CIESC Journal ›› 2024, Vol. 75 ›› Issue (4): 1519-1532.DOI: 10.11949/0438-1157.20240007
• Catalysis, kinetics and reactors • Previous Articles Next Articles
Xiaoqing YAN1( ), Ying ZHAO1(
), Ying ZHAO1( ), Yuzhe ZHANG1, Honghui OU1, Qizhong HUANG2, Huagui HU2, Guidong YANG1(
), Yuzhe ZHANG1, Honghui OU1, Qizhong HUANG2, Huagui HU2, Guidong YANG1( )
)
Received:2024-01-03
Revised:2024-03-22
Online:2024-06-07
Published:2024-04-25
Contact:
Guidong YANG
严孝清1( ), 赵瑛1(
), 赵瑛1( ), 张宇哲1, 欧鸿辉1, 黄起中2, 胡华贵2, 杨贵东1(
), 张宇哲1, 欧鸿辉1, 黄起中2, 胡华贵2, 杨贵东1( )
)
通讯作者:
杨贵东
作者简介:严孝清(1990—),男,博士研究生,助理教授,xq-yan@xjtu.edu.cn基金资助:CLC Number:
Xiaoqing YAN, Ying ZHAO, Yuzhe ZHANG, Honghui OU, Qizhong HUANG, Huagui HU, Guidong YANG. Preparation of five-fold twinned copper nanowires@polypyrrole and their electrocatalytic conversion of nitrate to ammonia[J]. CIESC Journal, 2024, 75(4): 1519-1532.
严孝清, 赵瑛, 张宇哲, 欧鸿辉, 黄起中, 胡华贵, 杨贵东. 五重孪晶铜纳米线@聚吡咯制备及其电催化硝酸盐还原制氨[J]. 化工学报, 2024, 75(4): 1519-1532.
Add to citation manager EndNote|Ris|BibTeX
| 样品编号 | T-CuNW/mg | 吡咯/μl | 过硫酸铵/mg | 碳酸氢钠/mg |
|---|---|---|---|---|
| T-CuNW | 50 | 0 | 0 | 0 |
| T-CuNW-5 | 50 | 5 | 0.0114 | 0.0084 |
| T-CuNW-10 | 50 | 10 | 0.0228 | 0.0168 |
| T-CuNW-20 | 50 | 20 | 0.0456 | 0.0336 |
Table 1 Preparation parameters of T-CuNW@ppy
| 样品编号 | T-CuNW/mg | 吡咯/μl | 过硫酸铵/mg | 碳酸氢钠/mg |
|---|---|---|---|---|
| T-CuNW | 50 | 0 | 0 | 0 |
| T-CuNW-5 | 50 | 5 | 0.0114 | 0.0084 |
| T-CuNW-10 | 50 | 10 | 0.0228 | 0.0168 |
| T-CuNW-20 | 50 | 20 | 0.0456 | 0.0336 |
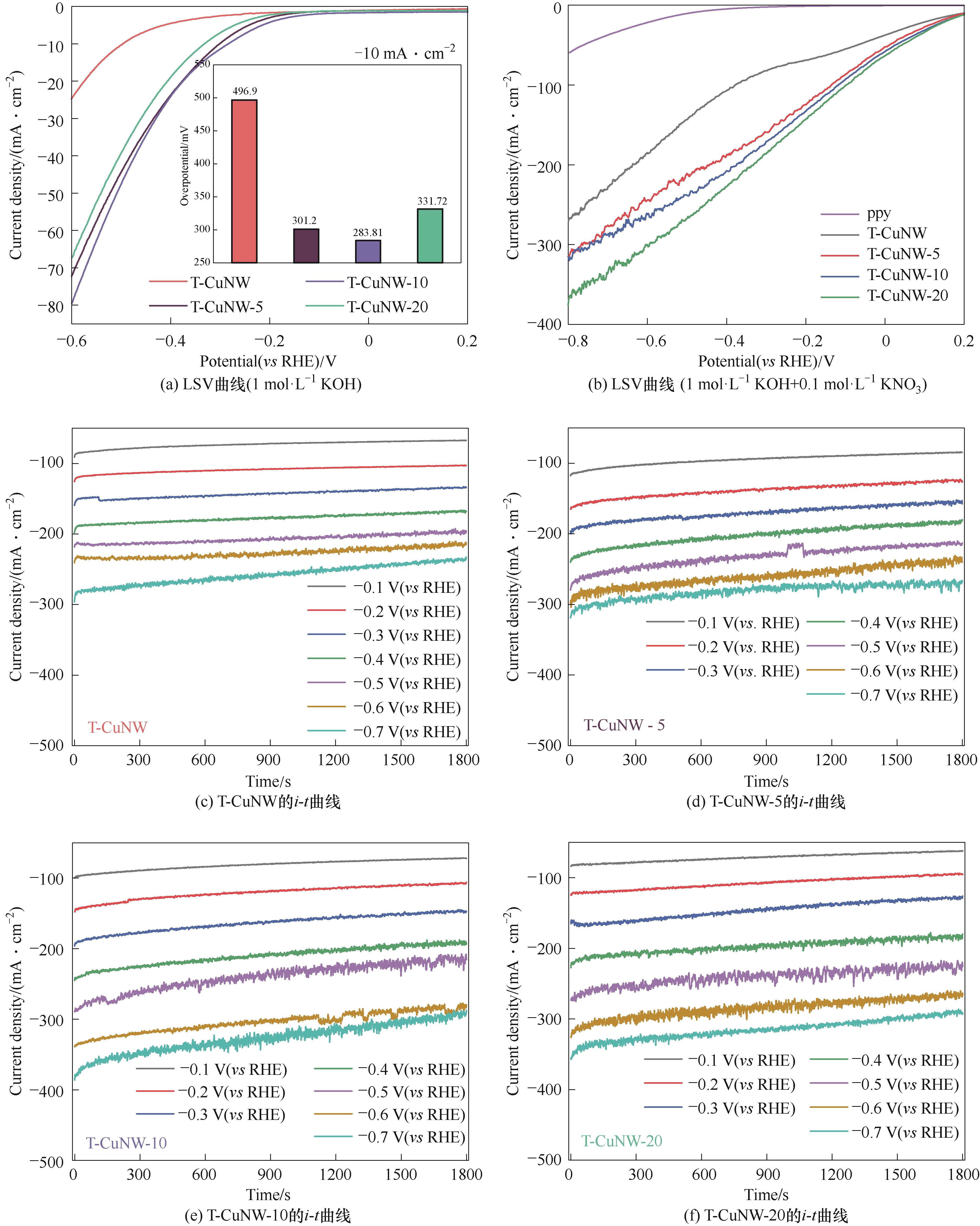
Fig.5 LSV polarization curves of as-synthesized samples in electrolytes using 0.1 mol·L-1 KOH as solvent without (a) and with (b) adding 0.1 mol·L-1NO3-; i-t curves of as-synthesized samples [(c) T-CuNW; (d) T-CuNW-5; (e) T-CuNW-10, (f) T-CuNW-20]; eNITRR performance of as-synthesized samples under different potential in 1 mol·L-1 KOH with 0.1 mol·L-1 KNO3 (g), FE of as-synthesized samples under different potential in 1 mol·L-1 KOH with 0.1 mol·L-1 KNO3 (h); eNITRR performance of T-CuNW-10 in electrolytes using 0.1 mol·L-1 KOH as solvent without and with adding 0.1 mol·L-1NO3- (i)
