CIESC Journal ›› 2022, Vol. 73 ›› Issue (8): 3338-3354.DOI: 10.11949/0438-1157.20220555
• Reviews and monographs • Previous Articles Next Articles
Xingang QI1( ), Libo LU1, Yunan CHEN1,2, Zhiwei GE1,2, Liejin GUO1,2,3(
), Libo LU1, Yunan CHEN1,2, Zhiwei GE1,2, Liejin GUO1,2,3( )
)
Received:2022-04-20
Revised:2022-06-24
Online:2022-09-06
Published:2022-08-05
Contact:
Liejin GUO
戚新刚1( ), 路利波1, 陈渝楠1,2, 葛志伟1,2, 郭烈锦1,2,3(
), 路利波1, 陈渝楠1,2, 葛志伟1,2, 郭烈锦1,2,3( )
)
通讯作者:
郭烈锦
作者简介:戚新刚(1993—),男,博士研究生,qxg0101@stu.xjtu.edu.cn
基金资助:CLC Number:
Xingang QI, Libo LU, Yunan CHEN, Zhiwei GE, Liejin GUO. Review of black liquor supercritical water gasification for hydrogen production with high value-added chemicals recovery[J]. CIESC Journal, 2022, 73(8): 3338-3354.
戚新刚, 路利波, 陈渝楠, 葛志伟, 郭烈锦. 造纸黑液超临界水气化制氢与高附加值化学品回收研究进展[J]. 化工学报, 2022, 73(8): 3338-3354.
Add to citation manager EndNote|Ris|BibTeX

Fig.1 Comparison of reaction potential energy and reaction process between supercritical water gasification and conventional pyrolysis gasification[31]
| 方案1 | 方案2 | 方案3 |
|---|---|---|
 2OH-+CO2 2OH-+CO2 | 2CO+2OH- 2HCOO- 2HCOO- |  |
2CO +2OH- 2HCOO- 2HCOO- | 2HCOO- HCOH + HCOH + | OH-+CO HCOO- HCOO- |
2HCOO-+2H2O 2HCOOH + 2OH- 2HCOOH + 2OH- | 2H2O+ 2OH-+H2CO3 2OH-+H2CO3 | HCOO-+H2O  |
2HCOOH  2CO2+ 2H2 2CO2+ 2H2 | H2CO3 H2O + CO2 H2O + CO2 | 2 H2O + H2O + |
2OH- + CO2 | HCOH  H2+ CO H2+ CO | H2O+COHCOOH H2+ CO H2+ CO |
Table 1 Mechanism of alkali-catalyzed water gas conversion reaction[50]
| 方案1 | 方案2 | 方案3 |
|---|---|---|
 2OH-+CO2 2OH-+CO2 | 2CO+2OH- 2HCOO- 2HCOO- |  |
2CO +2OH- 2HCOO- 2HCOO- | 2HCOO- HCOH + HCOH + | OH-+CO HCOO- HCOO- |
2HCOO-+2H2O 2HCOOH + 2OH- 2HCOOH + 2OH- | 2H2O+ 2OH-+H2CO3 2OH-+H2CO3 | HCOO-+H2O  |
2HCOOH  2CO2+ 2H2 2CO2+ 2H2 | H2CO3 H2O + CO2 H2O + CO2 | 2 H2O + H2O + |
2OH- + CO2 | HCOH  H2+ CO H2+ CO | H2O+COHCOOH H2+ CO H2+ CO |
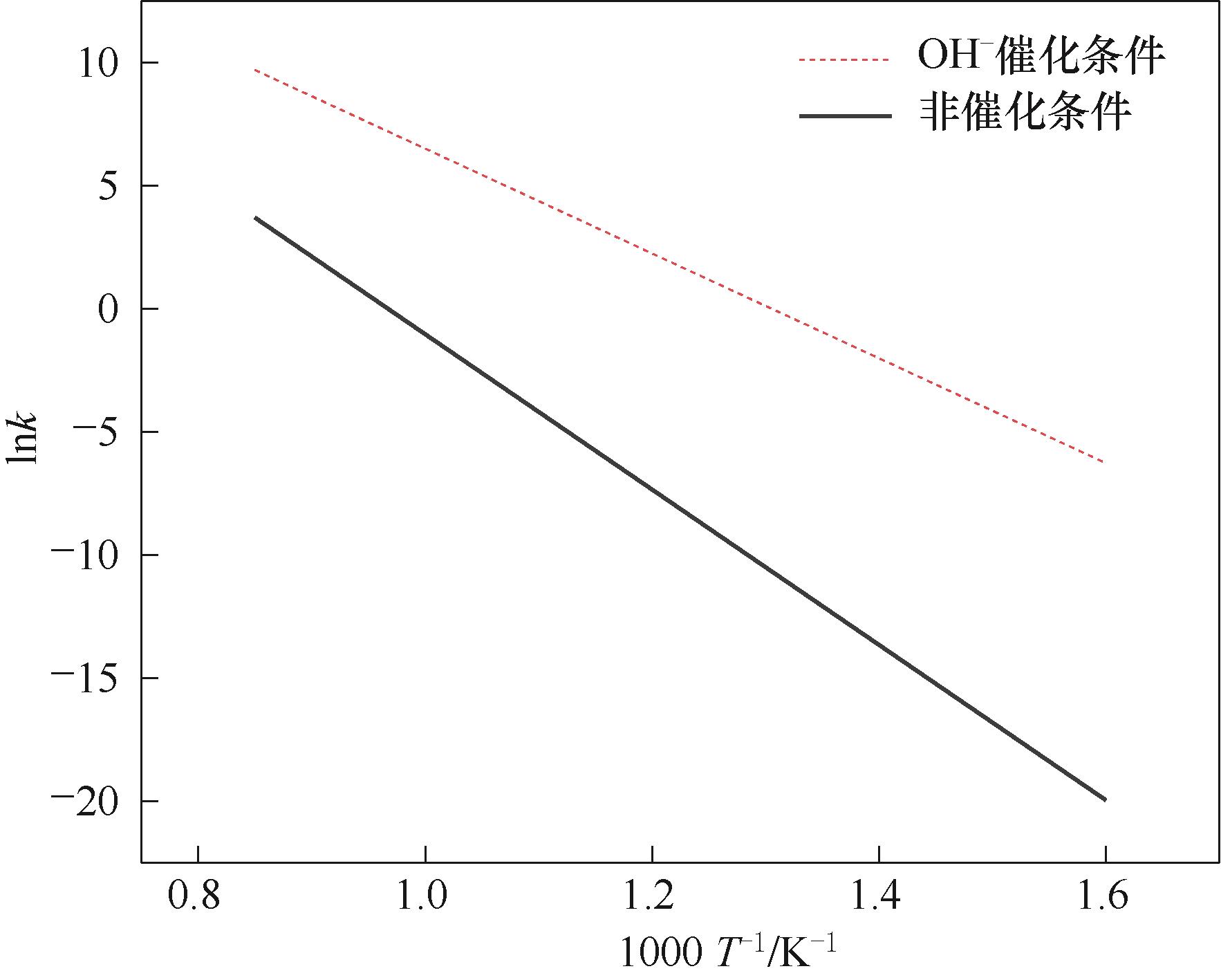
Fig.2 Comparison of fitted rate constants in the Arrhenius equation for water-gas conversion reactions under alkali-catalyzed and non-catalyzed conditions[50]

Fig.3 Supercritical water gasification of dilute black liquor in different continuous reactors(a) 23 MPa, 600—700℃, 0.81%(mass)[61]; (b) 25 MPa, 400—600℃, 2.5%(mass) [38]
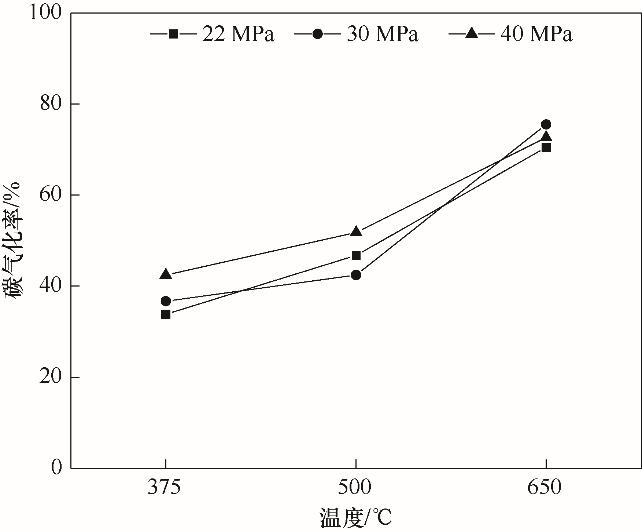
Fig.6 Carbon gasification efficiency in the quartz tube reactor at different temperature and pressure conditions [60](22—40 MPa, 375—650℃, 2.5%(mass))

Fig.8 Gas composition and carbon gasification efficiency at different concentration conditions in the intermittent reactor[62](23—26 MPa, 750℃, 2.5%—9.5%(mass))
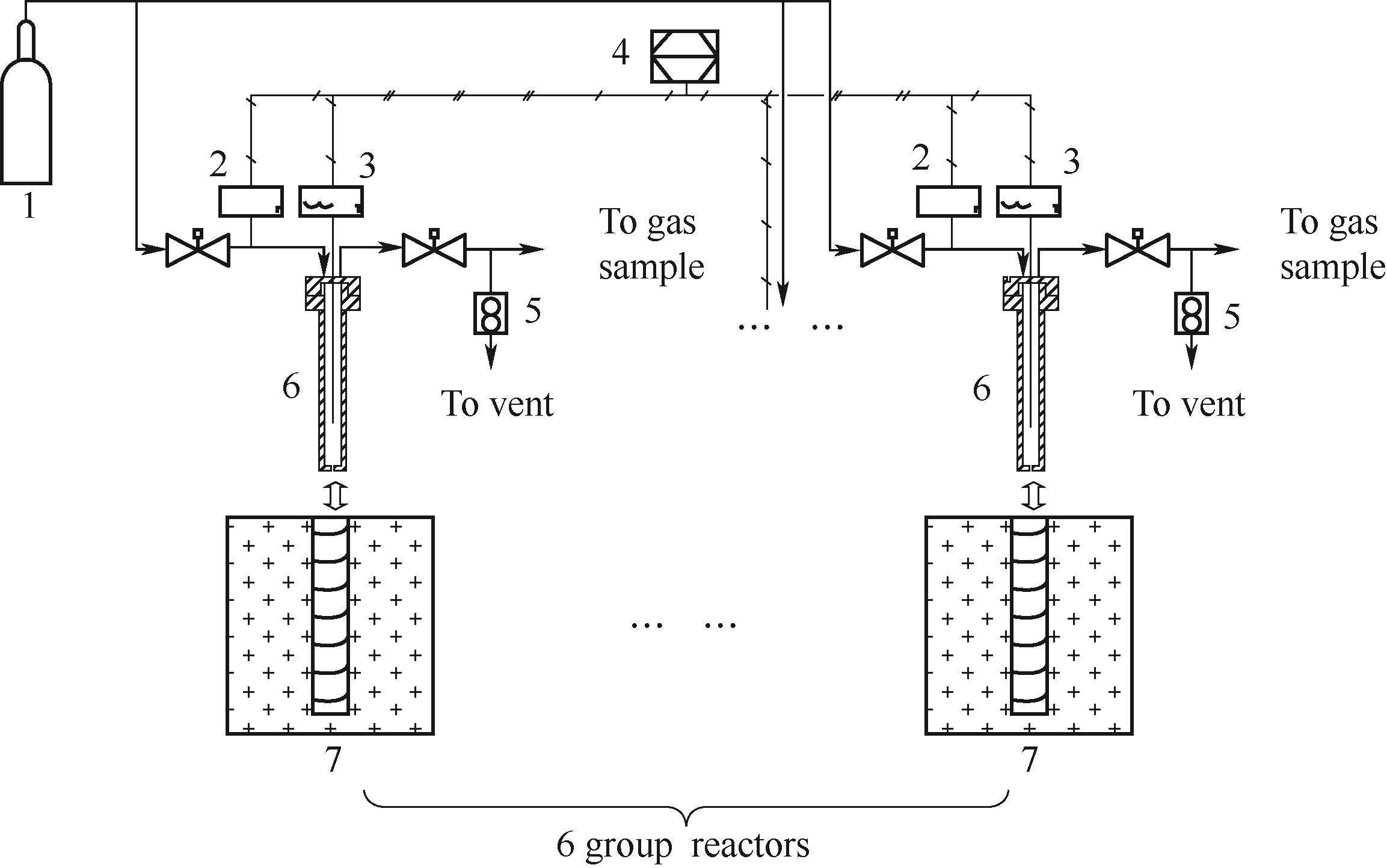
Fig.18 Schematic diagram of the intermittent reactor[14,40] system at the State Key Laboratory of Multiphase Flow in Power Engineering, Xi'an Jiaotong University1—Ar gas bottle; 2—pressure sensor; 3—temperature sensor; 4—data acquisition station; 5—wet gas flow meter; 6—intermittent kettle; 7—electric furnace
| 原料 | 国家 | 型式 | 操作参数 | 处理量 | 气化效果 | 盐回收 |
|---|---|---|---|---|---|---|
| 硫酸盐法黑液[ | 泰国 | 间歇反应器(石英毛细管,内径 1 mm;外径 2 mm,长度15 cm) | 650℃,40 MPa,无催化剂 | — | 碳气化率84.1% | 否 |
| 硫酸盐法黑液[ | 法国 | 间歇反应器(体积为5 ml) | 450℃,40 MPa,催化剂:CeO2 | — | 碳气化率30% | 否 |
| 硫酸盐法黑液[ | 中国 | 间歇反应器(体积为12 ml) | 600℃,23 MPa,催化剂:Co2O3等 | — | 碳气化率80.11%,H2产量 21.67mol/kg | 否 |
