CIESC Journal ›› 2022, Vol. 73 ›› Issue (1): 441-450.DOI: 10.11949/0438-1157.20211460
• Material science and engineering, nanotechnology • Previous Articles Next Articles
Zhenan ZHENG1,2( ),Xiang GAO1(
),Xiang GAO1( ),Yingwu LUO1,Jie HUANG2
),Yingwu LUO1,Jie HUANG2
Received:2021-10-13
Revised:2021-11-25
Online:2022-01-18
Published:2022-01-05
Contact:
Xiang GAO
通讯作者:
高翔
作者简介:郑哲楠(1991—),女,博士,讲师,基金资助:CLC Number:
Zhenan ZHENG, Xiang GAO, Yingwu LUO, Jie HUANG. Preparation of all-solid-state polymer electrolyte by ultraviolet cross-linking method[J]. CIESC Journal, 2022, 73(1): 441-450.
郑哲楠, 高翔, 罗英武, 黄杰. 紫外光交联法制备全固态聚合物电解质[J]. 化工学报, 2022, 73(1): 441-450.
Add to citation manager EndNote|Ris|BibTeX
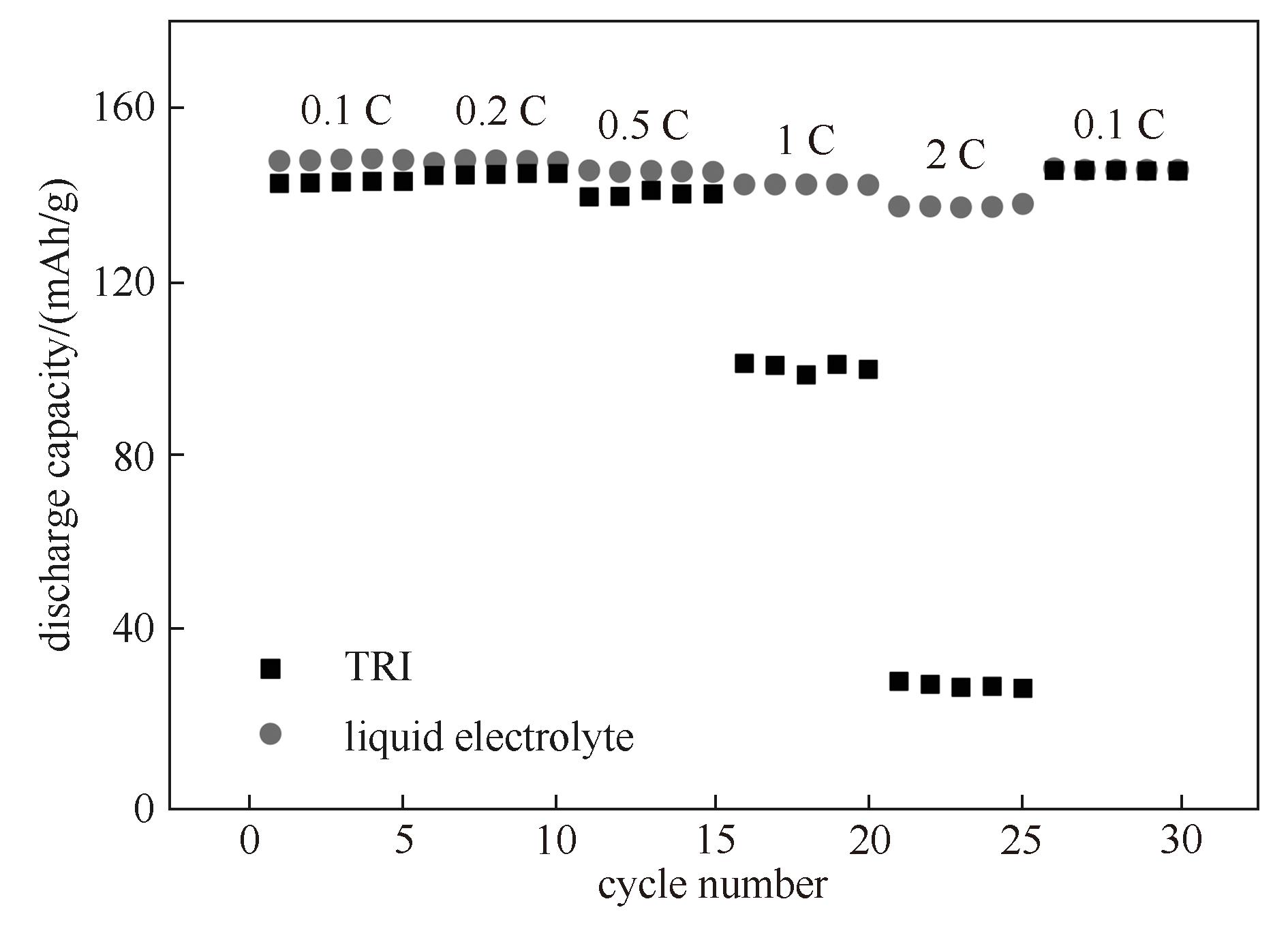
Fig.8 Rate performance of LiFePO4/Li cell using TRI and commercial liquid electrolyte (The test temperature for TRI was 60℃, while that for liquid electrolyte was 30℃)
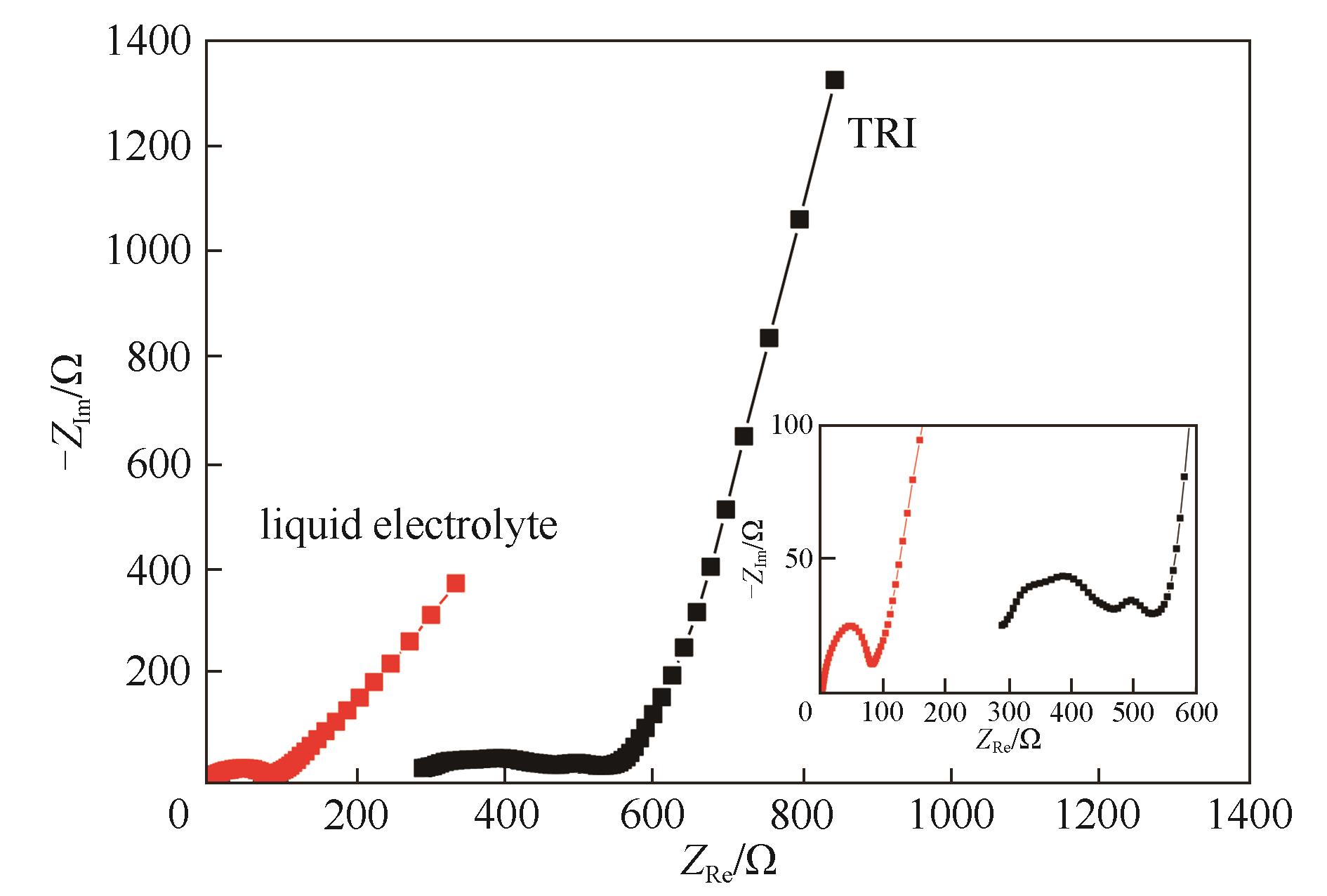
Fig.10 Nyquist plots of LiFePO4/Li cells using TRI and commercial liquid electrolyte after rate performance tests and at discharge state (the test temperature for TRI was 60℃, while that for liquid electrolyte was 30℃; the inset image is a partial enlargement of the semicircular)
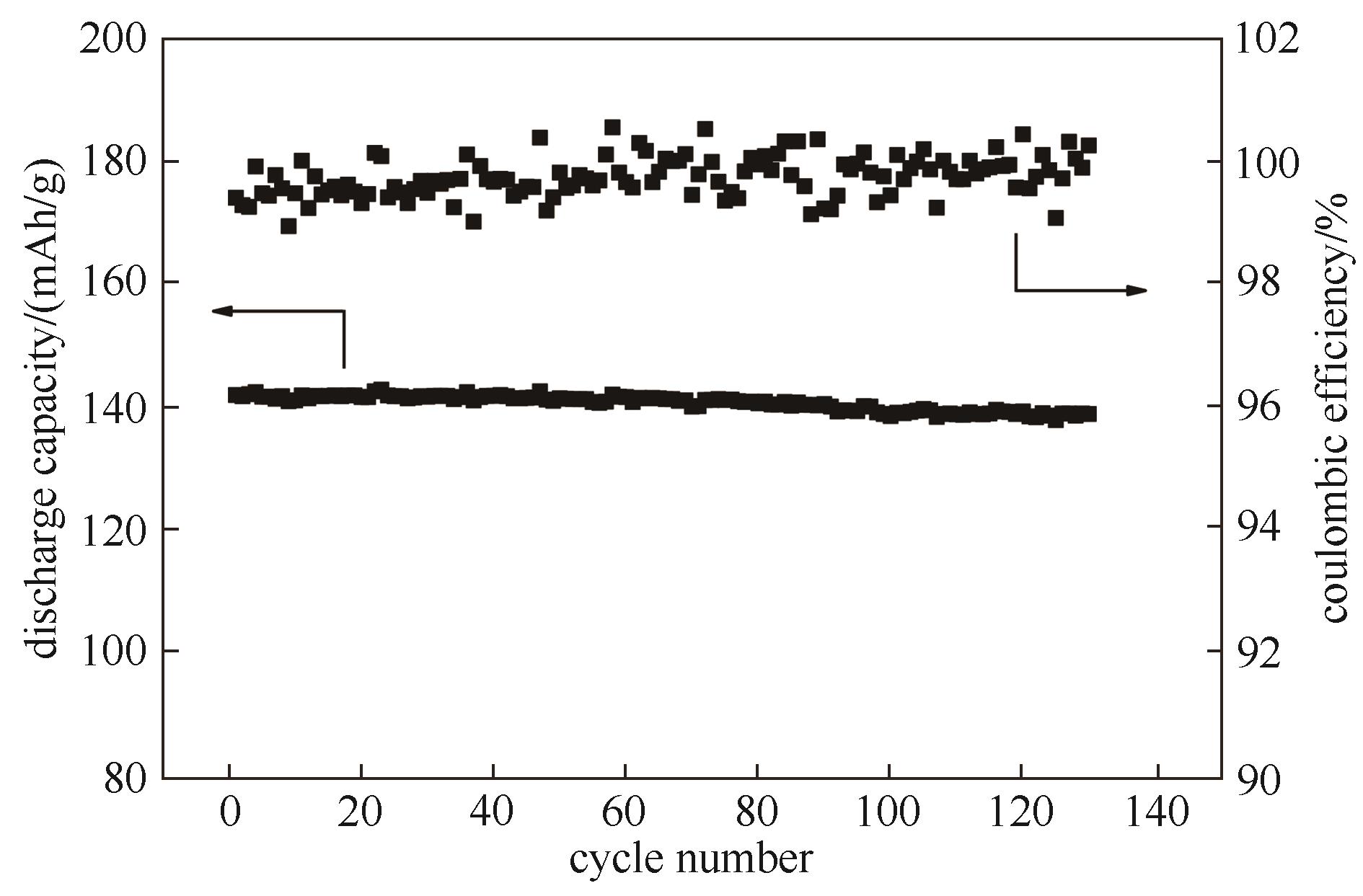
Fig.11 Cycling performance of LiFePO4/Li cell using TRI and its corresponding coulombic efficiencies (the test temperature was 60℃ and the test rate was 0.5 C for both charge and discharge procedures)
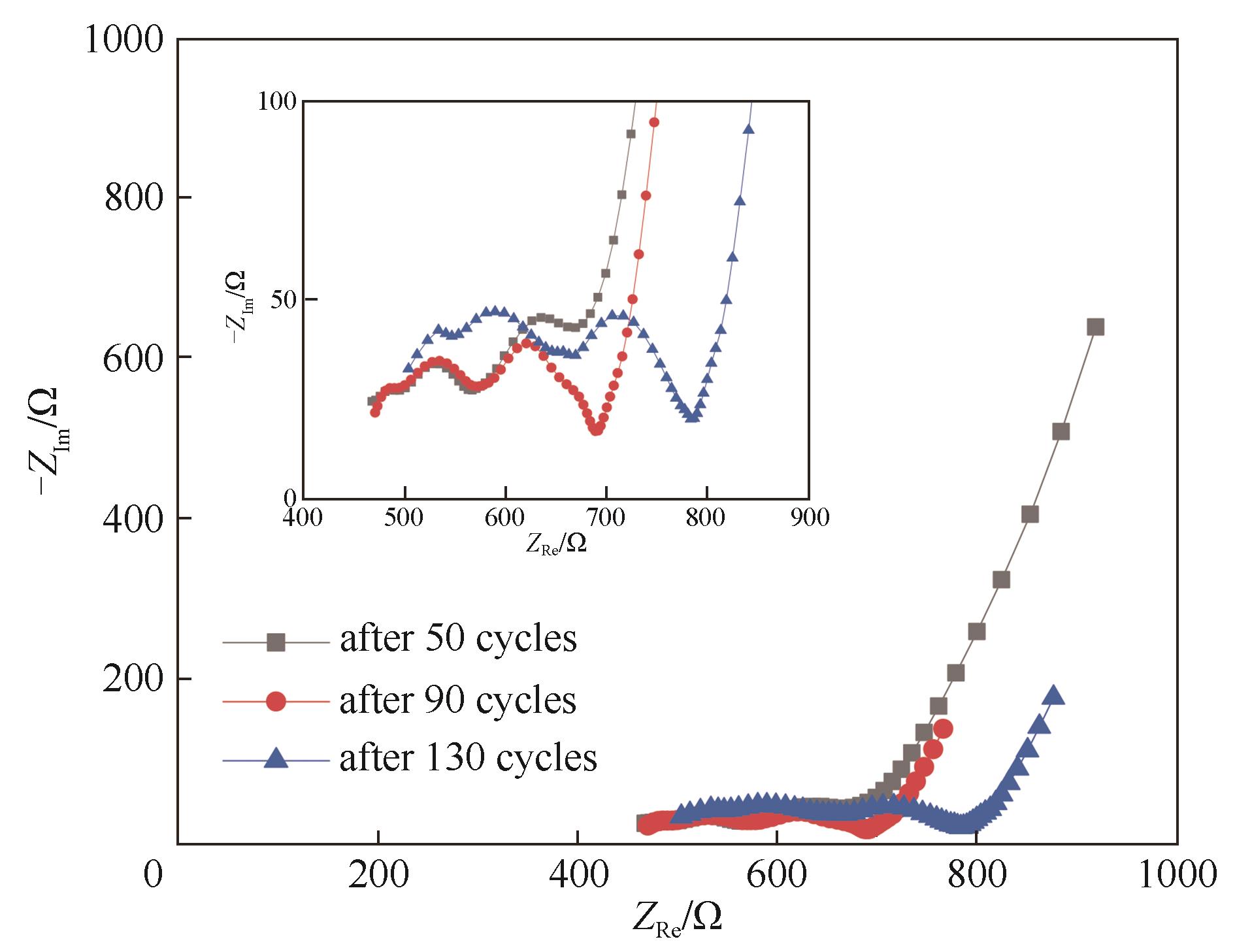
Fig.12 Nyquist plots of LiFePO4/Li cell using TRI after different amounts of cycles and at discharge state (the test temperature was 60℃, the inset image is a partial enlargement of the semicircular)
