CIESC Journal ›› 2022, Vol. 73 ›› Issue (3): 1403-1415.DOI: 10.11949/0438-1157.20211801
• Material science and engineering, nanotechnology • Previous Articles
Jian WANG1( ),Zixuan LEI1,Jiayu YAO1,Jian LI2,Yuhong LIU1(
),Zixuan LEI1,Jiayu YAO1,Jian LI2,Yuhong LIU1( )
)
Received:2021-12-22
Revised:2022-01-27
Online:2022-03-14
Published:2022-03-15
Contact:
Yuhong LIU
通讯作者:
刘育红
作者简介:王建(1996—),男,硕士研究生,基金资助:CLC Number:
Jian WANG, Zixuan LEI, Jiayu YAO, Jian LI, Yuhong LIU. Synthesis and curing kinetics of terephthalaldehyde phenolic resin[J]. CIESC Journal, 2022, 73(3): 1403-1415.
王建, 雷子萱, 姚家钰, 李建, 刘育红. 对苯二甲醛酚醛树脂的制备及其固化动力学研究[J]. 化工学报, 2022, 73(3): 1403-1415.
Add to citation manager EndNote|Ris|BibTeX
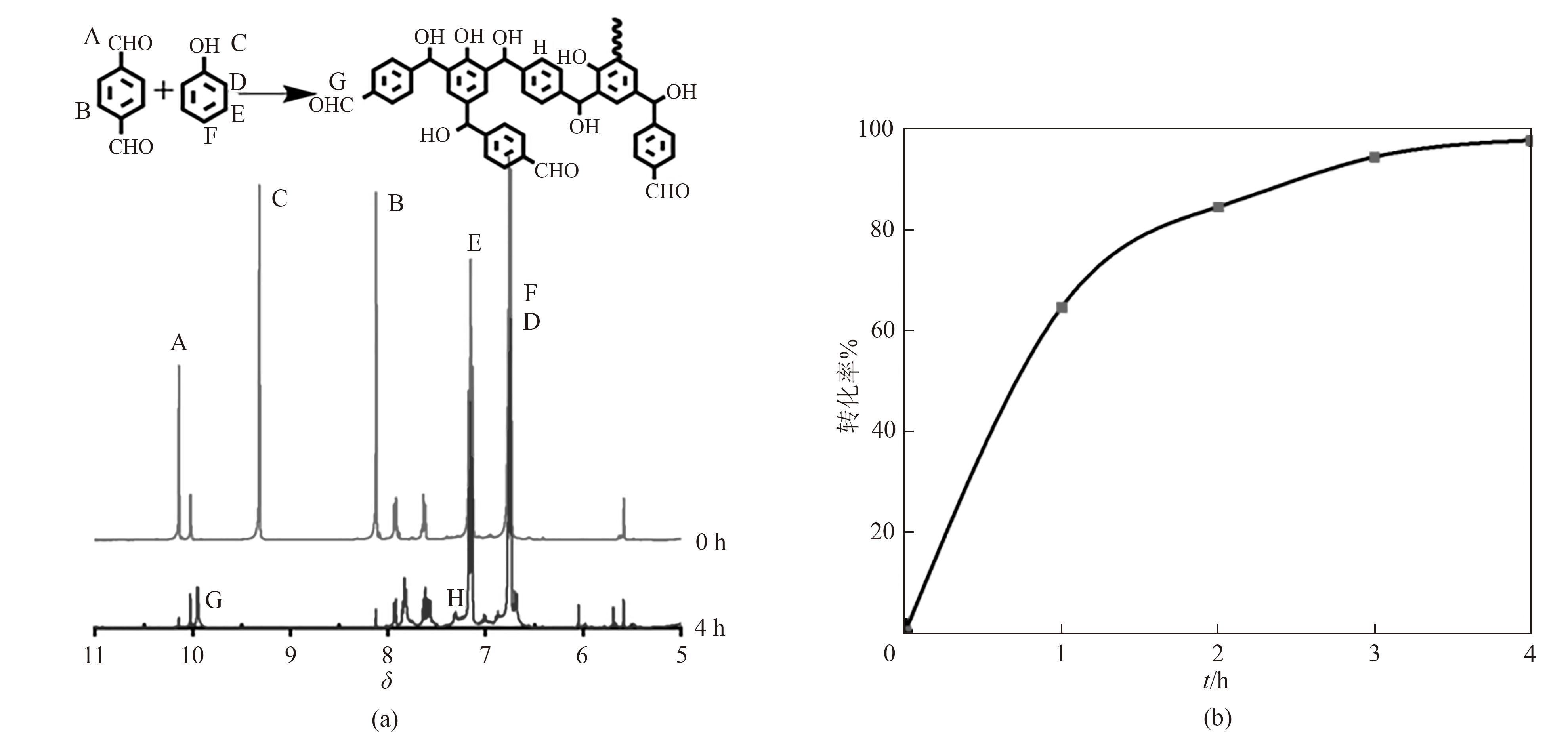
Fig.1 NMR images at 0 h and 4 h during the synthesis of terephthalaldehyde phenolic resin and possible prepolymer structure(a); The amount of terephthalaldehyde involved in the reaction process(b)
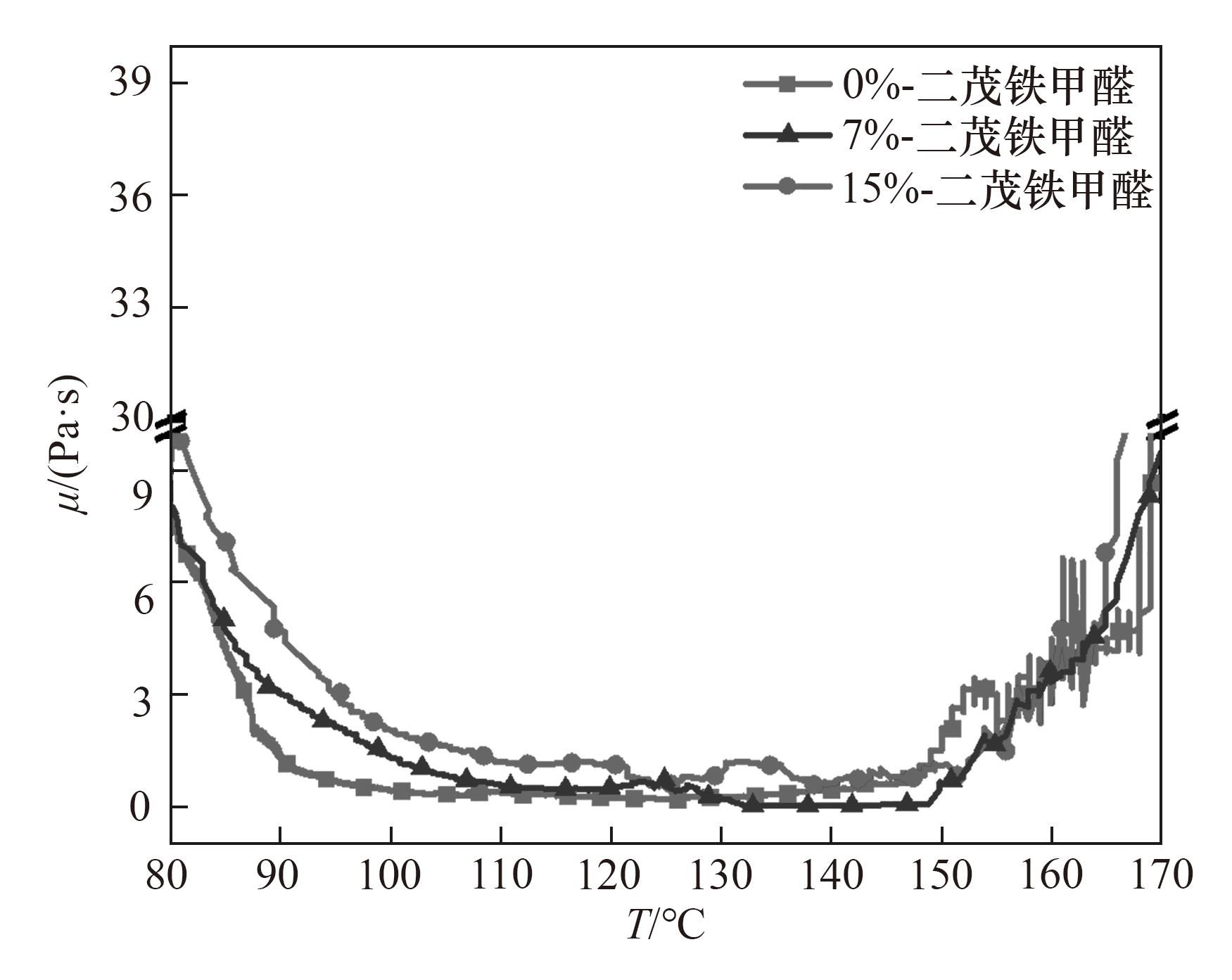
Fig.4 Rheological viscosity diagrams of terephthalaldehyde phenolic resins with different contents of ferrocenecarboxaldehyde (0%, 7%, 15%) during heating (80—170℃)
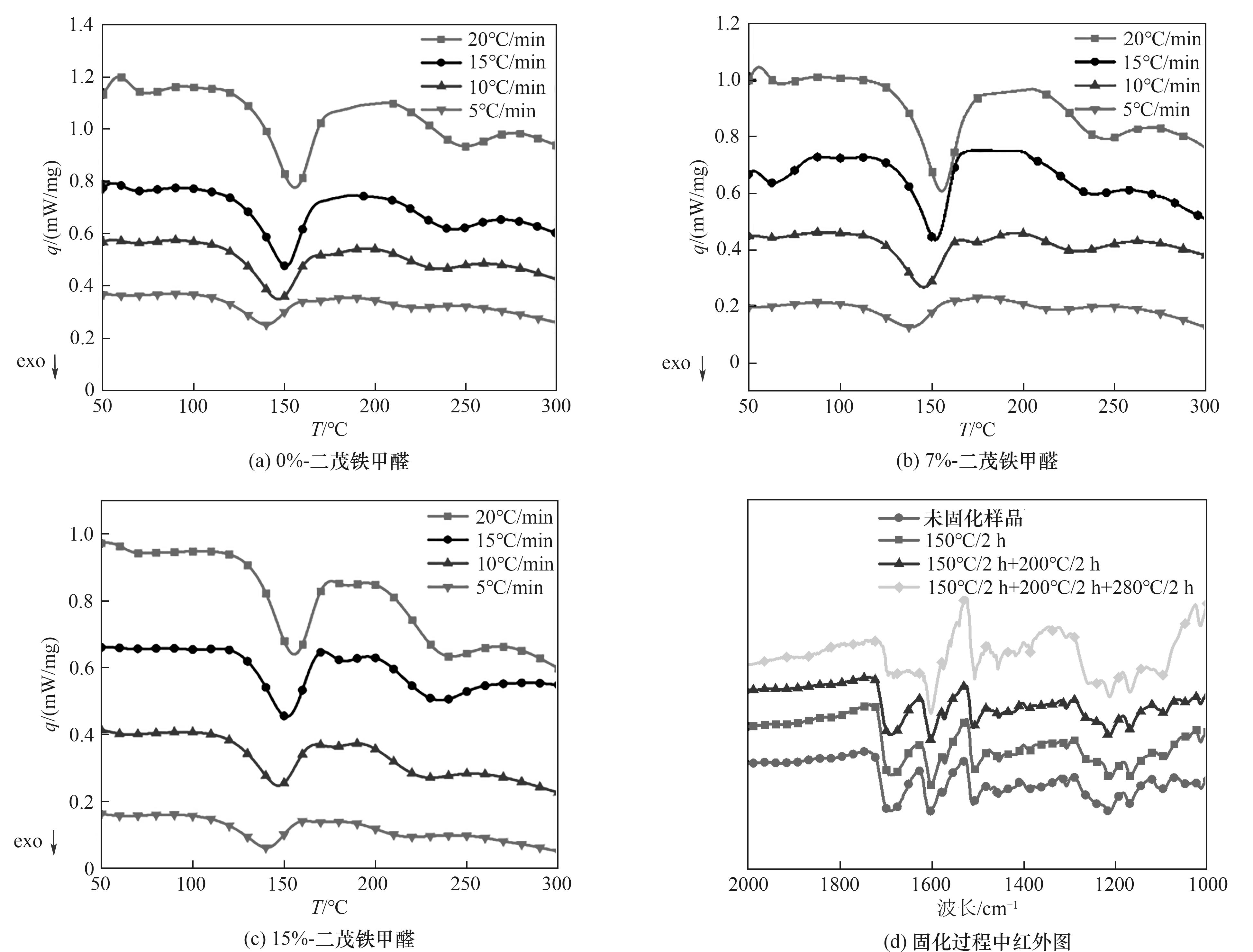
Fig.5 DSC of 0%-ferrocenecarboxaldehyde (a), 7%-ferrocenecarboxaldehyde (b), 15%-ferrocenecarboxaldehyde (c) modified terephthalaldehyde phenolic resin and FTIR diagram during resin curing process(d)

Fig.6 Curing mechanism diagram of terephthalaldehyde phenolic resin (a) and ferrocenecarboxaldehyde modified terephthalaldehyde phenolic resin (b) (R:H or its substitute)
| β/(℃/min) | 0% | 7% | 15% | |||
|---|---|---|---|---|---|---|
| Tp1/℃ | Tp2/℃ | Tp1/℃ | Tp2/℃ | Tp1/℃ | Tp2/℃ | |
| 5 | 142.1 | 222.8 | 141.3 | 218.8 | 142.8 | 218.4 |
| 10 | 148.6 | 235.7 | 147.5 | 230.6 | 148.7 | 228.8 |
| 15 | 152.5 | 242.7 | 152.0 | 238.8 | 153.2 | 236.0 |