| 烧碱法黑液[ | 中国 | 间歇反应器(体积为10 ml) | 700℃,23 MPa | — | 碳气化率98.17%,H2产量 62.38 mol/kg | 否 |
| 硫酸盐法黑液[ | 俄罗斯 | 连续管流式反应器(不锈钢AISI 321H,内径10 mm,长度 127 mm) | 750℃,30 MPa | 0.01 g/min | 碳气化率91% | 否 |
| 硫酸盐法黑液[ | 芬兰 | 连续管流式反应器(Inconel 625或不锈钢316,内径1.1 cm,长度50.8 cm) | 700℃,30 MPa | 0.63 g/min | 碳气化率75%,H2产量7.87 mol/kg | 否 |
| 硫酸盐法黑液[ | 西班牙 | 连续管流式反应器 (Inconel 625,内径13 mm, 长度0.373 m) | 700℃,23 MPa,催化剂:NaOH | 5 g/min | 碳气化率52.7%,H2产量38.68 mol/kg | 否 |
| 硫酸盐法黑液[ | 中国 | 连续管流式反应器 | 500℃,24 MPa,催化剂:Ni/ZrO2 | — | 碳气化率74.87% | 否 |
| 烧碱法黑液[ | 中国 | 连续管流式反应器(Hastelloy C-276, 内径 10.85 mm,长度1.24 m) | 600℃,23 MPa | 5 kg/h | 碳气化率67.89%,H2产量11.26 mol/kg | 否 |
| 硫酸盐法黑液 | 中国 | 流态化反应器 | 640℃,23 MPa | 30 g/min | 碳气化率98.41%,H2产量32.84 mol/kg | 是 |
Table 2 Comparison of black liquor supercritical water gasification reactors
| 原料 | 国家 | 型式 | 操作参数 | 处理量 | 气化效果 | 盐回收 |
|---|---|---|---|---|---|---|
| 硫酸盐法黑液[ | 泰国 | 间歇反应器(石英毛细管,内径 1 mm;外径 2 mm,长度15 cm) | 650℃,40 MPa,无催化剂 | — | 碳气化率84.1% | 否 |
| 硫酸盐法黑液[ | 法国 | 间歇反应器(体积为5 ml) | 450℃,40 MPa,催化剂:CeO2 | — | 碳气化率30% | 否 |
| 硫酸盐法黑液[ | 中国 | 间歇反应器(体积为12 ml) | 600℃,23 MPa,催化剂:Co2O3等 | — | 碳气化率80.11%,H2产量 21.67mol/kg | 否 |
| 烧碱法黑液[ | 中国 | 间歇反应器(体积为10 ml) | 700℃,23 MPa | — | 碳气化率98.17%,H2产量 62.38 mol/kg | 否 |
| 硫酸盐法黑液[ | 俄罗斯 | 连续管流式反应器(不锈钢AISI 321H,内径10 mm,长度 127 mm) | 750℃,30 MPa | 0.01 g/min | 碳气化率91% | 否 |
| 硫酸盐法黑液[ | 芬兰 | 连续管流式反应器(Inconel 625或不锈钢316,内径1.1 cm,长度50.8 cm) | 700℃,30 MPa | 0.63 g/min | 碳气化率75%,H2产量7.87 mol/kg | 否 |
| 硫酸盐法黑液[ | 西班牙 | 连续管流式反应器 (Inconel 625,内径13 mm, 长度0.373 m) | 700℃,23 MPa,催化剂:NaOH | 5 g/min | 碳气化率52.7%,H2产量38.68 mol/kg | 否 |
| 硫酸盐法黑液[ | 中国 | 连续管流式反应器 | 500℃,24 MPa,催化剂:Ni/ZrO2 | — | 碳气化率74.87% | 否 |
| 烧碱法黑液[ | 中国 | 连续管流式反应器(Hastelloy C-276, 内径 10.85 mm,长度1.24 m) | 600℃,23 MPa | 5 kg/h | 碳气化率67.89%,H2产量11.26 mol/kg | 否 |
| 硫酸盐法黑液 | 中国 | 流态化反应器 | 640℃,23 MPa | 30 g/min | 碳气化率98.41%,H2产量32.84 mol/kg | 是 |
| 1 | Bajpai P. Biermann's Handbook of Pulp and Paper: Volume 1: Raw Material and Pulp Making [M]. Amsterdam: Elsevier, 2018. |
| 2 | 陈俊峰, 李光荣. 碱法制浆造纸黑液的资源化利用[J]. 广东化工, 2018, 45(3): 94-95. |
| Chen J F, Li G R. Resource utilization of alkaline pulping black liquor[J]. Guangdong Chemical Industry, 2018, 45(3): 94-95. | |
| 3 | Darmawan A, Ajiwibowo M W, Yoshikawa K, et al. Energy-efficient recovery of black liquor through gasification and syngas chemical looping[J]. Applied Energy, 2018, 219: 290-298. |
| 4 | Darmawan A, Hardi F, Yoshikawa K, et al. Enhanced process integration of black liquor evaporation, gasification, and combined cycle[J]. Applied Energy, 2017, 204: 1035-1042. |
| 5 | 付丹. 造纸黑液处理技术研究进展[J]. 辽宁化工, 2015, 44(4): 385-386, 391. |
| Fu D. Research progress in the treatment technology of papermaking black liquor[J]. Liaoning Chemical Industry, 2015, 44(4): 385-386, 391. | |
| 6 | Dong X Y, Guo S Q, Li S, et al. Physicochemical characteristics and combustion reactivity of reed stalk hydrochar obtained under dilute black liquor condition[J]. Biomass Conversion and Biorefinery, 2022. DOI:10.1007/s13399-022-02394-4 . |
| 7 | 唐华, 王大伟, 栾小娟. 制浆黑液燃烧特性实验研究进展[J]. 中国造纸, 2020, 39(9): 68-73. |
| Tang H, Wang D W, Luan X J. Experimental research progress of combustion characteristics of pulping black liquor[J]. China Pulp & Paper, 2020, 39(9): 68-73. | |
| 8 | 靳福明. 碱回收炉烟气排放及控制措施可行性技术分析[J]. 中国造纸, 2018, 37(3): 64-71. |