| 1 | Wang H C, Wang Q, Cao X, et al. Thiol-branched solid polymer electrolyte featuring high strength, toughness, and lithium ionic conductivity for lithium-metal batteries[J]. Advanced Materials, 2020, 32(37): 2001259. |
| 2 | Xu W, Wang J L, Ding F, et al. Lithium metal anodes for rechargeable batteries[J]. Energy Environ. Sci., 2014, 7(2): 513-537. |
| 3 | Lin D C, Liu Y Y, Cui Y. Reviving the lithium metal anode for high-energy batteries[J]. Nature Nanotechnology, 2017, 12(3): 194-206. |
| 4 | Cheng X B, Zhang R, Zhao C Z, et al. Toward safe lithium metal anode in rechargeable batteries: a review[J]. Chemical Reviews, 2017, 117(15): 10403-10473. |
| 5 | Young W S, Kuan W F, Epps I. Block copolymer electrolytes for rechargeable lithium batteries[J]. Journal of Polymer Science Part B: Polymer Physics, 2014, 52(1): 1-16. |
| 6 | Agrawal R C, Pandey G P. Solid polymer electrolytes: materials designing and all-solid-state battery applications: an overview[J]. Journal of Physics D: Applied Physics, 2008, 41(22): 223001. |
| 7 | Meyer W H. Polymer electrolytes for lithium-ion batteries[J]. Advanced Materials, 1998, 10(6): 439-448. |
| 8 | Young W S, Albert J N L, Schantz A B, et al. Mixed-salt effects on the ionic conductivity of lithium-doped PEO-containing block copolymers[J]. Macromolecules, 2011, 44(20): 8116-8123. |
| 9 | van Krevelen D W, te Nijenhuis K. Properties of Polymers[M]. Beijing: Science Press, 2010: 926-927. |
| 10 | 董甜甜, 张建军, 柴敬超, 等. 聚碳酸酯基固态聚合物电解质的研究进展[J]. 高分子学报, 2017(6): 906-921. |
