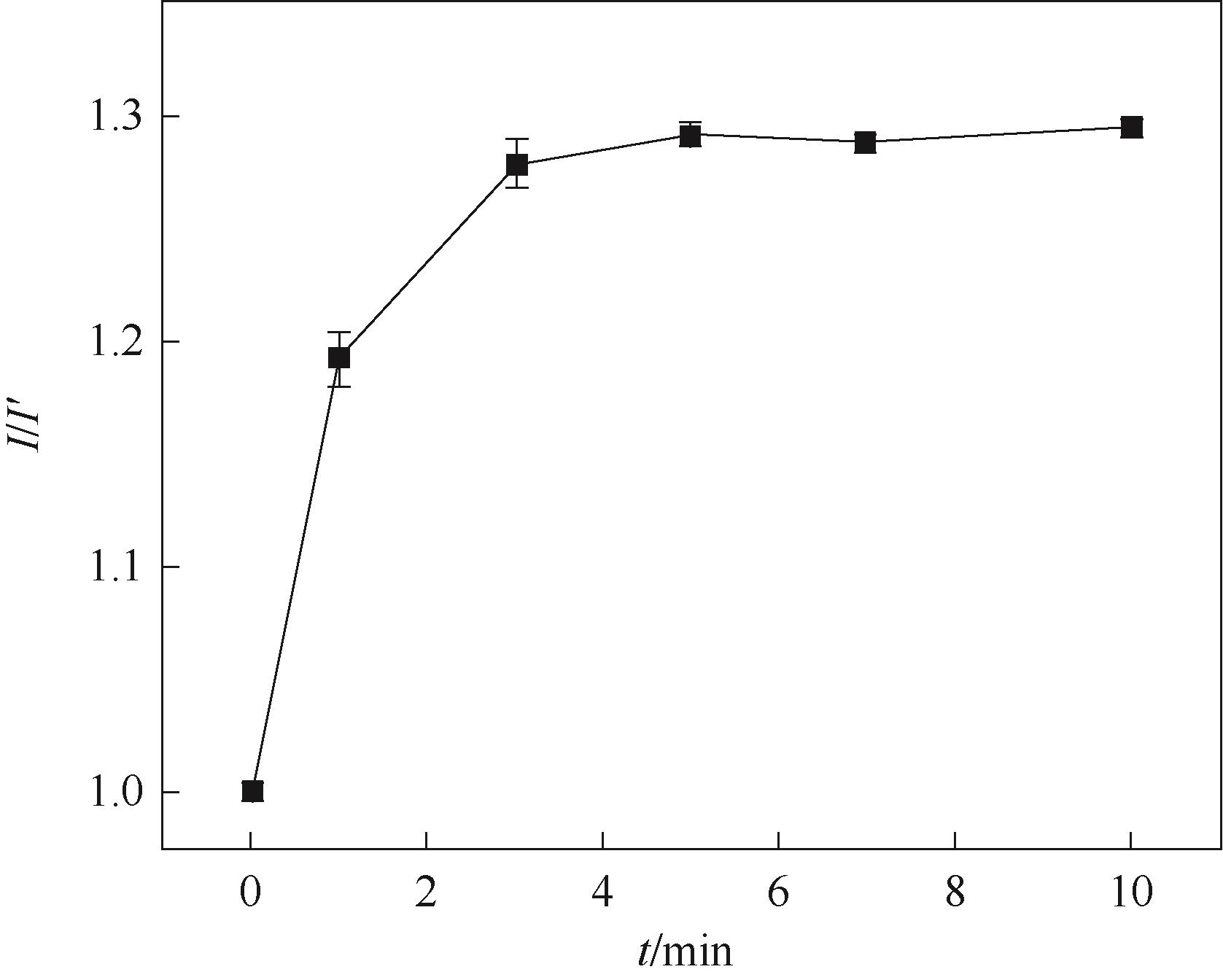
Fig.11 Effect of elution time on the peak current of CV response in the presence of 5.0 mmol/L K3[Fe(CN)6]/ 0.1 mol/L KCl (I′ is the current of the GCE/RGO-PEI/PPD after electropolymerization)
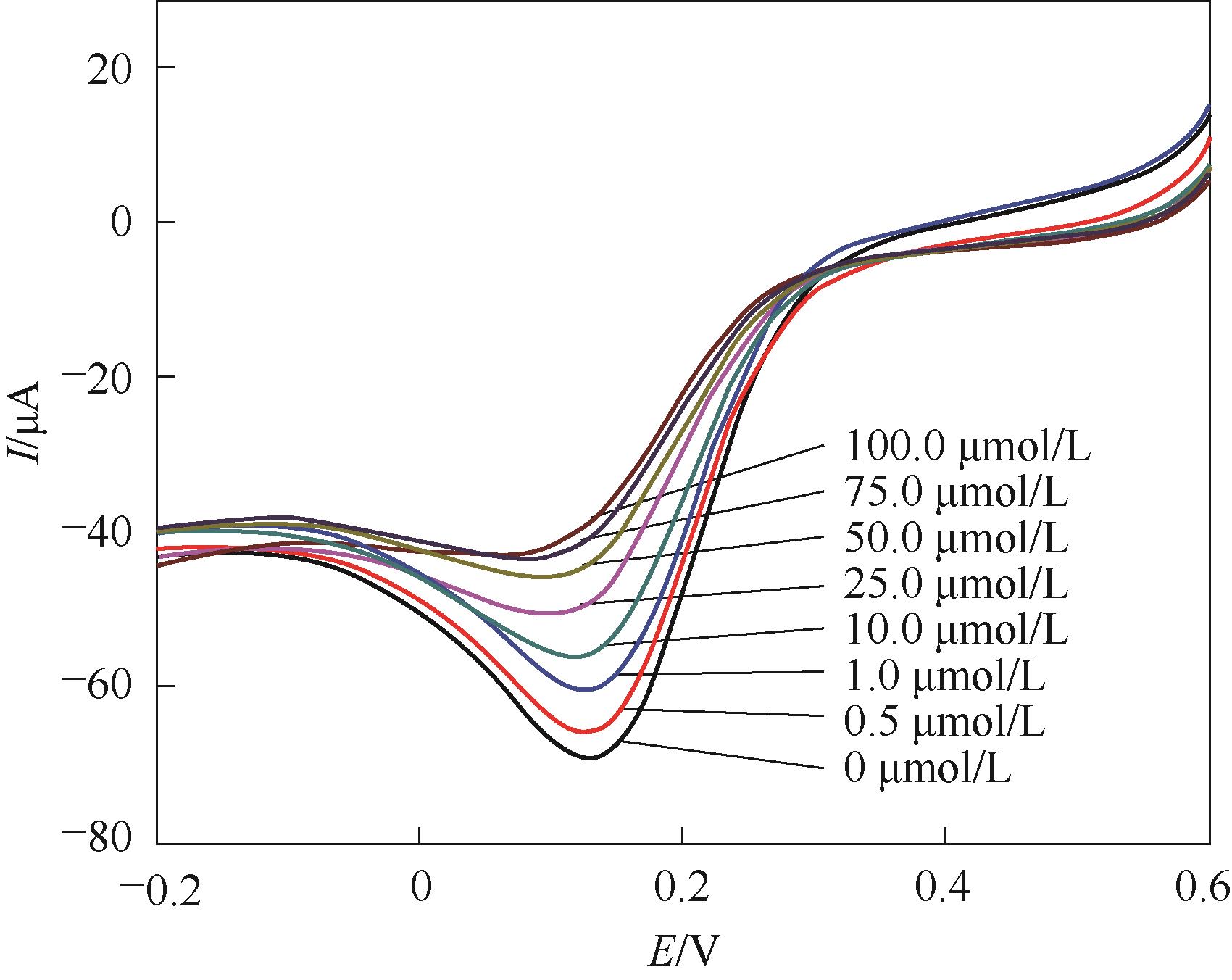
Fig.13 Linear sweep voltammograms of 5.0 mmol/L K3[Fe(CN)6]/0.1 mol/L KCl at 0.050 V/s for CTC-free MIP films after rebinding CTC at different concentrations
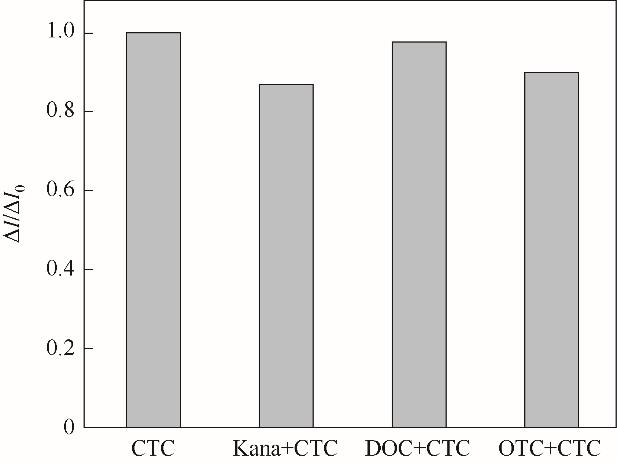
Fig.15 The the current change of MIP GCE/RGO-PEI/PPD in the presence of 5.0 mmol/L K3[Fe(CN)6]/ 0.1 mol/L KCl after incubation in 25.0 μmol/L CTC, 25.0 μmol/L CTC +100.0 μmol/L kanamycin, 25.0 μmol/L CTC + 100.0 μmol/L DOC, 25.0 μmol/L CTC + 100.0 μmol/L OTC(ΔI0 = I0 -ICTC; ΔI = I0 -ICTC+others)
| 样品 | 加入量/(μmol/L) | 测得量/(μmol/L) | 回收率/% | 平均回收率/% | RSD(n=3)/% |
|---|---|---|---|---|---|
| 1 | 10.0 | 10.51, 10.71, 10.47 | 105.1, 107.1, 104.7 | 104.7 | 1.22 |
| 2 | 50.0 | 52.61, 51.41, 50.05 | 105.2, 102.8, 100.1 | 102.7 | 2.49 |
Table 1 Recoveries for CTC detection in lake samples
| 样品 | 加入量/(μmol/L) | 测得量/(μmol/L) | 回收率/% | 平均回收率/% | RSD(n=3)/% |
|---|---|---|---|---|---|
| 1 | 10.0 | 10.51, 10.71, 10.47 | 105.1, 107.1, 104.7 | 104.7 | 1.22 |
| 2 | 50.0 | 52.61, 51.41, 50.05 | 105.2, 102.8, 100.1 | 102.7 | 2.49 |
| 1 | Qiao M, Ying G G, Singer A C, et al. Review of antibiotic resistance in China and its environment[J]. Environment International, 2018, 110: 160-172. |
| 2 | Pan L X, Feng X X, Cao M, et al. Determination and distribution of pesticides and antibiotics in agricultural soils from northern China[J]. RSC Advances, 2019, 9(28): 15686-15693. |
| 3 | Cai C Y, Li J Y, Wu D, et al. Spatial distribution, emission source and health risk of parent PAHs and derivatives in surface soils from the Yangtze River Delta, eastern China[J]. Chemosphere, 2017, 178: 301-308. |
| 4 | Lv J, Zhang L, Chen Y Y, et al. Occurrence and distribution of pharmaceuticals in raw, finished, and drinking water from seven large river basins in China[J]. Journal of Water and Health, 2019, 17(3): 477-489. |
| 5 | Han S, Li B Q, Song Z, et al. A kanamycin sensor based on an electrosynthesized molecularly imprinted poly-o-phenylenediamine film on a single-walled carbon nanohorn modified glassy carbon electrode[J]. Analyst, 2016, 142(1): 218-223. |