| 催化剂 | 偏压(vs RHE)/V | NH3产生速率 | 法拉第效率/% | 文献 |
|---|---|---|---|---|
| T-CuNW | -0.4 | 12.04 | 84.1 | 本论文 |
| T-CuNW@ppy | -0.4 | 13.83 | 83.0 | 本论文 |
| Cu49Fe1-NRA | -0.7 | 4.08 mg·cm-2·h-1 | 94.5 | [ |
| Cu SACs | -0.9 | 1.12 mg·cm-2·h-1 | 85.5 | [ |
| Cu1Co1HHTP | -0.6 | 5.09 mg·cm-2·h-1 | 96.4 | [ |
| Cu-Fe2O3 | -0.6 | 179.55 | 约100 | [ |
| Cu Ni NPS/CF | -0.48 | 94.57 mg· cm-2·h-1 | 97.0 | [ |
| T40-CuNCs | -0.6 | 2.62 mg·cm-2·h-1 | 96.8 | [ |
| Cu SCCs | -0.5 | 1.99 mg·cm-2·h-1 | 96.0 | [ |
| Cu-HTBs | -0.7 | 23789.8 | 90.0 | [ |
| Cu5Pd NCs | -0.7 | 32 mg·cm-2·h-1 | 95.5 | [ |
| Ru0.15Cu0.85 | -0.2 | 26.25 | 4.4 | [ |
Table 2 Comparison of eNITRR performance of T-CuNW, T-CuNW@ppy and other typical materials
| 催化剂 | 偏压(vs RHE)/V | NH3产生速率 | 法拉第效率/% | 文献 |
|---|---|---|---|---|
| T-CuNW | -0.4 | 12.04 | 84.1 | 本论文 |
| T-CuNW@ppy | -0.4 | 13.83 | 83.0 | 本论文 |
| Cu49Fe1-NRA | -0.7 | 4.08 mg·cm-2·h-1 | 94.5 | [ |
| Cu SACs | -0.9 | 1.12 mg·cm-2·h-1 | 85.5 | [ |
| Cu1Co1HHTP | -0.6 | 5.09 mg·cm-2·h-1 | 96.4 | [ |
| Cu-Fe2O3 | -0.6 | 179.55 | 约100 | [ |
| Cu Ni NPS/CF | -0.48 | 94.57 mg· cm-2·h-1 | 97.0 | [ |
| T40-CuNCs | -0.6 | 2.62 mg·cm-2·h-1 | 96.8 | [ |
| Cu SCCs | -0.5 | 1.99 mg·cm-2·h-1 | 96.0 | [ |
| Cu-HTBs | -0.7 | 23789.8 | 90.0 | [ |
| Cu5Pd NCs | -0.7 | 32 mg·cm-2·h-1 | 95.5 | [ |
| Ru0.15Cu0.85 | -0.2 | 26.25 | 4.4 | [ |
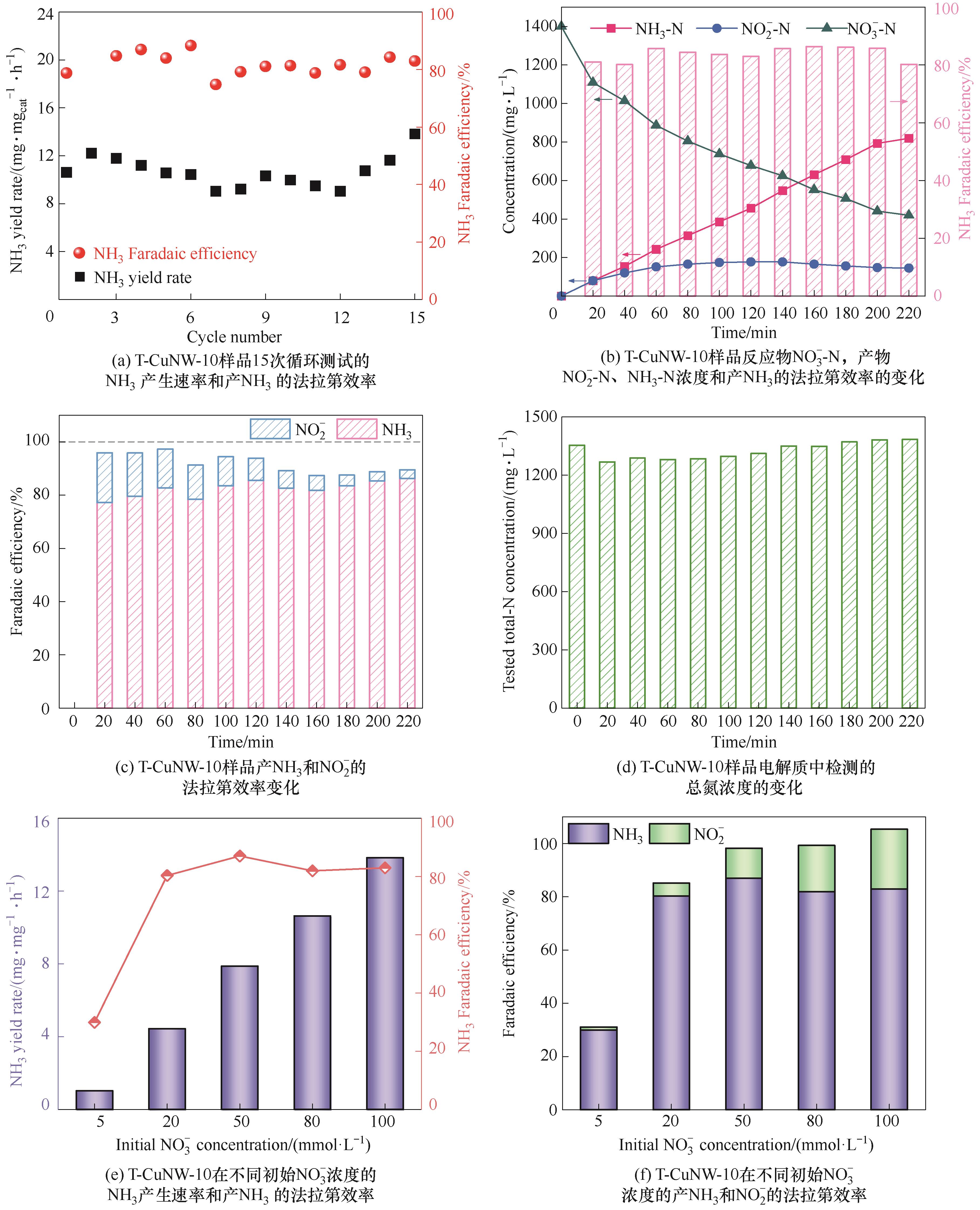
Fig.6 (a) Cycling tests of T-CuNW-10 for eNITRR tests at -0.4 V(vs RHE); (b) Time dependent concentration change of NO3-, NO2- and NH3 over T-CuNW-10 at -0.4 V(vs RHE); (c) Time dependent concentration change of FE; (d) Time dependent concentration change of total nitrogen at -0.4 V(vs RHE); (e) NH3 yield rate and FE of T-CuNW-10 with different concentrations of nitrate; (f) FE of NH3 and NO2- on T-CuNW-10 with different concentrations of nitrate
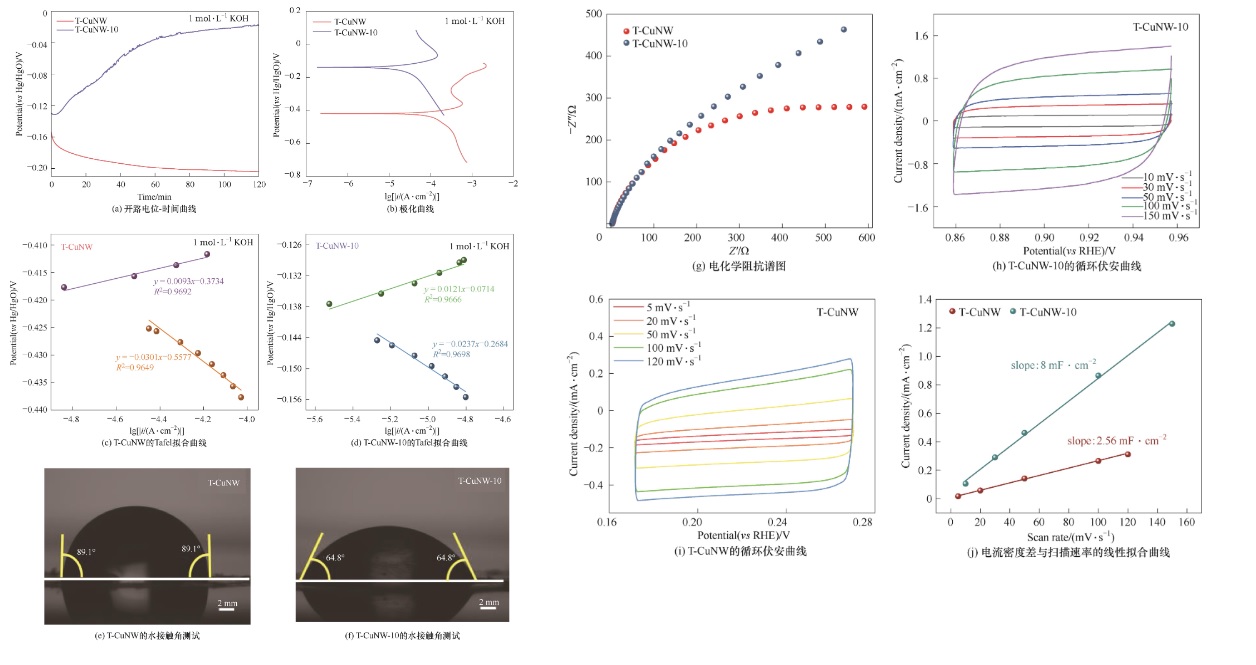