| Dong T T, Zhang J J, Chai J C, et al. Research progress on polycarbonate-based solid-state polymer electrolytes[J]. Acta Polymerica Sinica, 2017(6): 906-921. | |
| 11 | 张建军, 杨金凤, 吴瀚, 等. 二次电池用原位生成聚合物电解质的研究进展[J]. 高分子学报, 2019, 50(9): 890-914. |
| Zhang J J, Yang J F, Wu H, et al. Research progress of in situ generated polymer electrolyte for rechargeable batteries[J]. Acta Polymerica Sinica, 2019, 50(9): 890-914. | |
| 12 | Takahashi Y, Tadokoro H. Structural studies of polyethers, (-(CH2)m-O-)n. Ⅹ. Crystal structure of poly(ethylene oxide)[J]. Macromolecules, 1973, 6(5): 672-675. |
| 13 | Long L Z, Wang S J, Xiao M, et al. Polymer electrolytes for lithium polymer batteries[J]. Journal of Materials Chemistry A, 2016, 4(26): 10038-10069. |
| 14 | Xue Z G, He D, Xie X L. Poly(ethylene oxide)-based electrolytes for lithium-ion batteries[J]. Journal of Materials Chemistry A, 2015, 3(38): 19218-19253. |
| 15 | Zhang J X, Zhao N, Zhang M, et al. Flexible and ion-conducting membrane electrolytes for solid-state lithium batteries: dispersion of garnet nanoparticles in insulating polyethylene oxide[J]. Nano Energy, 2016, 28: 447-454. |
| 16 | Chen L, Li Y T, Li S P, et al. PEO/garnet composite electrolytes for solid-state lithium batteries: from “ceramic-in-polymer” to “polymer-in-ceramic”[J]. Nano Energy, 2018, 46: 176-184. |
| 17 | Abetz V, Simon P F W. Phase Behaviour and Morphologies of Block Copolymers[M]//Abetz V. Block Copolymers I. Berlin: Springer, 2005: 125-212. |
| 18 | Bouchet R, Phan T N T, Beaudoin E, et al. Charge transport in nanostructured PS-PEO-PS triblock copolymer electrolytes[J]. Macromolecules, 2014, 47(8): 2659-2665. |