| 6 | Han S, Zhang X, Sun H D, et al. Electrochemical behavior and voltammetric determination of chloramphenicol and doxycycline using a glassy carbon electrode modified with single-walled carbon nanohorns[J]. Electroanalysis, 2022, 34(4): 735-742. |
| 7 | Pulicharla R, Brar S K, Rouissi T, et al. Degradation of chlortetracycline in wastewater sludge by ultrasonication, Fenton oxidation, and Ferro-sonication[J]. Ultrasonics Sonochemistry, 2017, 34: 332-342. |
| 8 | Meng L, Lan C W, Liu Z H, et al. A novel ratiometric fluorescence probe for highly sensitive and specific detection of chlorotetracycline among tetracycline antibiotics[J]. Analytica Chimica Acta, 2019, 1089: 144-151. |
| 9 | Zhou Y T, Niu L L, Zhu S Y, et al. Occurrence, abundance, and distribution of sulfonamide and tetracycline resistance genes in agricultural soils across China[J]. Science of the Total Environment, 2017, 599/600: 1977-1983. |
| 10 | Pulicharla R, Das R K, Brar S K, et al. Toxicity of chlortetracycline and its metal complexes to model microorganisms in wastewater sludge[J]. Science of the Total Environment, 2015, 532: 669-675. |
| 11 | Han J, Jiang D, Chen T S, et al. Simultaneous determination of olaquindox, oxytetracycline and chlorotetracycline in feeds by high performance liquid chromatography with ultraviolet and fluorescence detection adopting online synchronous derivation and separation[J]. Journal of Chromatography B, 2020, 1152: 122253. |
| 12 | Weng R, Sun L S, Jiang L P, et al. Electrospun graphene oxide-doped nanofiber-based solid phase extraction followed by high-performance liquid chromatography for the determination of tetracycline antibiotic residues in food samples[J]. Food Analytical Methods, 2019, 12(7): 1594-1603. |
| 13 | Du F Y, Zheng X, Sun L, et al. Development and validation of polymerized high internal phase emulsion monoliths coupled with HPLC and fluorescence detection for the determination of trace tetracycline antibiotics in environmental water samples[J]. Journal of Separation Science, 2015, 38(21): 3774-3780. |
| 14 | Valverde R S, Pérez I S, Franceschelli F, et al. Determination of photoirradiated tetracyclines in water by high-performance liquid chromatography with chemiluminescence detection based reaction of Rhodamine B with cerium (Ⅳ)[J]. Journal of Chromatography A, 2007, 1167(1): 85-94. |
| 15 | Gajda A, Antczak M, Mitrowska K, et al. Development, validation and application to real samples of a liquid chromatography with tandem mass spectrometry method for the determination of tetracyclines in beeswax[J]. Journal of Separation Science, 2018, 41(20): 3821-3829. |