| 20 | 156.5 | 250.4 | 155.9 | 246.1 | 156.6 | 240.6 |
Table 1 Peak temperatures of two curing peaks of terephthalaldehyde phenolic resin with different contents of ferrocenecarboxaldehyde (0%, 7%, 15%)
| β/(℃/min) | 0% | 7% | 15% | |||
|---|---|---|---|---|---|---|
| Tp1/℃ | Tp2/℃ | Tp1/℃ | Tp2/℃ | Tp1/℃ | Tp2/℃ | |
| 5 | 142.1 | 222.8 | 141.3 | 218.8 | 142.8 | 218.4 |
| 10 | 148.6 | 235.7 | 147.5 | 230.6 | 148.7 | 228.8 |
| 15 | 152.5 | 242.7 | 152.0 | 238.8 | 153.2 | 236.0 |
| 20 | 156.5 | 250.4 | 155.9 | 246.1 | 156.6 | 240.6 |

Fig.7 Fitting curves of 0%- ferrocenecarboxaldehyde [(a),(b)], 7%- ferrocenecarboxaldehyde [(c),(d)], 15%-ferrocenecarboxaldehyde [(e),(f)] modified terephthalaldehyde phenolic resin
| Peak | Ea/(kJ/mol) | ||
|---|---|---|---|
| 0% | 7% | 15% | |
| peak 1 | 137.82 | 134.07 | 143.14 |
| peak 2 | 101.81 | 100.16 | 121.89 |
| total | 239.63 | 234.23 | 265.03 |
Table 2 Activation energy of terephthalaldehyde phenolic resin with different contents of ferrocenecarboxaldehyde (0%, 7%, 15%)
| Peak | Ea/(kJ/mol) | ||
|---|---|---|---|
| 0% | 7% | 15% | |
| peak 1 | 137.82 | 134.07 | 143.14 |
| peak 2 | 101.81 | 100.16 | 121.89 |
| total | 239.63 | 234.23 | 265.03 |

Fig.8 Activation energy of peak 1(a) and peak 2(b) of terephthalaldehyde phenolic resin with different contents of ferrocenecarboxaldehyde (0%, 7%, 15%)

Fig.9 Curves of curing degree and temperature of 0%- ferrocenecarboxaldehyde [(a),(b)], 7%- ferrocenecarboxaldehyde [(c),(d)], 15%- ferrocenecarboxaldehyde [(e),(f)] modified terephthalaldehyde phenolic resin
| Sample | β/(℃/min) | lnA1 | m1 | n1 | m1/n1 | lnA2 | m2 | n2 | m2/n2 |
|---|---|---|---|---|---|---|---|---|---|
| 0% | 5 | 39.39 | 0.75 | 0.08 | 7.90 | 23.94 | 0.78 | 0.28 | 3.53 |
| 10 | 39.37 | 0.73 | 0.04 | 23.86 | 0.78 | 0.25 | |||
| 15 | 39.56 | 0.79 | 0.11 | 23.77 | 0.76 | 0.17 | |||
