CIESC Journal ›› 2025, Vol. 76 ›› Issue (5): 2279-2293.DOI: 10.11949/0438-1157.20241210
• Separation engineering • Previous Articles Next Articles
Lei TANG( ), Zhenfei WANG, Congli LI, Jiahui YANG, Hao ZHENG, Qi SHI(
), Zhenfei WANG, Congli LI, Jiahui YANG, Hao ZHENG, Qi SHI( ), Jinxiang DONG
), Jinxiang DONG
Received:2024-10-31
Revised:2024-11-30
Online:2025-06-13
Published:2025-05-25
Contact:
Qi SHI
唐磊( ), 王振菲, 李聪利, 杨佳辉, 郑浩, 石琪(
), 王振菲, 李聪利, 杨佳辉, 郑浩, 石琪( ), 董晋湘
), 董晋湘
通讯作者:
石琪
作者简介:唐磊(1997—),男,硕士研究生,1193580514@qq.com
基金资助:CLC Number:
Lei TANG, Zhenfei WANG, Congli LI, Jiahui YANG, Hao ZHENG, Qi SHI, Jinxiang DONG. CO working capacity and operating conditions of Co-MOF-74 and Mg-MOF-74[J]. CIESC Journal, 2025, 76(5): 2279-2293.
唐磊, 王振菲, 李聪利, 杨佳辉, 郑浩, 石琪, 董晋湘. Co-MOF-74和Mg-MOF-74的CO工作吸附容量及操作条件[J]. 化工学报, 2025, 76(5): 2279-2293.
Add to citation manager EndNote|Ris|BibTeX
| 样品 | T/℃ | 吸附容量 | 工作吸附容量 | R/% | ||||
|---|---|---|---|---|---|---|---|---|
| p/bar | nCO/(mmol·g-1) | p/bar | ΔnCO/(mmol·g-1) | |||||
| 解吸 | 吸附 | 解吸 | 吸附 | |||||
| Co-MOF-74 | 25 | 0 | 0.5 | 5.38 | 0.1 | 0.5 | 1.04 | 19.33 |
| 60 | 0 | 0.5 | 4.75 | 0.1 | 0.5 | 1.70 | 35.79 | |
| 100 | 0 | 0.5 | 2.50 | 0.1 | 0.5 | 1.74 | 69.60 | |
| 140 | 0 | 0.5 | 0.93 | 0.1 | 0.5 | 0.77 | 82.80 | |
| 25 | 0 | 1.5 | 5.71 | 0.1 | 1.5 | 1.37 | 23.99 | |
| 60 | 0 | 1.5 | 5.44 | 0.1 | 1.5 | 2.39 | 43.93 | |
| 100 | 0 | 1.5 | 3.89 | 0.1 | 1.5 | 3.13 | 80.46 | |
| 140 | 0 | 1.5 | 2.10 | 0.1 | 1.5 | 1.94 | 92.38 | |
| 25 | 0 | 3.0 | 5.93 | 0.1 | 3.0 | 1.59 | 26.81 | |
| 60 | 0 | 3.0 | 5.75 | 0.1 | 3.0 | 2.70 | 46.96 | |
| 100 | 0 | 3.0 | 4.73 | 0.1 | 3.0 | 3.97 | 83.93 | |
| 140 | 0 | 3.0 | 3.12 | 0.1 | 3.0 | 2.96 | 94.87 | |
| Mg-MOF-74 | 25 | 0 | 0.5 | 2.83 | 0.1 | 0.5 | 1.86 | 65.72 |
| 60 | 0 | 0.5 | 1.17 | 0.1 | 0.5 | 0.88 | 75.21 | |
| 25 | 0 | 1.0 | 3.73 | 0.1 | 1.0 | 2.76 | 73.99 | |
| 60 | 0 | 1.0 | 1.92 | 0.1 | 1.0 | 1.63 | 84.90 | |
| 25 | 0 | 2.0 | 4.52 | 0.1 | 2.0 | 3.55 | 78.54 | |
| 60 | 0 | 2.0 | 2.82 | 0.1 | 2.0 | 2.53 | 89.72 | |
Table 1 Summary of static adsorption capacity, working capacity and regenerability of CO for Co-MOF-74 and Mg-MOF-74
| 样品 | T/℃ | 吸附容量 | 工作吸附容量 | R/% | ||||
|---|---|---|---|---|---|---|---|---|
| p/bar | nCO/(mmol·g-1) | p/bar | ΔnCO/(mmol·g-1) | |||||