| 16 | Tong L, Liu H, Xie C, et al. Quantitative analysis of antibiotics in aquifer sediments by liquid chromatography coupled to high resolution mass spectrometry[J]. Journal of Chromatography A, 2016, 1452: 58-66. |
| 17 | Jiao Z, Zhang S L, Chen H W. Determination of tetracycline antibiotics in fatty food samples by selective pressurized liquid extraction coupled with high-performance liquid chromatography and tandem mass spectrometry[J]. Journal of Separation Science, 2015, 38(1): 115-120. |
| 18 | Pamreddy A, Hidalgo M, Havel J, et al. Determination of antibiotics (tetracyclines and sulfonamides) in biosolids by pressurized liquid extraction and liquid chromatography-tandem mass spectrometry[J]. Journal of Chromatography A, 2013, 1298: 68-75. |
| 19 | Chang X S, Meyer M T, Liu X Y, et al. Determination of antibiotics in sewage from hospitals, nursery and slaughter house, wastewater treatment plant and source water in Chongqing region of Three Gorge Reservoir in China[J]. Environmental Pollution, 2010, 158(5): 1444-1450. |
| 20 | Patyra E, Kowalczyk E, Grelik A, et al. Screening method for the determination of tetracyclines and fluoroquinolones in animal drinking water by liquid chromatography with diode array detector[J]. Polish Journal of Veterinary Sciences, 2015, 18(2): 283-289. |
| 21 | Patyra E, Kowalczyk E, Kwiatek K. Screening method for the determination of selected tetracyclines in water by liquid chromatography with diode array detector[J]. Bulletin of the Veterinary Institute in Pulawy, 2014, 58(1): 65-70. |
| 22 | Karageorgou E, Armeni M, Moschou I, et al. Ultrasound-assisted dispersive extraction for the high pressure liquid chromatographic determination of tetracyclines residues in milk with diode array detection[J]. Food Chemistry, 2014, 150: 328-334. |
| 23 | Patyra E, Kowalczyk E, Kwiatek K. Development and validation method for the determination of selected tetracyclines in animal medicated feedingstuffs with the use of micellar liquid chromatography[J]. Analytical and Bioanalytical Chemistry, 2013, 405(21): 6799-6806. |
| 24 | Loetanantawong B, Suracheep C, Somasundrum M, et al. Electrocatalytic tetracycline oxidation at a mixed-valent ruthenium oxide: ruthenium cyanide-modified glassy carbon electrode and determination of tetracyclines by liquid chromatography with electrochemical detection[J]. Analytical Chemistry, 2004, 76(8): 2266-2272. |