| 19 | Wang C X, Sakai T, Watanabe O, et al. All solid-state lithium-polymer battery using a self-cross-linking polymer electrolyte[J]. Journal of the Electrochemical Society, 2003, 150(9): A1166. |
| 20 | Panday A, Mullin S, Gomez E D, et al. Effect of molecular weight and salt concentration on conductivity of block copolymer electrolytes[J]. Macromolecules, 2009, 42(13): 4632-4637. |
| 21 | Wanakule N S, Panday A, Mullin S A, et al. Ionic conductivity of block copolymer electrolytes in the vicinity of order-disorder and order-order transitions[J]. Macromolecules, 2009, 42(15): 5642-5651. |
| 22 | Schulze M W, McIntosh L D, Hillmyer M A, et al. High-modulus, high-conductivity nanostructured polymer electrolyte membranes via polymerization-induced phase separation[J]. Nano Letters, 2014, 14(1): 122-126. |
| 23 | Hamersky M W, Hillmyer M A, Tirrell M, et al. Block copolymer self-diffusion in the gyroid and cylinder morphologies[J]. Macromolecules, 1998, 31(16): 5363-5370. |
| 24 | Young W S, Epps T H. Ionic conductivities of block copolymer electrolytes with various conducting pathways: sample preparation and processing considerations[J]. Macromolecules, 2012, 45(11): 4689-4697. |
| 25 | Niitani T, Shimada M, Kawamura K, et al. Characteristics of new-type solid polymer electrolyte controlling nano-structure[J]. Journal of Power Sources, 2005, 146(1/2): 386-390. |
| 26 | Ferguson C J, Hughes R J, Nguyen D, et al. Ab initio emulsion polymerization by RAFT-controlled self-assembly[J]. Macromolecules, 2005, 38(6): 2191-2204. |
| 27 | Yang L, Xu J Q, Sun P, et al. Ab initio emulsion and miniemulsion polymerization of styrene mediated by a cyclohexenyl-functionalized amphiphilic RAFT agent[J]. Industrial & Engineering Chemistry Research, 2014, 53(28): 11259-11268. |