| Jin F M. Technical analysis on emission control method of recovery boiler[J]. China Pulp & Paper, 2018, 37(3): 64-71. | |
| 9 | Haddad M, Labrecque R, Bazinet L, et al. Effect of process variables on the performance of electrochemical acidification of Kraft black liquor by electrodialysis with bipolar membrane[J]. Chemical Engineering Journal, 2016, 304: 977-985. |
| 10 | Haddad M, Mikhaylin S, Bazinet L, et al. Electrochemical acidification of Kraft black liquor by electrodialysis with bipolar membrane: ion exchange membrane fouling identification and mechanisms[J]. Journal of Colloid and Interface Science, 2017, 488: 39-47. |
| 11 | Haddad M, Labrecque R, Bazinet L, et al. Effect of process variables on the performance of electrochemical acidification of Kraft black liquor by electrodialysis with bipolar membrane[J]. Chemical Engineering Journal, 2016, 304: 977-985. |
| 12 | Ma M S, Dai L, Si C L, et al. A facile preparation of super long-term stable lignin nanoparticles from black liquor[J]. ChemSusChem, 2019, 12(24): 5216. |
| 13 | 谭惠珊. 碱法制浆黑液中木质素的提取与纯化[D]. 天津: 天津科技大学, 2017. |
| Tan H S. The extraction and purification of lignin from alkaline pulping black liquor[D]. Tianjin: Tianjin University of Science & Technology, 2017. | |
| 14 | Chen Y N, He Y Y, Jin H, et al. Resource utilization of landfill leachate gasification in supercritical water[J]. Chemical Engineering Journal, 2020, 386: 124017. |
| 15 | Domínguez-Robles J, Palenzuela M D V, Sánchez R, et al. Coagulation–flocculation as an alternative way to reduce the toxicity of the black liquor from the paper industry: thermal valorization of the solid biomass recovered[J]. Waste and Biomass Valorization, 2020, 11(9): 4731-4742. |
| 16 | An X J, Zhong B, Chen G T, et al. Evaluation of bioremediation and detoxification potentiality for papermaking black liquor by a new isolated thermophilic and alkali-tolerant Serratia sp. AXJ-M[J]. Journal of Hazardous Materials, 2021, 406: 124285. |
| 17 | Narra M, Rudakiya D M, Macwan K, et al. Black liquor: a potential moistening agent for production of cost-effective hydrolytic enzymes by a newly isolated cellulo-xylano fungal strain Aspergillus tubingensis and its role in higher saccharification efficiency[J]. Bioresource Technology, 2020, 306: 123149. |
| 18 | Durai-Swamy K, Mansour M N, Warren D W. Pulsed combustion process for black liquor gasification[R]. Oak Ridge: Office of Scientific and Technical Information (OSTI), 1991. |
| 19 | Andersson J, Furusjö E, Wetterlund E, et al. Co-gasification of black liquor and pyrolysis oil: evaluation of blend ratios and methanol production capacities[J]. Energy Conversion and Management, 2016, 110: 240-248. |
| 20 | Naqvi M, Yan J, Dahlquist E. Black liquor gasification integrated in pulp and paper mills: a critical review[J]. Bioresource Technology, 2010, 101(21): 8001-8015. |
| 21 | Nohlgren I. Non-conventional causticization technology: a review[J]. Nordic Pulp & Paper Research Journal, 2004, 19(4): 470-480. |
| 22 | Zetterholm J, Pettersson K, Leduc S, et al. Resource efficiency or economy of scale: biorefinery supply chain configurations for co-gasification of black liquor and pyrolysis liquids[J]. Applied Energy, 2018, 230: 912-924. |
