CIESC Journal ›› 2023, Vol. 74 ›› Issue (6): 2264-2280.DOI: 10.11949/0438-1157.20230048
• Reviews and monographs • Previous Articles Next Articles
Tan ZHANG1,2,3( ), Guang LIU1(
), Guang LIU1( ), Jinping LI1,2,3(
), Jinping LI1,2,3( ), Yuhan SUN2,4
), Yuhan SUN2,4
Received:2023-01-19
Revised:2023-06-01
Online:2023-07-27
Published:2023-06-05
Contact:
Guang LIU, Jinping LI
张谭1,2,3( ), 刘光1(
), 刘光1( ), 李晋平1,2,3(
), 李晋平1,2,3( ), 孙予罕2,4
), 孙予罕2,4
通讯作者:
刘光,李晋平
作者简介:张谭(1995—),男,博士研究生,zhangtan1206@126.com
基金资助:CLC Number:
Tan ZHANG, Guang LIU, Jinping LI, Yuhan SUN. Performance regulation strategies of Ru-based nitrogen reduction electrocatalysts[J]. CIESC Journal, 2023, 74(6): 2264-2280.
张谭, 刘光, 李晋平, 孙予罕. Ru基氮还原电催化剂性能调控策略[J]. 化工学报, 2023, 74(6): 2264-2280.
Add to citation manager EndNote|Ris|BibTeX
| 序号 | 催化剂 | 产氨率 | FE/% | 电解液 | 电位/V(vs RHE) | 文献 | 调控策略 |
|---|---|---|---|---|---|---|---|
| 1 | LaFeO3-Ru | 137.5 μg·h-1·mg-1 | 56.9 | 0.1 mol·L-1 K2SO4 | -0.7 | [ | 单原子策略 |
| 2 | Ru SAs/g-C3N4 | 23.0 μg·h-1·mg-1 | 8.3 | 0.5 mol·L-1 NaOH | 0.05 | [ | |
| 3 | Ru SAs/N-C | 120.9 μg·h-1·mg-1 | 29.6 | 均可 | -0.2 | [ | |
| 4 | SA Ru-Mo2CT x | 40.57 μg·h-1·mg-1 | 25.77 | 0.5 mol·L-1 K2SO4 | -0.3 | [ | |
| 5 | Ru SAs/Ti3C2O | 27.56 μg·h-1·mg-1 | 23.3 | 0.1 mol·L-1 HCl | -0.2 | [ | |
| 6 | PdRu TPs | 37.23 μg·h-1·mg-1 | 1.85 | 0.1 mol·L-1 KOH | -0.2 | [ | 形貌/晶体调控策略 |
| 7 | PdRu BPNs | 25.92 μg·h-1·mg-1 | 1.53 | 0.1 mol·L-1 HCl | -0.1 | [ | |
| 8 | mRhRu/NF | 30.28 μg·h-1·mg-1 | 28.33 | 0.1 mol·L-1 Na2SO4 | -0.05 | [ | |
| 9 | a1-Ru/CNTs | 10.49 μg·h-1·mg-1 | 17.48 | 5 mmol·L-1 Cs2CO3 | -0.2 | [ | |
| 10 | Ru/rGO-C12 | 50 μg·h-1·mg-1 | 11 | 0.05 mol·L-1 H2SO4 | -0.1 | [ | 表/界面工程 |
| 11 | RuCu-FNs | 53.6 μg·h-1·mg-1 | 7.2 | 0.1 mol·L-1 HCl | -0.1 | [ | |
| 12 | Ru88Pt12 | 47.4 μg·h-1·mg-1 | 8.9 | 0.1 mol·L-1 KOH | -0.2 | [ | 杂原子掺杂 |
| 13 | Rh0.6Ru0.4 NAs/CP | 57.75 μg·h-1·mg-1 | 3.39 | 0.1 mol·L-1 Na2SO4 | -0.2 | [ | |