| 20 | 39.48 | 0.81 | 0.16 | 23.60 | 0.75 | 0.17 | |||
| 7% | 5 | 38.36 | 0.87 | 0.08 | 5.38 | 23.46 | 0.78 | 0.19 | 3.54 |
| 10 | 38.37 | 0.76 | 0.07 | 23.89 | 0.84 | 0.30 | |||
| 15 | 38.80 | 0.85 | 0.35 | 23.78 | 0.78 | 0.19 | |||
| 20 | 38.37 | 0.93 | 0.14 | 23.63 | 0.76 | 0.22 | |||
| 15% | 5 | 40.88 | 0.80 | 0.03 | 5.20 | 28.77 | 0.75 | 0.02 | 9.70 |
| 10 | 41.00 | 0.79 | 0.12 | 28.92 | 0.80 | 0.15 | |||
| 15 | 41.05 | 0.85 | 0.30 | 28.88 | 0.83 | 0.15 | |||
| 20 | 40.86 | 0.80 | 0.17 | 28.81 | 0.78 | 0.01 |
Table 3 Curing kinetic parameters of terephthalaldehyde phenolic resin with different contents of ferrocenecarboxaldehyde (0%, 7%, 15%)
| Sample | β/(℃/min) | lnA1 | m1 | n1 | m1/n1 | lnA2 | m2 | n2 | m2/n2 |
|---|---|---|---|---|---|---|---|---|---|
| 0% | 5 | 39.39 | 0.75 | 0.08 | 7.90 | 23.94 | 0.78 | 0.28 | 3.53 |
| 10 | 39.37 | 0.73 | 0.04 | 23.86 | 0.78 | 0.25 | |||
| 15 | 39.56 | 0.79 | 0.11 | 23.77 | 0.76 | 0.17 | |||
| 20 | 39.48 | 0.81 | 0.16 | 23.60 | 0.75 | 0.17 | |||
| 7% | 5 | 38.36 | 0.87 | 0.08 | 5.38 | 23.46 | 0.78 | 0.19 | 3.54 |
| 10 | 38.37 | 0.76 | 0.07 | 23.89 | 0.84 | 0.30 | |||
| 15 | 38.80 | 0.85 | 0.35 | 23.78 | 0.78 | 0.19 | |||
| 20 | 38.37 | 0.93 | 0.14 | 23.63 | 0.76 | 0.22 | |||
| 15% | 5 | 40.88 | 0.80 | 0.03 | 5.20 | 28.77 | 0.75 | 0.02 | 9.70 |
| 10 | 41.00 | 0.79 | 0.12 | 28.92 | 0.80 | 0.15 | |||
| 15 | 41.05 | 0.85 | 0.30 | 28.88 | 0.83 | 0.15 | |||
| 20 | 40.86 | 0.80 | 0.17 | 28.81 | 0.78 | 0.01 |
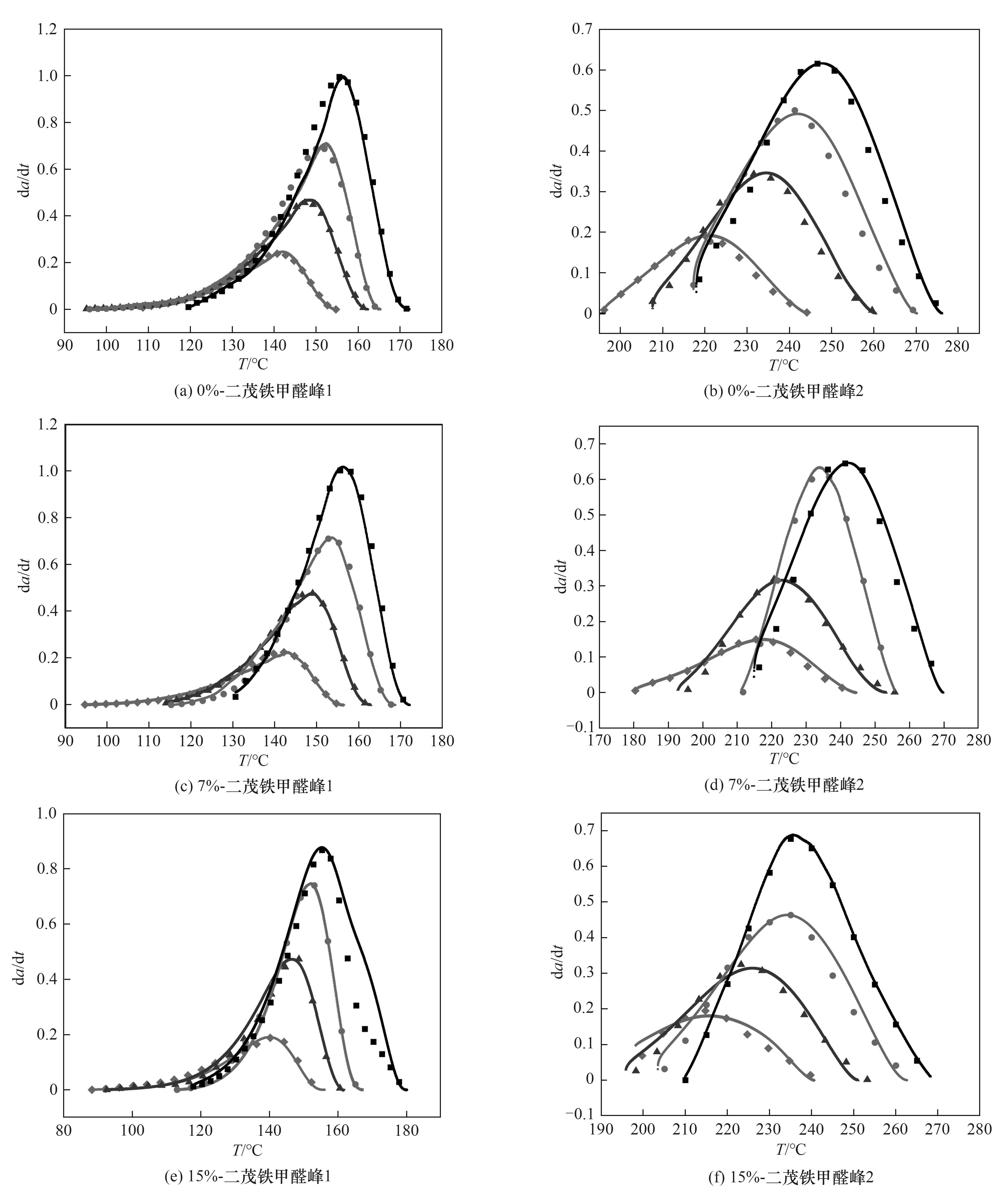
Fig.10 Fitting curves of 0%- ferrocenecarboxaldehyde[(a),(b)], 7%- ferrocenecarboxaldehyde[(c),(d)], 15%- ferrocenecarboxaldehyde[ (e),(f) ] modified terephthalaldehyde phenolic resin (the solid line is the curve obtained from simulated data, and the scattered point is the curve obtained from experimental data)