Fig.7 (a) Open circuit potentials decays with rest time; (b) Polarization curves; (c),(d) Curve of Tafel; (e),(f)Contact angle measurements; (g) Nyquist plots for the as-synthesized samples; (h),(i) Cyclic voltammograms; (j) Linear regression curve of difference in current density and scanning rate
| 样品名称 | Ecorr /mV | icorr/(μA·cm-2) |
|---|---|---|
| T-CuNW | -416.90 | 21.01 |
| T-CuNW-10 | -137.98 | 3.14 |
Table 3 Tafel curve fitting values of T-CUNW-10 and T-CuNW catalysts in 1 mol·L-1 KOH solution
| 样品名称 | Ecorr /mV | icorr/(μA·cm-2) |
|---|---|---|
| T-CuNW | -416.90 | 21.01 |
| T-CuNW-10 | -137.98 | 3.14 |
| 样品名称 | Cdl/(mF·cm-2) | ECSA/cm2 |
|---|---|---|
| T-CuNW | 2.56 | 64 |
| T-CuNW-10 | 8 | 200 |
Table 4 ECSA test of T-CUNW-10 and T-CuNW
| 样品名称 | Cdl/(mF·cm-2) | ECSA/cm2 |
|---|---|---|
| T-CuNW | 2.56 | 64 |
| T-CuNW-10 | 8 | 200 |
| 1 | Wang L, Xia M K, Wang H, et al. Greening ammonia toward the solar ammonia refinery[J]. Joule, 2018, 2(6): 1055-1074. |
| 2 | Guo J P, Chen P. Catalyst: NH3 as an energy carrier[J]. Chem, 2017, 3(5): 709-712. |
| 3 | Sun J, Alam D, Daiyan R, et al. A hybrid plasma electrocatalytic process for sustainable ammonia production[J]. Energy & Environmental Science, 2021, 14(2): 865-872. |
| 4 | 张谭, 刘光, 李晋平, 等. Ru基氮还原电催化剂性能调控策略[J]. 化工学报, 2023, 74(6): 2264-2280. |
| Zhang T, Liu G, Li J P, et al. Performance regulation strategies of Ru-based nitrogen reduction electrocatalysts[J]. CIESC Journal, 2023, 74(6): 2264-2280. | |
| 5 | Van Langevelde P H, Katsounaros I, Koper M T M. Electrocatalytic nitrate reduction for sustainable ammonia production[J]. Joule, 2021, 5(2): 290-294. |
| 6 | Fan S H, Hu Y N, Zhang T, et al. Highly selective environmental electrocatalytic nitrogen reduction to ammonia on Fe2(MoO4)3/C composite electrocatalyst[J]. International Journal of Hydrogen Energy, 2024, 51: 1198-1206. |
| 7 | Fan S H, Wang Q, Hu Y N, et al. Efficient electrocatalytic conversion of N2 to NH3 using oxygen-rich vacancy lithium niobate cubes[J]. Chinese Journal of Chemical Engineering, 2023, 62: 132-138. |