| 25 | Wu X Y, Xu Z Q, Huang Z, et al. Large volume sample stacking of cationic tetracycline antibiotics toward 10 ppb level analysis by capillary electrophoresis with UV detection[J]. Electrophoresis, 2016, 37(22): 2963-2969. |
| 26 | Guo Y X, Meng L, Zhang Y H, et al. Sensitive determination of four tetracycline antibiotics in pig plasma by field-amplified sample stacking open-tubular capillary electrochromatography with dimethylethanolamine aminated polychloromethyl styrene nano-latex coated capillary column[J]. Journal of Chromatography B, 2013, 942/943: 151-157. |
| 27 | Wangfuengkanagul N, Siangproh W, Chailapakul O. A flow injection method for the analysis of tetracycline antibiotics in pharmaceutical formulations using electrochemical detection at anodized boron-doped diamond thin film electrode[J]. Talanta, 2004, 64(5): 1183-1188. |
| 28 | Si X J, Wang H L, Wu T H, et al. Novel methods for the rapid detection of trace tetracyclines based on the fluorescence behaviours of Maillard reaction fluorescent nanoparticles[J]. RSC Advances, 2020, 10(71): 43256-43261. |
| 29 | Gan T, Lv Z, Liu N, et al. Electrochemical detection method for chlorotetracycline based on enhancement of yolk–shell structured carbon sphere@MnO2 [J]. Journal of the Electrochemical Society, 2015, 162(4): H200-H205. |
| 30 | Ni Y N, Li S Z, Kokot S. Simultaneous voltammetric analysis of tetracycline antibiotics in foods[J]. Food Chemistry, 2011, 124(3): 1157-1163. |
| 31 | Arabi M, Ostovan A, Li J H, et al. Molecular imprinting: green perspectives and strategies[J]. Advanced Materials, 2021, 33(30): 2100543. |
| 32 | Tarannum N, Khatoon S, Dzantiev B B. Perspective and application of molecular imprinting approach for antibiotic detection in food and environmental samples: a critical review[J]. Food Control, 2020, 118: 107381. |
| 33 | Li S P, Guan H M, Xu G B, et al. Progress in molecular imprinting electrochemiluminescence analysis[J]. Chinese Journal of Analytical Chemistry, 2015, 43(2): 294-299. |
| 34 | Li W, Zheng Y P, Zhang T W, et al. A surface plasmon resonance-based optical fiber probe fabricated with electropolymerized molecular imprinting film for melamine detection[J]. Sensors, 2018, 18(3): 828. |