| 解吸 | 吸附 | 解吸 | 吸附 | |||||
| Co-MOF-74 | 25 | 0 | 0.5 | 5.38 | 0.1 | 0.5 | 1.04 | 19.33 |
| 60 | 0 | 0.5 | 4.75 | 0.1 | 0.5 | 1.70 | 35.79 | |
| 100 | 0 | 0.5 | 2.50 | 0.1 | 0.5 | 1.74 | 69.60 | |
| 140 | 0 | 0.5 | 0.93 | 0.1 | 0.5 | 0.77 | 82.80 | |
| 25 | 0 | 1.5 | 5.71 | 0.1 | 1.5 | 1.37 | 23.99 | |
| 60 | 0 | 1.5 | 5.44 | 0.1 | 1.5 | 2.39 | 43.93 | |
| 100 | 0 | 1.5 | 3.89 | 0.1 | 1.5 | 3.13 | 80.46 | |
| 140 | 0 | 1.5 | 2.10 | 0.1 | 1.5 | 1.94 | 92.38 | |
| 25 | 0 | 3.0 | 5.93 | 0.1 | 3.0 | 1.59 | 26.81 | |
| 60 | 0 | 3.0 | 5.75 | 0.1 | 3.0 | 2.70 | 46.96 | |
| 100 | 0 | 3.0 | 4.73 | 0.1 | 3.0 | 3.97 | 83.93 | |
| 140 | 0 | 3.0 | 3.12 | 0.1 | 3.0 | 2.96 | 94.87 | |
| Mg-MOF-74 | 25 | 0 | 0.5 | 2.83 | 0.1 | 0.5 | 1.86 | 65.72 |
| 60 | 0 | 0.5 | 1.17 | 0.1 | 0.5 | 0.88 | 75.21 | |
| 25 | 0 | 1.0 | 3.73 | 0.1 | 1.0 | 2.76 | 73.99 | |
| 60 | 0 | 1.0 | 1.92 | 0.1 | 1.0 | 1.63 | 84.90 | |
| 25 | 0 | 2.0 | 4.52 | 0.1 | 2.0 | 3.55 | 78.54 | |
| 60 | 0 | 2.0 | 2.82 | 0.1 | 2.0 | 2.53 | 89.72 | |
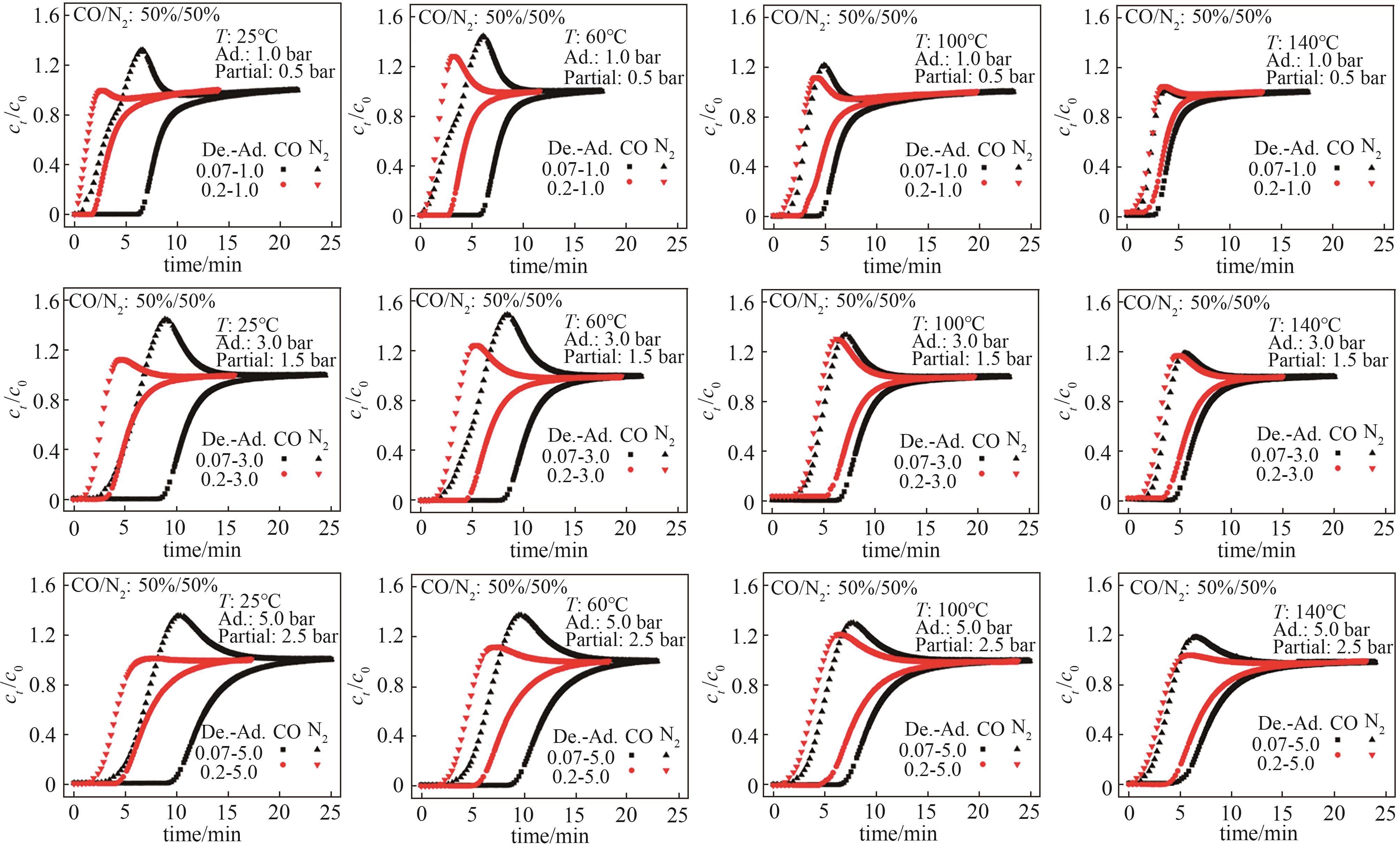
Fig.10 CO/N2 (50%/50%, vol) breakthrough curves of Co-MOF-74T—operating temperature; Partial—CO adsorption partial pressure; Ad.—total adsorption pressure; De.—total desorption pressure
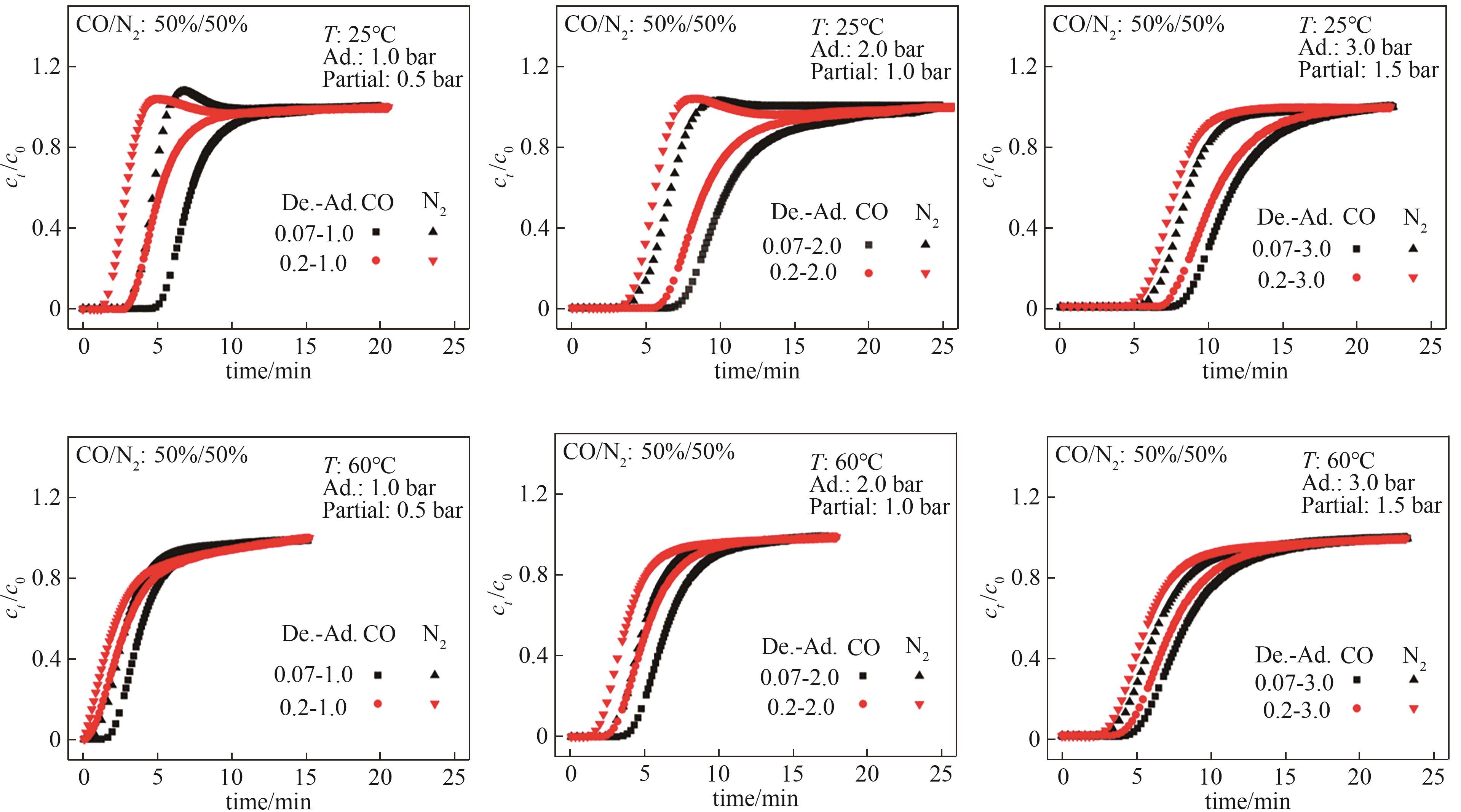
Fig.11 CO/N2 (50%/50%, vol) breakthrough curves of Mg-MOF-74T—operating temperature; Partial—CO adsorption partial pressure; Ad.—total adsorption pressure; De.—total desorption pressure
| 样品 | 进样组成(CO/N2) | T/℃ | 吸附容量 | 工作吸附容量 | R/% | ||||
|---|---|---|---|---|---|---|---|---|---|
| p/bar | nCO/(mmol·g-1) | p/bar | ΔnCO/(mmol·g-1) | ||||||
| 解吸 | 吸附 | 解吸 | 吸附 | ||||||
| Co-MOF-74 | 50%/50% | 25 | 0.07① 0② | 1.0① 0.5② | 3.30 | 0.2① 0.1② | 1.0① 0.5② | 1.51 | 45.76 |
| 60 | 3.00 | 1.74 | 58.00 | ||||||
| 100 | 2.70 | 2.10 | 77.78 | ||||||
| 140 | 1.74 | 1.39 | 79.88 | ||||||
| 25 | 0.07① 0② | 3.0① 1.5② | 4.25 | 0.2① 0.1② | 3.0① 1.5② | 2.13 | 50.12 | ||
| 60 | 4.02 | 2.66 | 66.17 | ||||||
| 100 | 3.40 | 2.85 | 83.82 | ||||||
| 140 | 2.60 | 2.27 | 87.31 | ||||||
| 25 | 0.07① 0② | 5.0① 2.5② | 4.92 | 0.2① 0.1② | 5.0① 2.5② | 2.86 | 58.13 | ||
| 60 | 4.61 | 3.30 | 71.58 | ||||||
| 100 | 3.82 | 3.44 | 90.05 | ||||||
| 140 | 3.08 | 2.82 | 91.56 | ||||||
| Mg-MOF-74 | 50%/50% | 25 | 0.07① | 1.0① | 1.40 | 0.2① | 1.0① | 0.93 | 66.43 |