| 23 | Marklund M, Tegman R, Gebart R. CFD modelling of black liquor gasification: identification of important model parameters[J]. Fuel, 2007, 86(12/13): 1918-1926. |
| 24 | Maxim F, Contescu C, Boillat P, et al. Visualization of supercritical water pseudo-boiling at Widom line crossover[J]. Nature Communications, 2019, 10: 4114. |
| 25 | Ai L Q, Zhou Y S, Chen M. A reactive force field molecular dynamics simulation of the dynamic properties of hydrogen bonding in supercritical water[J]. Journal of Molecular Liquids, 2019, 276: 83-92. |
| 26 | Andreani C, Romanelli G, Parmentier A, et al. Hydrogen dynamics in supercritical water probed by neutron scattering and computer simulations[J]. The Journal of Physical Chemistry Letters, 2020, 11(21): 9461-9467. |
| 27 | Savage P E. Organic chemical reactions in supercritical water[J]. Chemical Reviews, 1999, 99(2): 603-622. |
| 28 | Hou R, Quan Y H, Pan D. Dielectric constant of supercritical water in a large pressure-temperature range[J]. The Journal of Chemical Physics, 2020, 153(10): 101103. |
| 29 | Ren C, Ge Z W, Ou G B, et al. Supercritical water partial oxidation mechanism of ethanol[J]. International Journal of Hydrogen Energy, 2021, 46(44): 22777-22788. |
| 30 | Chen Y N, Yi L, Yin J R, et al. Sewage sludge gasification in supercritical water with fluidized bed reactor: reaction and product characteristics[J]. Energy, 2022, 239: 122115. |
| 31 | Jin H, Wu Y, Guo L J, et al. Molecular dynamic investigation on hydrogen production by polycyclic aromatic hydrocarbon gasification in supercritical water[J]. International Journal of Hydrogen Energy, 2016, 41(6): 3837-3843. |
| 32 | Chen Y N, Yi L, Wei W W, et al. Hydrogen production by sewage sludge gasification in supercritical water with high heating rate batch reactor[J]. Energy, 2022, 238: 121740. |
| 33 | Ge Z W, Guo L J, Jin H. Catalytic supercritical water gasification mechanism of coal[J]. International Journal of Hydrogen Energy, 2020, 45(16): 9504-9511. |
| 34 | Ge Z W, Guo S H, Ren C, et al. Non-catalytic supercritical water partial oxidation mechanism of coal[J]. International Journal of Hydrogen Energy, 2020, 45(41): 21178-21185. |
| 35 | Guo S H, Meng F R, Peng P, et al. Thermodynamic analysis of the superiority of the direct mass transfer design in the supercritical water gasification system[J]. Energy, 2022, 244: 122722. |
| 36 | Ren C, Guo S H, Wang Y, et al. Thermodynamic analysis and optimization of auto-thermal supercritical water gasification polygeneration system of pig manure[J]. Chemical Engineering Journal, 2022, 427: 131938. |
| 37 | Barros T V, Carregosa J D C, Wisniewski Jr A, et al. Assessment of black liquor hydrothermal treatment under sub- and supercritical conditions: products distribution and economic perspectives[J]. Chemosphere, 2022, 286: 131774. |
| 38 | Cao C Q, Guo L J, Chen Y N, et al. Hydrogen production from supercritical water gasification of alkaline wheat straw pulping black liquor in continuous flow system[J]. International Journal of Hydrogen Energy, 2011, 36(21): 13528-13535. |
| 39 | Cao C Q, Xie Y P, Mao L H, et al. Hydrogen production from supercritical water gasification of soda black liquor with various metal oxides[J]. Renewable Energy, 2020, 157: 24-32. |