| 8 | Chen G F, Yuan Y F, Jiang H F, et al. Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper-molecular solid catalyst[J]. Nature Energy, 2020, 5: 605-613. |
| 9 | 杨通, 何小波, 银凤翔. M-MOF-74 (M=Ni, Co, Zn) 的制备及其电化学催化合成氨性能[J]. 化工学报, 2020, 71(6): 2857-2870. |
| Yang T, He X B, Yin F X. Preparation of M-MOF-74 (M=Ni, Co, Zn) and its performance in electrocatalytic synthesis of ammonia[J]. CIESC Journal, 2020, 71(6): 2857-2870. | |
| 10 | Gao J N, Jiang B, Ni C C, et al. Enhanced reduction of nitrate by noble metal-free electrocatalysis on P doped three-dimensional Co3O4 cathode: mechanism exploration from both experimental and DFT studies[J]. Chemical Engineering Journal, 2020, 382: 123034. |
| 11 | Pérez-Gallent E, Figueiredo M C, Katsounaros I, et al. Electrocatalytic reduction of nitrate on copper single crystals in acidic and alkaline solutions[J]. Electrochimica Acta, 2017, 227: 77-84. |
| 12 | Min B, Gao Q, Yan Z H, et al. Powering the remediation of the nitrogen cycle: progress and perspectives of electrochemical nitrate reduction[J]. Industrial & Engineering Chemistry Research, 2021, 60(41): 14635-14650. |
| 13 | Yao Q F, Chen J B, Xiao S Z, et al. Selective electrocatalytic reduction of nitrate to ammonia with nickel phosphide[J]. ACS Applied Materials & Interfaces, 2021, 13(26): 30458-30467. |
| 14 | Lv C D, Zhong L X, Liu H J, et al. Selective electrocatalytic synthesis of urea with nitrate and carbon dioxide[J]. Nature Sustainability, 2021, 4: 868-876. |
| 15 | Tao Z X, Wu Y S, Wu Z S, et al. Cascade electrocatalytic reduction of carbon dioxide and nitrate to ethylamine[J]. Journal of Energy Chemistry, 2022, 65: 367-370. |
| 16 | Dima G E, de Vooys A C A, Koper M T M. Electrocatalytic reduction of nitrate at low concentration on coinage and transition-metal electrodes in acid solutions[J]. Journal of Electroanalytical Chemistry, 2003, 554/555: 15-23. |