| 35 | Tlili A, Attia G, Khaoulani S, et al. Contribution to the understanding of the interaction between a polydopamine molecular imprint and a protein model: ionic strength and pH effect investigation[J]. Sensors, 2021, 21(2): 619. |
| 36 | Tang X S, Zhang D, Zhou T S, et al. Fe3O4@Au sphere molecular imprinting with self-assembled monolayer for the recognition of parathion-methyl[J]. Analytical Methods, 2011, 3(10): 2313. |
| 37 | Yu Y J, Zhang Q, Buscaglia J, et al. Quantitative real-time detection of carcinoembryonic antigen (CEA) from pancreatic cyst fluid using 3-D surface molecular imprinting[J]. The Analyst, 2016, 141(14): 4424-4431. |
| 38 | Yin D X, Ulbricht M. Protein-selective adsorbers by molecular imprinting via a novel two-step surface grafting method[J]. Journal of Materials Chemistry B, 2013, 1(25): 3209. |
| 39 | Zhao M, Chen X J, Zhang H T, et al. Well-defined hydrophilic molecularly imprinted polymer microspheres for efficient molecular recognition in real biological samples by facile RAFT coupling chemistry[J]. Biomacromolecules, 2014, 15(5): 1663-1675. |
| 40 | Blanco-López M C, Lobo-Castañón M J, Miranda-Ordieres A J, et al. Voltammetric sensor for vanillylmandelic acid based on molecularly imprinted polymer-modified electrodes[J]. Biosensors and Bioelectronics, 2003, 18(4): 353-362. |
| 41 | Li H D, Guan H M, Dai H, et al. An amperometric sensor for the determination of benzophenone in food packaging materials based on the electropolymerized molecularly imprinted poly-o-phenylenediamine film[J]. Talanta, 2012, 99: 811-815. |
| 42 | Wu S X, He Q Y, Tan C L, et al. Graphene-based electrochemical sensors[J]. Small, 2013, 9(8): 1160-1172. |
| 43 | Chen D, Feng H B, Li J H. Graphene oxide: preparation, functionalization, and electrochemical applications[J]. Chemical Reviews, 2012, 112(11): 6027-6053. |
| 44 | Geim A K, Novoselov K S. The rise of graphene[J]. Nature Materials, 2007, 6(3): 183-191. |
| 45 | Lawal A T. Graphene-based nano composites and their applications. A review[J]. Biosensors and Bioelectronics, 2019, 141: 111384. |
| 46 | Huang X, Qi X Y, Boey F, et al. Graphene-based composites[J]. Chemical Society Reviews, 2012, 41(2): 666-686. |
| 47 | Atar N, Yola M L, Eren T. Sensitive determination of citrinin based on molecular imprinted electrochemical sensor[J]. Applied Surface Science, 2016, 362(1): 315-322. |