| 60 | 0② | 0.5② | 0.81 | 0.1② | 0.5② | 0.63 | 77.77 | ||
| 25 | 0.07① | 2.0① | 2.05 | 0.2① | 2.0① | 1.63 | 79.51 | ||
| 60 | 0② | 1.0② | 1.27 | 0.1② | 1.0② | 1.02 | 80.31 | ||
| 25 | 0.07① | 3.0① | 2.20 | 0.2① | 3.0① | 1.96 | 89.09 | ||
| 60 | 0② | 1.5② | 1.61 | 0.1② | 1.5② | 1.45 | 90.06 | ||
Table 2 Summary of dynamic adsorption capacity, working capacity and regenerability of CO for Co-MOF-74 and Mg-MOF-74
| 样品 | 进样组成(CO/N2) | T/℃ | 吸附容量 | 工作吸附容量 | R/% | ||||
|---|---|---|---|---|---|---|---|---|---|
| p/bar | nCO/(mmol·g-1) | p/bar | ΔnCO/(mmol·g-1) | ||||||
| 解吸 | 吸附 | 解吸 | 吸附 | ||||||
| Co-MOF-74 | 50%/50% | 25 | 0.07① 0② | 1.0① 0.5② | 3.30 | 0.2① 0.1② | 1.0① 0.5② | 1.51 | 45.76 |
| 60 | 3.00 | 1.74 | 58.00 | ||||||
| 100 | 2.70 | 2.10 | 77.78 | ||||||
| 140 | 1.74 | 1.39 | 79.88 | ||||||
| 25 | 0.07① 0② | 3.0① 1.5② | 4.25 | 0.2① 0.1② | 3.0① 1.5② | 2.13 | 50.12 | ||
| 60 | 4.02 | 2.66 | 66.17 | ||||||
| 100 | 3.40 | 2.85 | 83.82 | ||||||
| 140 | 2.60 | 2.27 | 87.31 | ||||||
| 25 | 0.07① 0② | 5.0① 2.5② | 4.92 | 0.2① 0.1② | 5.0① 2.5② | 2.86 | 58.13 | ||
| 60 | 4.61 | 3.30 | 71.58 | ||||||
| 100 | 3.82 | 3.44 | 90.05 | ||||||
| 140 | 3.08 | 2.82 | 91.56 | ||||||
| Mg-MOF-74 | 50%/50% | 25 | 0.07① | 1.0① | 1.40 | 0.2① | 1.0① | 0.93 | 66.43 |
| 60 | 0② | 0.5② | 0.81 | 0.1② | 0.5② | 0.63 | 77.77 | ||
| 25 | 0.07① | 2.0① | 2.05 | 0.2① | 2.0① | 1.63 | 79.51 | ||
| 60 | 0② | 1.0② | 1.27 | 0.1② | 1.0② | 1.02 | 80.31 | ||
| 25 | 0.07① | 3.0① | 2.20 | 0.2① | 3.0① | 1.96 | 89.09 | ||
| 60 | 0② | 1.5② | 1.61 | 0.1② | 1.5② | 1.45 | 90.06 | ||
| 1 | Martinelli M, Gnanamani M K, LeViness S, et al. An overview of Fischer-Tropsch synthesis: XtL processes, catalysts and reactors[J]. Applied Catalysis A: General, 2020, 608: 117740. |
| 2 | Liu Y T, Deng D H, Bao X H. Catalysis for selected C1 chemistry[J]. Chem, 2020, 6(10): 2497-2514. |
| 3 | 霍猛, 彭晓婉, 赵金, 等. 基于COSMO-RS的离子液体吸收CO的溶剂筛选及H2/CO分离实验[J]. 化工学报, 2022, 73(12): 5305-5313. |
| Huo M, Peng X W, Zhao J, et al. COSMO-RS based solvent screening and H2/CO separation experiments for CO absorption by ionic liquids[J]. CIESC Journal, 2022, 73(12): 5305-5313. | |
| 4 | Ramírez-Santos Á A, Castel C, Favre E. A review of gas separation technologies within emission reduction programs in the iron and steel sector: current application and development perspectives[J]. Separation and Purification Technology, 2018, 194: 425-442. |