| 17 | Garcia-Segura S, Lanzarini-Lopes M, Hristovski K, et al. Electrocatalytic reduction of nitrate: fundamentals to full-scale water treatment applications[J]. Applied Catalysis B: Environmental, 2018, 236: 546-568. |
| 18 | Fu X B, Zhao X G, Hu X B, et al. Alternative route for electrochemical ammonia synthesis by reduction of nitrate on copper nanosheets[J]. Applied Materials Today, 2020, 19: 100620. |
| 19 | Gao W S, Xie K F, Xie J, et al. Alloying of Cu with Ru enabling the relay catalysis for reduction of nitrate to ammonia[J]. Advanced Materials, 2023, 35(19): e2202952. |
| 20 | Wang Y T, Zhang P, Lin X Y, et al. Wide-pH-range adaptable ammonia electrosynthesis from nitrate on Cu-Pd interfaces[J]. Science China Chemistry, 2023, 66(3): 913-922. |
| 21 | Song M, Zhou G, Lu N, et al. Oriented attachment induces fivefold twins by forming and decomposing high-energy grain boundaries[J]. Science, 2020, 367(6473): 40-45. |
| 22 | Tang C, Chen Z, Wang Y J, et al. Atomic editing copper twin boundary for precision CO2 reduction[J]. ACS Catalysis, 2022, 12(19): 11838-11844. |
| 23 | Li Y F, Cui F, Ross M B, et al. Structure-sensitive CO2 electroreduction to hydrocarbons on ultrathin 5-fold twinned copper nanowires[J]. Nano Letters, 2017, 17(2): 1312-1317. |
| 24 | Choi C, Kwon S, Cheng T, et al. Highly active and stable stepped Cu surface for enhanced electrochemical CO2 reduction to C2H4 [J]. Nature Catalysis, 2020, 3: 804-812. |
| 25 | Cai J, Zhao Q, Hsu W Y, et al. Highly selective electrochemical reduction of CO2 into methane on nanotwinned Cu[J]. Journal of the American Chemical Society, 2023, 145(16): 9136-9143. |
| 26 | Bouzek K, Paidar M, Sadílková A, et al. Electrochemical reduction of nitrate in weakly alkaline solutions[J]. Journal of Applied Electrochemistry, 2001, 31(11): 1185-1193. |
| 27 | Paidar M, Roušar I, Bouzek K. Electrochemical removal of nitrate ions in waste solutions after regeneration of ion exchange columns[J]. Journal of Applied Electrochemistry, 1999, 29(5): 611-617. |