| 28 | Ma J, Cheng C, Wooley K L. Cycloalkenyl-functionalized polymers and block copolymers: syntheses via selective RAFT polymerizations and demonstration of their versatile reactivity[J]. Macromolecules, 2009, 42(5): 1565-1573. |
| 29 | Dondoni A. The emergence of thiol-ene coupling as a click process for materials and bioorganic chemistry[J]. Angewandte Chemie International Edition, 2008, 47(47): 8995-8997. |
| 30 | Lowe A B. Thiol-ene “click” reactions and recent applications in polymer and materials synthesis[J]. Polym. Chem., 2010, 1(1): 17-36. |
| 31 | Yang L, Xu J Q, Han J L, et al. A novel method for preparing click-ready latex and latex with stability against high electrolyte concentrations[J]. Industrial & Engineering Chemistry Reaction, 2015, 54(20): 5536-5542. |
| 32 | Xie P L, Yang X X, Li T F, et al. Highly stretchable, transparent, and colorless electrodes from a diblock copolymer electrolyte[J]. Journal of Materials Chemistry C, 2017, 38(5): 9865-9872. |
| 33 | Timachova K, Watanabe H, Balsara N P. Effect of molecular weight and salt concentration on ion transport and the transference number in polymer electrolytes[J]. Macromolecules, 2015, 48(21): 7882-7888. |
| 34 | Petrucci R H, Herring F G, Bissonnette C, et al. General Chemistry: Principles and Modern Applications[M]. 11th ed. Toronto: Pearson Canada Inc., 2017. |
| 35 | Dehmelt H. A single atomic particle forever floating at rest in free space: new value for electron radius[J]. Physica Scripta, 1988, T22: 102. |