| 5 | Mondal P, Dang G S, Garg M O. Syngas production through gasification and cleanup for downstream applications—recent developments[J]. Fuel Processing Technology, 2011, 92(8): 1395-1410. |
| 6 | Flores-Granobles M, Saeys M. Dynamic pressure-swing chemical looping process for the recovery of CO from blast furnace gas[J]. Energy Conversion and Management, 2022, 258: 115515. |
| 7 | Lee H H, Lee J C, Joo Y J, et al. Dynamic modeling of Shell entrained flow gasifier in an integrated gasification combined cycle process[J]. Applied Energy, 2014, 131: 425-440. |
| 8 | Li Y X, Li S S, Xue D M, et al. Incorporation of Cu(Ⅱ) and its selective reduction to Cu(Ⅰ) within confined spaces: efficient active sites for CO adsorption[J]. Journal of Materials Chemistry A, 2018, 6(19): 8930-8939. |
| 9 | Fakhraei Ghazvini M, Vahedi M, Najafi Nobar S, et al. Investigation of the MOF adsorbents and the gas adsorptive separation mechanisms[J]. Journal of Environmental Chemical Engineering, 2021, 9(1): 104790. |
| 10 | Oh H, Beum H T, Yoon Y S, et al. Experiment and modeling of adsorption of CO from blast furnace gas onto CuCl/boehmite[J]. Industrial & Engineering Chemistry Research, 2020, 59(26): 12176-12185. |
| 11 | 蔺彩虹, 王丽, 吴瑜, 等. 沸石中碱金属阳离子对CO2/N2O吸附分离性能的影响[J]. 化工学报, 2023, 74(5): 2013-2021. |
| Lin C H, Wang L, Wu Y, et al. Effect of alkali cations in zeolites on adsorption and separation of CO2/N2O[J]. CIESC Journal, 2023, 74(5): 2013-2021. | |
| 12 | Oh H, Tae Beum H, Lee S Y, et al. Bed configurations in CO vacuum pressure swing adsorption process for basic oxygen furnace gas utilization: experiment, simulation, and techno-economic analysis[J]. Chemical Engineering Journal, 2023, 454: 140432. |
| 13 | He Y B, Xiang S C, Chen B L. A microporous hydrogen-bonded organic framework for highly selective C2H2/C2H4 separation at ambient temperature[J]. Journal of the American Chemical Society, 2011, 133(37): 14570-14573. |
| 14 | Ko K J, Kim H, Cho Y H, et al. Overview of carbon monoxide adsorption performance of pristine and modified adsorbents[J]. Journal of Chemical & Engineering Data, 2022, 67(7): 1599-1616. |