Fig.12 TG(a) and DTG(b) curves of terephthalaldehyde phenolic resin modified with different contents of ferrocenecarboxaldehyde (0%, 7%, 15%) in N2 atmosphere
| 1 | Reghunadhan Nair C P, Bindu R L, Ninan K N. Thermal characteristics of addition-cure phenolic resins[J]. Polymer Degradation and Stability, 2001, 73(2): 251-257. |
| 2 | Yang W S, Jiao L, Wang X, et al. Formaldehyde-free self-polymerization of lignin-derived monomers for synthesis of renewable phenolic resin[J]. International Journal of Biological Macromolecules, 2021, 166: 1312-1319. |
| 3 | Sarika P R, Nancarrow P, Khansaheb A, et al. Bio-based alternatives to phenol and formaldehyde for the production of resins[J]. Polymers, 2020, 12(10): 2237. |
| 4 | Rivero G, Fasce L A, Ceré S M, et al. Furan resins as replacement of phenolic protective coatings: structural, mechanical and functional characterization[J]. Progress in Organic Coatings, 2014, 77(1): 247-256. |
| 5 | Younesi-Kordkheili H. Ionic liquid modified lignin-phenol-glyoxal resin: a green alternative resin for production of particleboards[J]. The Journal of Adhesion, 2019, 95(12): 1075-1087. |
| 6 | Foyer G, Chanfi B H, Virieux D, et al. Aromatic dialdehyde precursors from lignin derivatives for the synthesis of formaldehyde-free and high char yield phenolic resins[J]. European Polymer Journal, 2016, 77: 65-74. |
| 7 | 朱其仁, 李锦春, 王丽娟, 等. 苯酚-亚联苯型酚醛树脂的合成与表征[J]. 化工学报, 2009, 60(4): 1052-1056. |
| Zhu Q R, Li J C, Wang L J, et al. Synthesis and characterization of phenol-biphenylene resin[J]. CIESC Journal, 2009, 60(4): 1052-1056. | |
| 8 | Yun J, Chen L X, Zhang X F, et al. The effects of silicon and ferrocene on the char formation of modified novolac resin with high char yield[J]. Polymer Degradation and Stability, 2017, 139: 97-106. |
| 9 | Xu S H, Zhang F Y, Kang Q, et al. The effect of magnetic field on the catalytic graphitization of phenolic resin in the presence of Fe-Ni[J]. Carbon, 2009, 47(14): 3233-3237. |