| 40 | Cao C Q, Zhang Y, Li L H, et al. Supercritical water gasification of black liquor with wheat straw as the supplementary energy resource[J]. International Journal of Hydrogen Energy, 2019, 44(30): 15737-15745. |
| 41 | de Blasio C, de Gisi S, Molino A, et al. Concerning operational aspects in supercritical water gasification of kraft black liquor[J]. Renewable Energy, 2019, 130: 891-901. |
| 42 | Özdenkçi K, Prestipino M, Björklund-Sänkiaho M, et al. Alternative energy valorization routes of black liquor by stepwise supercritical water gasification: effect of process parameters on hydrogen yield and energy efficiency[J]. Renewable and Sustainable Energy Reviews, 2020, 134: 110146. |
| 43 | Akiya N, Savage P E. Roles of water for chemical reactions in high-temperature water[J]. ChemInform, 2002, 33(43): 293. |
| 44 | Kruse A, Dahmen N. Water — a magic solvent for biomass conversion[J]. The Journal of Supercritical Fluids, 2015, 96: 36-45. |
| 45 | Güvenatam B, Heeres E H J, Pidko E A, et al. Lewis-acid catalyzed depolymerization of protobind lignin in supercritical water and ethanol[J]. Catalysis Today, 2016, 259: 460-466. |
| 46 | Kruse A, Bernolle P, Dahmen N, et al. Hydrothermal gasification of biomass: consecutive reactions to long-living intermediates[J]. Energy Environ. Sci., 2010, 3(1): 136-143. |
| 47 | Brock E E, Savage P E. Detailed chemical kinetics model for supercritical water oxidation of C1 compounds and H2 [J]. AIChE Journal, 1995, 41(8): 1874-1888. |
| 48 | Park K C, Tomiyasu H. Gasification reaction of organic compounds catalyzed by RuO2 in supercritical water[J]. Chemical Communications, 2003(6): 694-695. |
| 49 | Watanabe M, Hirakoso H, Sawamoto S, et al. Polyethylene conversion in supercritical water[J]. The Journal of Supercritical Fluids, 1998, 13(1/2/3): 247-252. |
| 50 | Wang R Y, Guo L J, Jin H, et al. DFT study of the enhancement on hydrogen production by alkaline catalyzed water gas shift reaction in supercritical water[J]. International Journal of Hydrogen Energy, 2018, 43(30): 13879-13886. |
| 51 | Ding Z Y, Frisch M A, Li L X, et al. Catalytic oxidation in supercritical water[J]. Industrial & Engineering Chemistry Research, 1996, 35(10): 3257-3279. |
| 52 | Akiya N, Savage P E. Kinetics and mechanism of cyclohexanol dehydration in high-temperature water[J]. Industrial & Engineering Chemistry Research, 2001, 40(8): 1822-1831. |
| 53 | Wang B S, Hou H, Gu Y S. Water-catalyzed mechanism for the pyrolysis of formic acid[J]. Chemical Physics, 1999, 243(1/2): 27-34. |
| 54 | Akiya N, Savage P E. Role of water in formic acid decomposition[J]. AIChE Journal, 1998, 44(2): 405-415. |
| 55 | Elliott D C. Catalytic hydrothermal gasification of biomass[J]. Biofuels, Bioproducts and Biorefining, 2008, 2(3): 254-265. |
| 56 | Elliott D C, Sealock L J. Aqueous catalyst systems for the water-gas shift reaction(1): Comparative catalyst studies[J]. Industrial & Engineering Chemistry Product Research and Development, 1983, 22(3): 426-431. |
| 57 | Sınağ A, Kruse A, Schwarzkopf V. Key compounds of the hydropyrolysis of glucose in supercritical water in the presence of K2CO3 [J]. Industrial & Engineering Chemistry Research, 2003, 42(15): 3516-3521. |
| 58 | Kruse A, Gawlik A. Biomass conversion in water at 330—410 ℃ and 30—50 MPa. Identification of key compounds for indicating different chemical reaction pathways[J]. Industrial & Engineering Chemistry Research, 2003, 42(2): 267-279. |