| [1] | Yepin CHENG, Daqing HU, Yisha XU, Huayan LIU, Hanfeng LU, Guokai CUI. Application of ionic liquid-based deep eutectic solvents for CO2 conversion [J]. CIESC Journal, 2023, 74(9): 3640-3653. |
| [2] | Yali HU, Junyong HU, Suxia MA, Yukun SUN, Xueyi TAN, Jiaxin HUANG, Fengyuan YANG. Development of novel working fluid and study on electrochemical characteristics of reverse electrodialysis heat engine [J]. CIESC Journal, 2023, 74(8): 3513-3521. |
| [3] | Mengmeng ZHANG, Dong YAN, Yongfeng SHEN, Wencui LI. Effect of electrolyte types on the storage behaviors of anions and cations for dual-ion batteries [J]. CIESC Journal, 2023, 74(7): 3116-3126. |
| [4] | Jiali GE, Tuxiang GUAN, Xinmin QIU, Jian WU, Liming SHEN, Ningzhong BAO. Synthesis of FeF3 nanoparticles covered by vertical porous carbon for high performance Li-ion battery cathode [J]. CIESC Journal, 2023, 74(7): 3058-3067. |
| [5] | Yuanhao QU, Wenyi DENG, Xiaodan XIE, Yaxin SU. Study on electro-osmotic dewatering of sludge assisted by activated carbon/graphite [J]. CIESC Journal, 2023, 74(7): 3038-3050. |
| [6] | Jie LIU, Lisheng WU, Jinjin LI, Zhenghong LUO, Yinning ZHOU. Preparation and properties of polyether-based vinylogous urethane reversible crosslinked polymers [J]. CIESC Journal, 2023, 74(7): 3051-3057. |
| [7] | Tan ZHANG, Guang LIU, Jinping LI, Yuhan SUN. Performance regulation strategies of Ru-based nitrogen reduction electrocatalysts [J]. CIESC Journal, 2023, 74(6): 2264-2280. |
| [8] | Shaoyun CHEN, Dong XU, Long CHEN, Yu ZHANG, Yuanfang ZHANG, Qingliang YOU, Chenglong HU, Jian CHEN. Preparation and adsorption properties of monolayer polyaniline microsphere arrays [J]. CIESC Journal, 2023, 74(5): 2228-2238. |