| 10 | Zhang Y, Shen S H, Liu Y J. The effect of titanium incorporation on the thermal stability of phenol-formaldehyde resin and its carbonization microstructure[J]. Polymer Degradation and Stability, 2013, 98(2): 514-518. |
| 11 | Naderi A, Mazinani S, Javad Ahmadi S, et al. Modified thermo-physical properties of phenolic resin/carbon fiber composite with nano zirconium dioxide[J]. Journal of Thermal Analysis and Calorimetry, 2014, 117(1): 393-401. |
| 12 | Zhou J, Li X G, Du J Z, et al. Conversion of phenolic mixture to refractory resins: a resourcezation strategy for phenolic distillation residues[J]. Journal of Hazardous Materials, 2021, 414: 125357. |
| 13 | Granado L, Tavernier R, Foyer G, et al. Catalysis for highly thermostable phenol-terephthalaldehyde polymer networks[J]. Chemical Engineering Journal, 2020, 379: 122237. |
| 14 | Yi C, Rostron P, Vahdati N, et al. Curing kinetics and mechanical properties of epoxy based coatings: the influence of added solvent[J]. Progress in Organic Coatings, 2018, 124: 165-174. |
| 15 | Granado L, Tavernier R, Foyer G, et al. Comparative curing kinetics study of high char yield formaldehyde- and terephthalaldehyde-phenolic thermosets[J]. Thermochimica Acta, 2018, 667: 42-49. |
| 16 | 张西莹, 刘育红. 酚醛树脂/碳化硼/聚硼氮烷复合物的固化行为及其热解性能[J]. 化工学报, 2014, 65(8): 3268-3276. |
| Zhang X Y, Liu Y H. Curing and pyrolysis behavior of PF/B4C/PBZ composite[J]. CIESC Journal, 2014, 65(8): 3268-3276. | |
| 17 | 代洁, 李鹏程, 朱蓉琪, 等. 一种高残炭新型苯并𫫇嗪树脂的固化及热解动力学[J]. 功能高分子学报, 2018, 31(2): 114-120. |
| Dai J, Li P C, Zhu R Q, et al. Curing and pyrolysis kinetics of a new benzoxazine resin with high char yield[J]. Journal of Functional Polymers, 2018, 31(2): 114-120. | |