| 15 | Lopes F V S, Grande C A, Rodrigues A E. Activated carbon for hydrogen purification by pressure swing adsorption: multicomponent breakthrough curves and PSA performance[J]. Chemical Engineering Science, 2011, 66(3): 303-317. |
| 16 | Huang H Y, Padin J, Yang R T. Comparison of π-complexations of ethylene and carbon monoxide with Cu+ and Ag+ [J]. Industrial & Engineering Chemistry Research, 1999, 38(7): 2720-2725. |
| 17 | Feyzbar-Khalkhali-Nejad F, Hassani E, Rashti A, et al. Adsorption-based CO removal: principles and materials[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105317. |
| 18 | Peng J J, Xian S K, Xiao J, et al. A supported Cu(Ⅰ)@MIL-100(Fe) adsorbent with high CO adsorption capacity and CO/N2 selectivity[J]. Chemical Engineering Journal, 2015, 270: 282-289. |
| 19 | 梁晓武. Co-MOF-74的合成及CO/N2吸附分离性能的研究[D]. 太原: 太原理工大学, 2021. |
| Liang X W. Synthesis of Co-MOF-74 and study on adsorption and separation performance of CO/N2 [D]. Taiyuan: Taiyuan University of Technology, 2021. | |
| 20 | Evans A, Cummings M S, Luebke R, et al. Screening metal-organic frameworks for dynamic CO/N2 separation using complementary adsorption measurement techniques[J]. Industrial & Engineering Chemistry Research, 2019, 58(39): 18336-18344. |
| 21 | Bloch E D, Hudson M R, Mason J A, et al. Reversible CO binding enables tunable CO/H₂ and CO/N₂ separations in metal-organic frameworks with exposed divalent metal cations[J]. Journal of the American Chemical Society, 2014, 136(30): 10752-10761. |
| 22 | Evans A, Luebke R, Petit C. The use of metal-organic frameworks for CO purification[J]. Journal of Materials Chemistry A, 2018, 6(23): 10570-10594. |
| 23 | Kim H, Sohail M, Yim K, et al. Effective CO2 and CO separation using[M2(DOBDC)] (M = Mg, Co, Ni) with unsaturated metal sites and excavation of their adsorption sites[J]. ACS Applied Materials & Interfaces, 2019, 11(7): 7014-7021. |
| 24 | Pandey I, Lin L C, Chen C C, et al. Understanding carbon monoxide binding and interactions in M-MOF-74 (M = Mg, Mn, Ni, Zn)[J]. Langmuir, 2023, 39(50): 18187-18197. |
| 25 | Cheng M, Wang S H, Zhang Z Y, et al. High-throughput virtual screening of metal-organic frameworks for xenon recovery from exhaled anesthetic gas mixture[J]. Chemical Engineering Journal, 2023, 451: 138218. |