| 48 | Yola M L, Eren T, Atar N. A sensitive molecular imprinted electrochemical sensor based on gold nanoparticles decorated graphene oxide: application to selective determination of tyrosine in milk[J]. Sensors and Actuators B, 2015, 210: 149-157. |
| 49 | Zhou X H, Chen Z X, Yan D H, et al. Deposition of Fe-Ni nanoparticles on polyethyleneimine-decorated graphene oxide and application in catalytic dehydrogenation of ammonia borane[J]. Journal of Materials Chemistry, 2012, 22(27): 13506. |
| 50 | Gan S Y, Zhong L J, Han D X, et al. Probing bio-nano interactions between blood proteins and monolayer-stabilized graphene sheets[J]. Small, 2015, 11(43): 5814-5825. |
| 51 | Kim H, Namgung R, Singha K, et al. Graphene oxide-polyethylenimine nanoconstruct as a gene delivery vector and bioimaging tool[J]. Bioconjugate Chemistry, 2011, 22(12): 2558-2567. |
| 52 | Guo H L, Wang X F, Qian Q Y, et al. A green approach to the synthesis of graphene nanosheets[J]. ACS Nano, 2009, 3(9): 2653-2659. |
| 53 | Zhang Y, Chen B, Zhang L M, et al. Controlled assembly of Fe3O4 magnetic nanoparticles on graphene oxide[J]. Nanoscale, 2011, 3(4): 1446-1450. |
| 54 | Yang S, Yue W B, Huang D Z, et al. A facile green strategy for rapid reduction of graphene oxide by metallic zinc[J]. RSC Advances, 2012, 2(23): 8827-8832. |
| 55 | Gao Y, Li Y, Zhang L, et al. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide[J]. Journal of Colloid and Interface Science, 2012, 368(1): 540-546. |
| [1] | Jiali GE, Tuxiang GUAN, Xinmin QIU, Jian WU, Liming SHEN, Ningzhong BAO. Synthesis of FeF3 nanoparticles covered by vertical porous carbon for high performance Li-ion battery cathode [J]. CIESC Journal, 2023, 74(7): 3058-3067. |
| [2] | Qiyu ZHANG, Lijun GAO, Yuhang SU, Xiaobo MA, Yicheng WANG, Yating ZHANG, Chao HU. Recent advances in carbon-based catalysts for electrochemical reduction of carbon dioxide [J]. CIESC Journal, 2023, 74(7): 2753-2772. |
| [3] | Hao GU, Fujian ZHANG, Zhen LIU, Wenxuan ZHOU, Peng ZHANG, Zhongqiang ZHANG. Desalination performance and mechanism of porous graphene membrane in temporal dimension under mechanical-electrical coupling [J]. CIESC Journal, 2023, 74(5): 2067-2074. |
| [4] | Jianglong DU, Wenqi YANG, Kai HUANG, Cheng LIAN, Honglai LIU. Heat dissipation performance of the module combined CPCM with air cooling for lithium-ion batteries [J]. CIESC Journal, 2023, 74(2): 674-689. |
| [5] | Houchuan YU, Teng REN, Ning ZHANG, Xiaobin JIANG, Yan DAI, Xiaopeng ZHANG, Junjiang BAO, Gaohong HE. Advances in two-dimensional graphene oxide membrane for ion selective transport [J]. CIESC Journal, 2023, 74(1): 303-312. |