| 18 | Gao J G, Huo L, Du Y G. Nonisothermal cure kinetics and diffusion effect of liquid-crystalline epoxy sulfonyl bis(1, 4-phenylene)bis[4-(2, 3-epoxypropyloxy)benzoate]resin with aromatic diamine[J]. Journal of Applied Polymer Science, 2012, 125(5): 3329-3334. |
| 19 | Xu M Z, Luo Y S, Lei Y X, et al. Phthalonitrile-based resin for advanced composite materials: curing behavior studies[J]. Polymer Testing, 2016, 55: 38-43. |
| 20 | Li C, Ma Z Z, Zhang X W, et al. Silicone-modified phenolic resin: relationships between molecular structure and curing behavior[J]. Thermochimica Acta, 2016, 639: 53-65. |
| 21 | Lei Z X, Jiang X, Lv Y, et al. Time-temperature-transformation diagram of modified resol phenolic resin and the thermomechanical performance of resol phenolic resin/glass fabric composite[J]. Polymers for Advanced Technologies, 2018, 29(11): 2827-2837. |
| 22 | Asaro L, Manfredi L B, Pellice S, et al. Innovative ablative fire resistant composites based on phenolic resins modified with mesoporous silica particles[J]. Polymer Degradation and Stability, 2017, 144: 7-16. |
| 23 | Wang S J, Jia Q X, Liu Y H, et al. An investigation on the effect of phenylboronic acid on the processibilities and thermal properties of bis-benzoxazine resins[J]. Reactive and Functional Polymers, 2015, 93: 111-119. |
| 24 | Wang S J, Jing X L, Wang Y, et al. Synthesis and characterization of novel phenolic resins containing aryl-boron backbone and their utilization in polymeric composites with improved thermal and mechanical properties[J]. Polymers for Advanced Technologies, 2014, 25(2): 152-159. |
| 25 | Fox T G, Loshaek S. Influence of molecular weight and degree of crosslinking on the specific volume and glass temperature of polymers[J]. Journal of Polymer Science, 1955, 15(80): 371-390. |
| 26 | Trick K A, Saliba T E. Mechanisms of the pyrolysis of phenolic resin in a carbon/phenolic composite[J]. Carbon, 1995, 33(11): 1509-1515. |