| [9] | Chengze WANG, Kaili GU, Jinhua ZHANG, Jianxuan SHI, Yiwei LIU, Jinxiang LI. Sulfidation couples with aging to enhance the reactivity of zerovalent iron toward Cr(Ⅵ) in water [J]. CIESC Journal, 2023, 74(5): 2197-2206. |
| [10] | Xu GUO, Yongzheng ZHANG, Houbing XIA, Na YANG, Zhenzhen ZHU, Jingyao QI. Research progress in the removal of water pollutants by carbon-based materials via electrooxidation [J]. CIESC Journal, 2023, 74(5): 1862-1874. |
| [11] | Zheng ZHANG, Yongping HE, Haidong SUN, Rongzi ZHANG, Zhengping SUN, Jinlan CHEN, Yixuan ZHENG, Xiao DU, Xiaogang HAO. Electrochemically switched ion exchange device with serpentine flow field for selective extraction of lithium [J]. CIESC Journal, 2023, 74(5): 2022-2033. |
| [12] | Ruikang LI, Yingying HE, Weipeng LU, Yuanyuan WANG, Haodong DING, Yongming LUO. Study on the electrochemical enhanced cobalt-based cathode to activate peroxymonosulfate [J]. CIESC Journal, 2023, 74(5): 2207-2216. |
| [13] | Xuehong WU, Linlin LUAN, Yanan CHEN, Min ZHAO, Cai LYU, Yong LIU. Preparation and thermal properties of degradable flexible phase change films [J]. CIESC Journal, 2023, 74(4): 1818-1826. |
| [14] | Ruiqi LIU, Xitong ZHOU, Yue ZHANG, Ying HE, Jing GAO, Li MA. The construction and application of biosensor based on gold nanoparticles loaded SiO2-nanoflowers [J]. CIESC Journal, 2023, 74(3): 1247-1259. |
| [15] | Dong XU, Du TIAN, Long CHEN, Yu ZHANG, Qingliang YOU, Chenglong HU, Shaoyun CHEN, Jian CHEN. Preparation and electrochemical energy storage of polyaniline/manganese dioxide/polypyrrole composite nanospheres [J]. CIESC Journal, 2023, 74(3): 1379-1389. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||