| 59 | Kang S M, Li X L, Fan J, et al. Hydrothermal conversion of lignin: a review[J]. Renewable and Sustainable Energy Reviews, 2013, 27: 546-558. |
| 60 | Sricharoenchaikul V. Assessment of black liquor gasification in supercritical water[J]. Bioresource Technology, 2009, 100(2): 638-643. |
| 61 | Casademont P, Sánchez-Oneto J, Scandelai A P J, et al. Hydrogen production by supercritical water gasification of black liquor: use of high temperatures and short residence times in a continuous reactor[J]. The Journal of Supercritical Fluids, 2020, 159: 104772. |
| 62 | Cao C Q, Xu L C, He Y Y, et al. High-efficiency gasification of wheat straw black liquor in supercritical water at high temperatures for hydrogen production[J]. Energy & Fuels, 2017, 31(4): 3970-3978. |
| 63 | Bühler W, Dinjus E, Ederer H J, et al. Ionic reactions and pyrolysis of glycerol as competing reaction pathways in near- and supercritical water[J]. The Journal of Supercritical Fluids, 2002, 22(1): 37-53. |
| 64 | Ibrahim A B A, Akilli H. Supercritical water gasification of wastewater sludge for hydrogen production[J]. International Journal of Hydrogen Energy, 2019, 44(21): 10328-10349. |
| 65 | He C, Wang K, Giannis A, et al. Products evolution during hydrothermal conversion of dewatered sewage sludge in sub- and near-critical water: effects of reaction conditions and calcium oxide additive[J]. International Journal of Hydrogen Energy, 2015, 40(17): 5776-5787. |
| 66 | Zhang L H, Xu C B, Champagne P. Energy recovery from secondary pulp/paper-mill sludge and sewage sludge with supercritical water treatment[J]. Bioresource Technology, 2010, 101(8): 2713-2721. |
| 67 | Yan Z Y, Tan X Y. Hydrogen generation from oily wastewater via supercritical water gasification (SCWG)[J]. Journal of Industrial and Engineering Chemistry, 2015, 23: 44-49. |
| 68 | 晏波, 吴君章, 何凤媚, 等. Ni/ZrO2催化超临界水气化造纸黑液产氢资源化研究[C]//第八届全国氢能学术会议. 2007. |
| Yan B, Wu J Z, He F M, et al.Study on the hydrogen production from pulp and paper millwastewater by supercritical water gasification (SCWG) in a continuous flow reactor with Ni/ZrO2 catalyst[C]// Proceedings of the 8th National Conference on Hydrogen Energy. 2007. | |
| 69 | de Blasio C, Lucca G, Özdenkci K, et al. A study on supercritical water gasification of black liquor conducted in stainless steel and nickel-chromium-molybdenum reactors[J]. Journal of Chemical Technology & Biotechnology, 2016, 91(10): 2664-2678. |
| 70 | Boucard H, Watanabe M, Takami S, et al. Beneficial use of CeO2 nanocatalyst for black liquor conversion under sub and supercritical conditions[J]. The Journal of Supercritical Fluids, 2015, 105: 66-76. |
| 71 | Pokhrel D, Viraraghavan T. Treatment of pulp and paper mill wastewater—a review[J]. Science of the Total Environment, 2004, 333(1/2/3): 37-58. |
| 72 | Saari J, Sermyagina E, Kaikko J, et al. Evaluation of the energy efficiency improvement potential through back-end heat recovery in the kraft recovery boiler[J]. Energies, 2021, 14(6): 1550. |
| 73 | Lin J H, Liao Q X, Hu Y P, et al. Effects of process parameters on sulfur migration and H2S generation during supercritical water gasification of sludge[J]. Journal of Hazardous Materials, 2021, 403: 123678. |