| 27 | Xing X L, Zhang P, Zhao Y H, et al. Pyrolysis mechanism of phenylboronic acid modified phenolic resin[J]. Polymer Degradation and Stability, 2021, 191: 109672. |
| 28 | Wang S J, Wang Y, Bian C, et al. The thermal stability and pyrolysis mechanism of boron-containing phenolic resins: the effect of phenyl borates on the char formation[J]. Applied Surface Science, 2015, 331: 519-529. |
| 29 | Amer W A, Wang L, Amin A M, et al. Study on the electrochemical, thermal, and liquid crystalline properties of poly(diethyleneglycol 1, 1'-ferrocene dicarboxylate)[J]. Designed Monomers and Polymers, 2013, 16(2): 160-169. |
| 30 | Talabi S I, Luz A P, Pandolfelli V C, et al. Synthesis and graphitization of resole resins by ferrocene[J]. Progress in Natural Science: Materials International, 2019, 29(1): 71-80. |
| [1] | Xueyan WEI, Yong QIAN. Experimental study on the low to medium temperature oxidation characteristics and kinetics of micro-size iron powder [J]. CIESC Journal, 2023, 74(6): 2624-2638. |
| [2] | Jieyuan ZHENG, Xianwei ZHANG, Jintao WAN, Hong FAN. Synthesis and curing kinetic analysis of eugenol-based siloxane epoxy resin [J]. CIESC Journal, 2023, 74(2): 924-932. |
| [3] | Zhe WANG, Yuan ZU, Fangyuan HU, Xigao JIAN. Kinetic analysis of curing of epoxy resin containing phthalazinone structure [J]. CIESC Journal, 2022, 73(2): 681-688. |
| [4] | Zhenan ZHENG, Xiang GAO, Yingwu LUO, Jie HUANG. Preparation of all-solid-state polymer electrolyte by ultraviolet cross-linking method [J]. CIESC Journal, 2022, 73(1): 441-450. |
| [5] | ZHANG Rui, SHAO Qi, ZHANG Huayu, JIN Zelong, ZHANG Xiaoliang. Fabrication of boron-doped hybrid silica membranes for pervaporation desalination [J]. CIESC Journal, 2021, 72(4): 2317-2327. |
| [6] | LIU Ning, CHU Changhui, WANG Qian, SUN Shipeng. Preparation of nanofiltration membrane for separation of mixed monovalent salts [J]. CIESC Journal, 2021, 72(1): 578-588. |