| 28 | Liu Y, Liu Z, Lu N, et al. Facile synthesis of polypyrrole coated copper nanowires: a new concept to engineered core-shell structures[J]. Chemical Communications, 2012, 48(20): 2621-2623. |
| 29 | Wang W, Yan X Q, Geng J F, et al. Engineering a copper@polypyrrole nanowire network in the near field for plasmon-enhanced solar evaporation[J]. ACS Nano, 2021, 15(10): 16376-16394. |
| 30 | Niu Z Q, Chen S P, Yu Y, et al. Morphology-controlled transformation of Cu@Au core-shell nanowires into thermally stable Cu3Au intermetallic nanowires[J]. Nano Research, 2020, 13(9): 2564-2569. |
| 31 | Zeng G F, Sun Q, Horta S, et al. A layered Bi2Te3@PPy cathode for aqueous zinc-ion batteries: mechanism and application in printed flexible batteries[J]. Advanced Materials, 2024, 36(1): e2305128. |
| 32 | Wang C H, Liu Z Y, Hu T, et al. Metasequoia-like nanocrystal of iron-doped copper for efficient electrocatalytic nitrate reduction into ammonia in neutral media[J]. ChemSusChem, 2021, 14(8): 1825-1829. |
| 33 | Xu Y T, Xie M Y, Zhong H Q, et al. In situ clustering of single-atom copper precatalysts in a metal-organic framework for efficient electrocatalytic nitrate-to-ammonia reduction[J]. ACS Catalysis, 2022, 12(14): 8698-8706. |
| 34 | Liu H M, Lang X Y, Zhu C, et al. Efficient electrochemical nitrate reduction to ammonia with copper-supported rhodium cluster and single-atom catalysts[J]. Angewandte Chemie International Edition, 2022, 61(23): e202202556. |
| 35 | Fan K, Xie W F, Li J Z, et al. Active hydrogen boosts electrochemical nitrate reduction to ammonia[J]. Nature Communications, 2022, 13: 7958. |
| 36 | Wang Y L, Yin H B, Dong F, et al. N-coordinated Cu-Ni dual-single-atom catalyst for highly selective electrocatalytic reduction of nitrate to ammonia[J]. Small, 2023, 19(20): e2207695. |
| 37 | Li K, Ding L, Xie Z Q, et al. Robust copper-based nanosponge architecture decorated by ruthenium with enhanced electrocatalytic performance for ambient nitrogen reduction to ammonia[J]. ACS Applied Materials & Interfaces, 2023, 15(9): 11703-11712. |