| [6] | Wenzhang JIN, Yuling ZHANG, Xiaoyu JIA. Study on degradation efficiency of hydroxyethylidene diphosphonic acid by electrochemical advanced oxidation [J]. CIESC Journal, 2022, 73(9): 4062-4069. |
| [7] | Kai HUANG, Sijie WANG, Haiping SU, Cheng LIAN, Honglai LIU. First principle study on inhibition of lithium dendrites growth by regulating graphene layer spacings [J]. CIESC Journal, 2022, 73(8): 3501-3510. |
| [8] | Hongchao LIU, Suhang CHEN, Xianli DUAN, Fan WU, Xiaofei XU, Xianyu SONG, Shuangliang ZHAO, Honglai LIU. Transport behavior of Janus graphene quantum dots in biomembrane: a molecular dynamics simulation [J]. CIESC Journal, 2022, 73(7): 2835-2843. |
| [9] | Wenjing ZHANG, Jing LI, Zidong WEI. Electrocatalysis from a mesoscale perspective: interface, membrane and porous electrode [J]. CIESC Journal, 2022, 73(6): 2289-2305. |
| [10] | Zhichao LI, Yu ZHENG, Runnan ZHANG, Zhongyi JIANG. Research progress of high flux and antifouling graphene oxide membranes [J]. CIESC Journal, 2022, 73(6): 2370-2380. |
| [11] | Yuzhe LIU, Chengcai LI, Lin LI, Shaohui WANG, Peihui LIU, Tonghua WANG. Structure-property relationship between microstructure of activated carbon and supercapacitor performance [J]. CIESC Journal, 2022, 73(4): 1807-1816. |
| [12] | Miao ZHANG, Honghai YANG, Yong YIN, Yue XU, Junjie SHEN, Xincheng LU, Weigang SHI, Jun WANG. Start-up and heat transfer characteristics of a pulsating heat pipe with graphene oxide nanofluids [J]. CIESC Journal, 2022, 73(3): 1136-1146. |
| [13] | Xuemei CHEN, Tong WANG, Yubo GAO, Dingcheng PENG, Yuting LUO. Efficient solar interfacial evaporation using laser-induced graphene [J]. CIESC Journal, 2022, 73(12): 5648-5659. |
| [14] | Huan XU, Lyu KE, Shenghui ZHANG, Zilin ZHANG, Guangdong HAN, Jinsheng CUI, Daoyuan TANG, Donghui HUANG, Jiefeng GAO, Xinjian HE. Upgrading dispersion and interfacial morphologies for thermally conductive polypropylene composites by in situ growth of carbon nanotubes at graphene oxide [J]. CIESC Journal, 2022, 73(11): 5150-5157. |
| [15] | Haibo LIU, Nan WANG, Hongzhou LIU, Tiezhu CHEN, Jianchang LI. Effects of voltage perturbation on the activities of microorganisms and key enzymes in EAD metabolic flux [J]. CIESC Journal, 2022, 73(10): 4603-4612. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||