| [7] | Yifan ZHOU, Congxue YAO, Jingwen WANG, Wenwen GUO, Lei SONG, Xiaowei MU, Yuan HU. Study on thermal decomposition characteristics and kinetics of soybean [J]. CIESC Journal, 2020, 71(S2): 187-194. |
| [8] | Di CAI, Jing LI. Study on thermal properties of stearyl alcohol modified graphene oxide/ n-octadecane composite phase change materials [J]. CIESC Journal, 2020, 71(10): 4826-4835. |
| [9] | Liping WEI,Guodong JIANG,Yukuan GU,Haipeng TENG. Evaluation and application of pyrolysis kinetic model of Wucaiwan coal and Tulufan coal [J]. CIESC Journal, 2019, 70(S2): 275-286. |
| [10] | ZHENG Lifang, WANG Zhaozhong, XIE Yajie, YUE Lina, WANG Li. Kinetic study on thermal decomposition of GFRP under γ irradiation [J]. CIESC Journal, 2018, 69(S1): 161-169. |
| [11] | ZHANG Jizong, CHANG Houchun, CHANG Jianmin, LONG Jinxing, LI Xuehui. Curing characteristics of bio-oil starch adhesive [J]. CIESC Journal, 2018, 69(12): 5309-5315. |
| [12] | YANG Ze, HU Dongdong, LIU Tao, CAO Kun, ZHAO Ling. Non-isothermal curing kinetics of polyurethane under high-pressure gas atmosphere [J]. CIESC Journal, 2018, 69(11): 4728-4736. |
| [13] | SU Jiale, ZHOU Junhu, ZHANG Xing, ZHAO Qingchen, WANG Yefeng, YANG Weijuan. Kinetics of ethanol oxidation over Pt/ZSM-5 catalyst [J]. CIESC Journal, 2018, 69(10): 4269-4275. |
| [14] | XU Shang, ZHAO Lingling, CAI Zhuangli, CHEN Chao. Modeling study on thermal conductivity of two-dimensional hexagonal aluminum nitride [J]. CIESC Journal, 2017, 68(9): 3321-3327. |
| [15] | XIE Jingchao, TANG Yiling, ZHANG Zhaofeng, WANG Wei, LIU Jiaping, WANG Jianping. Optimum heat storage performance of building envelope under coupling condition of ventilation and phase change [J]. CIESC Journal, 2017, 68(7): 2684-2695. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||