| 38 | Li R, Gao T T, Qiu W X, et al. Unveiling the size effect of nitrogen-doped carbon-supported copper-based catalysts on nitrate-to-ammonia electroreduction[J]. Nano Research, 2023: 1-6. |
| 39 | Hu Q, Huo Q H, Qi S, et al. Unconventional synthesis of hierarchically twinned copper as efficient electrocatalyst for nitrate-ammonia conversion[J]. Advanced Materials, 2024, 36(11): 2311375. |
| 40 | Song Z M, Qin L, Liu Y, et al. Efficient electroreduction of nitrate to ammonia with CuPd nanoalloy catalysts[J]. ChemSusChem, 2023, 16(22): e202300202. |
| 41 | Luo W J, Wu S L, Jiang Y Y, et al. Efficient electrocatalytic nitrate reduction to ammonia based on DNA-templated copper nanoclusters[J]. ACS Applied Materials & Interfaces, 2023, 15(15): 18928-18939. |
| [1] | Tianyi LI, Yutai WU, Yongsheng WANG, Jiarui GU, Yiheng SONG, Fengcheng YANG, Guangping HAO. Advances in light isotopes separation and catalytic labeling [J]. CIESC Journal, 2024, 75(4): 1284-1301. |
| [2] | Mingze SUN, Helai HUANG, Zhiqiang NIU. Pt-based oxygen reduction reaction catalysts: from single crystal electrode to nanostructured extended surface [J]. CIESC Journal, 2024, 75(4): 1256-1269. |
| [3] | Yiwei FAN, Wei LIU, Yingying LI, Peixia WANG, Jisong ZHANG. Research progress on catalytic dehydrogenation of dodecahydro-N-ethylcarbazole as liquid organic hydrogen carrier [J]. CIESC Journal, 2024, 75(4): 1198-1208. |
| [4] | Youming SI, Lingfeng ZHENG, Pengzhong CHEN, Jiangli FAN, Xiaojun PENG. Performance and mechanism of novel antimony oxo cluster photoresist [J]. CIESC Journal, 2024, 75(4): 1705-1717. |
| [5] | Xudong JIA, Bolong YANG, Qian CHENG, Xueli LI, Zhonghua XIANG. Preparation of high-efficiency iron-cobalt bimetallic site oxygen reduction electrocatalysts by step-by-step metal loading method [J]. CIESC Journal, 2024, 75(4): 1578-1593. |
| [6] | Ang LI, Zhenyu ZHAO, Hong LI, Xin GAO. Microwave induced construction of highly dispersed Pd/FeP catalysts and their electrocatalytic performance [J]. CIESC Journal, 2024, 75(4): 1594-1606. |