| 26 | Raganati F, Chirone R, Ammendola P. CO2 capture by temperature swing adsorption: working capacity As affected by temperature and CO2 partial pressure[J]. Industrial & Engineering Chemistry Research, 2020, 59(8): 3593-3605. |
| 27 | Wang Z F, Li C L, Tang L, et al. The CO working capacity of Ni-MOF-74 and corresponding operating conditions for CO/N2 adsorption separation[J]. Industrial & Engineering Chemistry Research, 2024, 63(31): 13776-13786. |
| 28 | Rowsell J L C, Yaghi O M. Effects of functionalization, catenation, and variation of the metal oxide and organic linking units on the low-pressure hydrogen adsorption properties of metal-organic frameworks[J]. Journal of the American Chemical Society, 2006, 128(4): 1304-1315. |
| 29 | Caskey S R, Wong-Foy A G, Matzger A J. Dramatic tuning of carbon dioxide uptake via metal substitution in a coordination polymer with cylindrical pores[J]. Journal of the American Chemical Society, 2008, 130(33): 10870-10871. |
| 30 | Xue C L, Hao W M, Cheng W P, et al. CO adsorption performance of CuCl/activated carbon by simultaneous Reduction-Dispersion of mixed Cu(Ⅱ) salts[J]. Materials, 2019, 12(10): 1605. |
| 31 | Oliveira M L M, Miranda A A L, Barbosa C M B M, et al. Adsorption of thiophene and toluene on NaY zeolites exchanged with Ag(Ⅰ), Ni(Ⅱ) and Zn(Ⅱ)[J]. Fuel, 2009, 88(10): 1885-1892. |
| 32 | Cessford N F, Seaton N A, Düren T. Evaluation of ideal adsorbed solution theory as a tool for the design of metal-organic framework materials[J]. Industrial & Engineering Chemistry Research, 2012, 51(13): 4911-4921. |
| 33 | Bae Y S, Lee C Y, Kim K C, et al. High propene/propane selectivity in isostructural metal-organic frameworks with high densities of open metal sites[J]. Angewandte Chemie (International Ed), 2012, 51(8): 1857-1860. |
| 34 | Choma J, Stachurska K, Marszewski M, et al. Equilibrium isotherms and isosteric heat for CO2 adsorption on nanoporous carbons from polymers[J]. Adsorption, 2016, 22(4): 581-588. |
| 35 | Tao L R, You Y Y, Liu X J. Numerical studies of CO separation and enrichment from blast furnace gas by using a CuCl/Y fixed bed[J]. Ironmaking & Steelmaking, 2021, 48(10): 1187-1199. |
| 36 | Basdogan Y, Sezginel K B, Keskin S. Identifying highly selective metal organic frameworks for CH4/H2 separations using computational tools[J]. Industrial & Engineering Chemistry Research, 2015, 54(34): 8479-8491. |