| 74 | Liu S K, Li L H, Guo L J, et al. Sulfur transformation characteristics and mechanisms during hydrogen production by coal gasification in supercritical water[J]. Energy & Fuels, 2017, 31(11): 12046-12053. |
| 75 | Ding K L, Li S Y, Yue C T, et al. Simulation experiments on the reaction system of CH4-MgSO4-H2O[J]. Chinese Science Bulletin, 2008, 53(7): 1071-1078. |
| 76 | Wang R Y, Lu L B, Chen J, et al. Combining experiment and density functional theory to study the mechanism of thermochemical sulfate reduction by hydrogen in supercritical water[J]. Journal of Molecular Liquids, 2021, 330: 115654. |
| 77 | Yue C T, Li S Y, Ding K L, et al. Thermodynamics and kinetics of reactions between C1—C3 hydrocarbons and calcium sulfate in deep carbonate reservoirs[J]. Geochemical Journal, 2006, 40(1): 87-94. |
| 78 | Duverger-Nédellec E, Voisin T, Erriguible A, et al. Unveiling the complexity of salt(s) in water under transcritical conditions[J]. The Journal of Supercritical Fluids, 2020, 165: 104977. |
| 79 | Valyashko V M. Phase equilibria in water-salt systems: some problems of solubility at elevated temperature and pressure[J]. High Temperature High Pressure Electrochemistry in Aqueous Solutions, 1976, 4: 153-157. |
| 80 | 王润宇. 碱及无机硫在超临界水气化过程中反应机理的研究 [D]. 西安; 西安交通大学, 2021. |
| Wang R Y. Study on the reaction mechanism of alkali and inorganic sulfur in supercritical water gasification process [D]. Xi'an: Xi'an Jiaotong University, 2021. | |
| 81 | Marshall W L. Water and its solutions at high temperatures and pressures[J]. Chemistry, 1975, 48: 6-12. |
| 82 | Schubert M, Aubert J, Müller J B, et al. Continuous salt precipitation and separation from supercritical water(Part 3): Interesting effects in processing type 2 salt mixtures[J]. The Journal of Supercritical Fluids, 2012, 61: 44-54. |
| 83 | Schubert M, Regler J W, Vogel F. Continuous salt precipitation and separation from supercritical water(Part 1): Type 1 salts[J]. The Journal of Supercritical Fluids, 2010, 52(1): 99-112. |
| 84 | Schubert M, Regler J W, Vogel F. Continuous salt precipitation and separation from supercritical water(Part 2): Type 2 salts and mixtures of two salts[J]. The Journal of Supercritical Fluids, 2010, 52(1): 113-124. |
| 85 | Schubert M, Müller J B, Vogel F. Continuous hydrothermal of glycerol and its degradation products on the continuous salt separation and the enhancing effect of K3PO4 on the glycerol degradation[J]. The Journal of Supercritical Fluids, 2014, 95: 364-372. |
| 86 | Wang R Y, Deplazes R, Vogel F, et al. Continuous extraction of black liquor salts under hydrothermal conditions[J]. Industrial & Engineering Chemistry Research, 2021, 60(10): 4072-4085. |
| 87 | Azadi P, Farnood R. Review of heterogeneous catalysts for sub- and supercritical water gasification of biomass and wastes[J]. International Journal of Hydrogen Energy, 2011, 36(16): 9529-9541. |
| 88 | Fedyaeva O N, Vostrikov A A, Shishkin A V, et al. Conjugated processes of black liquor mineral and organic components conversion in supercritical water[J]. The Journal of Supercritical Fluids, 2019, 143: 191-197. |
| [1] | Congqi HUANG, Yimei WU, Jianye CHEN, Shuangquan SHAO. Simulation study of thermal management system of alkaline water electrolysis device for hydrogen production [J]. CIESC Journal, 2023, 74(S1): 320-328. |