| [7] | Yuwei YANG, Min LI, Zhiying YAO, Qinlin SUN, Yang LIU, Dan GE, Bingbing SUN. Application and prospect of organoids-on-chip in the study of nano-drug delivery systems [J]. CIESC Journal, 2024, 75(4): 1209-1221. |
| [8] | Yu HAN, Le ZHOU, Xin ZHANG, Yong LUO, Baochang SUN, Haikui ZOU, Jianfeng CHEN. Preparation of high adhesion Pd/SiO2/NF monolithic catalyst and its hydrogenation performance [J]. CIESC Journal, 2024, 75(4): 1533-1542. |
| [9] | Xiaokai CHENG, Wei LI, Jingdai WANG, Yongrong YANG. Advances in nickel catalyzed controlled/living radical polymerization reactions [J]. CIESC Journal, 2024, 75(4): 1105-1117. |
| [10] | Zhiming CHEN, Zefeng WANG, Gaoqi MA, Liangbo WANG, Chengtao YU, Pengju PAN. Research progress on improving thermal stability of polylactic acid based on stannous inactivation and chain end-group modification [J]. CIESC Journal, 2024, 75(3): 760-767. |
| [11] | Yuexing WEI, Ziyue HE, Kezhou YAN, Linyu LI, Yuhong QIN, Chong HE, Luchang JIAO. Catalytic degradation of bisphenol A by modified coal gasification slag [J]. CIESC Journal, 2024, 75(3): 877-889. |
| [12] | Yu CAO, Guohui ZHANG, Ang GAO, Xinyu DU, Jing ZHOU, Yongmao CAI, Xuan YU, Xiaoming YU. Research progress of two-dimensional MXene materials in solar cells and metal-ion batteries [J]. CIESC Journal, 2024, 75(2): 412-428. |
| [13] | Yuhua YIN, Can FANG, Qingfeng YI, Guang LI. Impact of different carbon conductive agents on performance of iron-air battery [J]. CIESC Journal, 2024, 75(2): 685-694. |
| [14] | Xingyu GAI, Yuxue YUE, Chunhua YANG, Zilong ZHANG, Tianzi CAI, Haifeng ZHANG, Bolin WANG, Xiaonian LI. Carbon supported Cs- and Cu-based catalysts for gas-phase dehydrochlorination of 1,1,2-trichloroethane [J]. CIESC Journal, 2024, 75(2): 575-583. |
| [15] | Jialin ZHANG, Dawei XU, Yue GAO, Xingang LI. Performance of soot combustion over CeO2 modified CuO catalysts supported on nickel foams [J]. CIESC Journal, 2024, 75(1): 312-321. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||