| 37 | Bae Y S, Snurr R Q. Development and evaluation of porous materials for carbon dioxide separation and capture[J]. Angewandte Chemie International Edition, 2011, 50(49): 11586-11596. |
| 38 | Tong M M, Yang Q Y, Xiao Y L, et al. Revealing the structure-property relationship of covalent organic frameworks for CO₂ capture from postcombustion gas: a multi-scale computational study[J]. Physical Chemistry Chemical Physics, 2014, 16(29): 15189-15198. |
| 39 | Jiang H X, Wang Q Y, Wang H Q, et al. MOF-74 as an efficient catalyst for the low-temperature selective catalytic reduction of NO x with NH3 [J]. ACS Applied Materials & Interfaces, 2016, 8(40): 26817-26826. |
| 40 | Sun H, Ren D N, Kong R Q, et al. Tuning 1-hexene/n-hexane adsorption on MOF-74 via constructing Co-Mg bimetallic frameworks[J]. Microporous and Mesoporous Materials, 2019, 284: 151-160. |
| 41 | Wu Y Q, Chen Z A, Li B, et al. Highly selective adsorption of CO over N2 on CuCl-loaded SAPO-34 adsorbent[J]. Journal of Energy Chemistry, 2019, 36: 122-128. |
| 42 | Álvarez-Gutiérrez N, Gil M V, Rubiera F, et al. Adsorption performance indicators for the CO2/CH4 separation: application to biomass-based activated carbons[J]. Fuel Processing Technology, 2016, 142: 361-369. |
| [1] | Ruijie MA, Zixuan HUANG, Xueqian GUAN, Guangjin CHEN, Bei LIU. Efficient ethane and methane separation using ZIF-8/DMPU slurry [J]. CIESC Journal, 2025, 76(5): 2262-2269. |
| [2] | Zhichao XU, Zhendong YU, Haofeng WU, Peiwen WU, Hongxiang WU, Yanhong CHAO, Wenshuai ZHU, Zhichang LIU, Chunming XU. Preparation of acid-rich 13X molecular sieve and its ultra-deep adsorption removal of mercaptan in biodiesel [J]. CIESC Journal, 2025, 76(5): 2198-2208. |
| [3] | Pengtao GUO, Ting WANG, Bo XUE, Yunpan YING, Dahuan LIU. Ultramicroporous MOF with multiple adsorption sites for CH4/N2 separation [J]. CIESC Journal, 2025, 76(5): 2304-2312. |
| [4] | Yan LI, Meili LEI, Xingang LI. Regulation strategy of sequential simulated moving bed structure based on separation performance [J]. CIESC Journal, 2025, 76(5): 2219-2229. |
| [5] | Yaqi BA, Tao WU, Andi DI, Anhui LU. Progress in porous carbons for efficient separation of gaseous light hydrocarbon [J]. CIESC Journal, 2025, 76(5): 2136-2157. |