| [2] | Zhewen CHEN, Junjie WEI, Yuming ZHANG. System integration and energy conversion mechanism of the power technology with integrated supercritical water gasification of coal and SOFC [J]. CIESC Journal, 2023, 74(9): 3888-3902. |
| [3] | Chen HAN, Youmin SITU, Bin ZHU, Jianliang XU, Xiaolei GUO, Haifeng LIU. Study of reaction and flow characteristics in multi-nozzle pulverized coal gasifier with co-processing of wastewater [J]. CIESC Journal, 2023, 74(8): 3266-3278. |
| [4] | Yuyuan ZHENG, Zhiwei GE, Xiangyu HAN, Liang WANG, Haisheng CHEN. Progress and prospect of medium and high temperature thermochemical energy storage of calcium-based materials [J]. CIESC Journal, 2023, 74(8): 3171-3192. |
| [5] | Ming DONG, Jinliang XU, Guanglin LIU. Molecular dynamics study on heterogeneous characteristics of supercritical water [J]. CIESC Journal, 2023, 74(7): 2836-2847. |
| [6] | Zhenghao YANG, Zhen HE, Yulong CHANG, Ziheng JIN, Xia JIANG. Research progress in downer fluidized bed reactor for biomass fast pyrolysis [J]. CIESC Journal, 2023, 74(6): 2249-2263. |
| [7] | Xiaowen ZHOU, Jie DU, Zhanguo ZHANG, Guangwen XU. Study on the methane-pulsing reduction characteristics of Fe2O3-Al2O3 oxygen carrier [J]. CIESC Journal, 2023, 74(6): 2611-2623. |
| [8] | Yanhui LI, Shaoming DING, Zhouyang BAI, Yinan ZHANG, Zhihong YU, Limei XING, Pengfei GAO, Yongzhen WANG. Corrosion micro-nano scale kinetics model development and application in non-conventional supercritical boilers [J]. CIESC Journal, 2023, 74(6): 2436-2446. |
| [9] | Yong LI, Jiaqi GAO, Chao DU, Yali ZHAO, Boqiong LI, Qianqian SHEN, Husheng JIA, Jinbo XUE. Construction of Ni@C@TiO2 core-shell dual-heterojunctions for advanced photo-thermal catalytic hydrogen generation [J]. CIESC Journal, 2023, 74(6): 2458-2467. |
| [10] | Bimao ZHOU, Shisen XU, Xiaoxiao WANG, Gang LIU, Xiaoyu LI, Yongqiang REN, Houzhang TAN. Effect of burner bias angle on distribution characteristics of gasifier slag layer [J]. CIESC Journal, 2023, 74(5): 1939-1949. |
| [11] | Zedong WANG, Zhiping SHI, Liyan LIU. Numerical simulation and optimization of acoustic streaming considering inhomogeneous bubble cloud dissipation in rectangular reactor [J]. CIESC Journal, 2023, 74(5): 1965-1973. |
| [12] | Jianhua ZHANG, Mengmeng CHEN, Yawen SUN, Yongzhen PENG. Efficient nitrogen and phosphorus removal from domestic wastewater via simultaneous partial nitritation and phosphorus removal combined Anammox [J]. CIESC Journal, 2023, 74(5): 2147-2156. |
| [13] | Zefeng GE, Yuqing WU, Mingxun ZENG, Zhenting ZHA, Yuna MA, Zenghui HOU, Huiyan ZHANG. Effect of ash chemical components on biomass gasification properties [J]. CIESC Journal, 2023, 74(5): 2136-2146. |
| [14] | Lanhe ZHANG, Qingyi LAI, Tiezheng WANG, Xiaozhuo GUAN, Mingshuang ZHANG, Xin CHENG, Xiaohui XU, Yanping JIA. Effect of H2O2 on nitrogen removal and sludge properties in SBR [J]. CIESC Journal, 2023, 74(5): 2186-2196. |
| [15] | Sheng’an ZHANG, Guilian LIU. Multi-objective optimization of high-efficiency solar water electrolysis hydrogen production system and its performance [J]. CIESC Journal, 2023, 74(3): 1260-1274. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||