| [6] | Peng TAN, Xuemei LI, Xiaoqin LIU, Linbing SUN. Study on magnetically responsive composite materials based on flexible MOFs and their propylene adsorption performance [J]. CIESC Journal, 2025, 76(5): 2230-2240. |
| [7] | Hao QI, Yujie WANG, Shenhui LI, Qi ZOU, Yiqun LIU, Zhiping ZHAO. Molecular simulation study on adsorption and diffusion of C3H6 and C3H8 on Co/Zn-ZIFs [J]. CIESC Journal, 2025, 76(5): 2313-2326. |
| [8] | Chunhui TAO, Yinhui LI, Yu FU, Ran DUAN, Zeyi ZHAO, Yufeng TANG, Gang ZHANG, Heping MA. Selective adsorption and purification of low-concentration Kr gas using various adsorbents [J]. CIESC Journal, 2025, 76(5): 2358-2366. |
| [9] | Yue ZHANG, Jiaxin LIU, Jing MA, Yi LIU. Recent progress on metal-organic framework membranes towards uranium separation from seawater [J]. CIESC Journal, 2025, 76(5): 2087-2100. |
| [10] | Jialang HU, Mingyuan JIANG, Lyuming JIN, Yonggang ZHANG, Peng HU, Hongbing JI. Machine learning-assisted high-throughput computational screening of MOFs and advances in gas separation research [J]. CIESC Journal, 2025, 76(5): 1973-1996. |
| [11] | Junde ZHAO, Aiguo ZHOU, Yanlin CHEN, Jiale ZHENG, Tianshu GE. Current status of energy consumption of adsorption CO2 direct air capture [J]. CIESC Journal, 2025, 76(4): 1375-1390. |
| [12] | Yihao JIN, Junxin LUO, Zhangmao HU, Wei WANG, Qian YIN. Experimental investigation on hydrophilic functionalized MgSO4/expanded vermiculite composites for water adsorption and heat storage [J]. CIESC Journal, 2025, 76(4): 1852-1862. |
| [13] | Tianzi CAI, Haifeng ZHANG, Haidan LIN, Zilong ZHANG, Pengyu ZHOU, Bolin WANG, Xiaonian LI. A density functional theory study on the sensing of dissolved gases CO and CO2 in transformer oil using boron-doped nitrogen-based graphene [J]. CIESC Journal, 2025, 76(4): 1841-1851. |
| [14] | Yao FU, Yingjuan SHAO, Wenqi ZHONG. Experimental study on cyclic heat storage performance of TiO2-doped calcium based materials under pressurized carbonation [J]. CIESC Journal, 2025, 76(3): 1180-1190. |
| [15] | Liwen ZHAO, Guilian LIU. Performance enhancement and parameter optimization of complex catalytic reaction system based on system integration [J]. CIESC Journal, 2025, 76(3): 1111-1119. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||