| 14 | Ru-Cu NPs | 73 μg·h-1·cm-2 | 30.95 | 0.1 mol·L-1 HCl | -0.1 | [ | |
| 15 | Ru2P-rGO | 32.8 μg·h-1·mg-1 | 13.04 | 0.1 mol·L-1 HCl | -0.05 | [ | |
| 16 | Ru/CeO2-Vo | 9.87×10-8 mmol·s-1·cm-2 | 11.7 | 0.05 mol·L-1 H2SO4 | -0.25 | [ | 空位调控 |
| 17 | Ru/2H-MoS2 | 1.14×10-10 mmol·s-1·cm-2 | 17.6 | 10 mmol·L-1 HCl | -0.15 | [ |
Table 1 Summary of performance of partial Ru-based catalysts guided by performance-improving strategies
| 序号 | 催化剂 | 产氨率 | FE/% | 电解液 | 电位/V(vs RHE) | 文献 | 调控策略 |
|---|---|---|---|---|---|---|---|
| 1 | LaFeO3-Ru | 137.5 μg·h-1·mg-1 | 56.9 | 0.1 mol·L-1 K2SO4 | -0.7 | [ | 单原子策略 |
| 2 | Ru SAs/g-C3N4 | 23.0 μg·h-1·mg-1 | 8.3 | 0.5 mol·L-1 NaOH | 0.05 | [ | |
| 3 | Ru SAs/N-C | 120.9 μg·h-1·mg-1 | 29.6 | 均可 | -0.2 | [ | |
| 4 | SA Ru-Mo2CT x | 40.57 μg·h-1·mg-1 | 25.77 | 0.5 mol·L-1 K2SO4 | -0.3 | [ | |
| 5 | Ru SAs/Ti3C2O | 27.56 μg·h-1·mg-1 | 23.3 | 0.1 mol·L-1 HCl | -0.2 | [ | |
| 6 | PdRu TPs | 37.23 μg·h-1·mg-1 | 1.85 | 0.1 mol·L-1 KOH | -0.2 | [ | 形貌/晶体调控策略 |
| 7 | PdRu BPNs | 25.92 μg·h-1·mg-1 | 1.53 | 0.1 mol·L-1 HCl | -0.1 | [ | |
| 8 | mRhRu/NF | 30.28 μg·h-1·mg-1 | 28.33 | 0.1 mol·L-1 Na2SO4 | -0.05 | [ | |
| 9 | a1-Ru/CNTs | 10.49 μg·h-1·mg-1 | 17.48 | 5 mmol·L-1 Cs2CO3 | -0.2 | [ | |
| 10 | Ru/rGO-C12 | 50 μg·h-1·mg-1 | 11 | 0.05 mol·L-1 H2SO4 | -0.1 | [ | 表/界面工程 |
| 11 | RuCu-FNs | 53.6 μg·h-1·mg-1 | 7.2 | 0.1 mol·L-1 HCl | -0.1 | [ | |
| 12 | Ru88Pt12 | 47.4 μg·h-1·mg-1 | 8.9 | 0.1 mol·L-1 KOH | -0.2 | [ | 杂原子掺杂 |
| 13 | Rh0.6Ru0.4 NAs/CP | 57.75 μg·h-1·mg-1 | 3.39 | 0.1 mol·L-1 Na2SO4 | -0.2 | [ | |
| 14 | Ru-Cu NPs | 73 μg·h-1·cm-2 | 30.95 | 0.1 mol·L-1 HCl | -0.1 | [ | |
| 15 | Ru2P-rGO | 32.8 μg·h-1·mg-1 | 13.04 | 0.1 mol·L-1 HCl | -0.05 | [ | |
| 16 | Ru/CeO2-Vo | 9.87×10-8 mmol·s-1·cm-2 | 11.7 | 0.05 mol·L-1 H2SO4 | -0.25 | [ | 空位调控 |
| 17 | Ru/2H-MoS2 | 1.14×10-10 mmol·s-1·cm-2 | 17.6 | 10 mmol·L-1 HCl | -0.15 | [ |
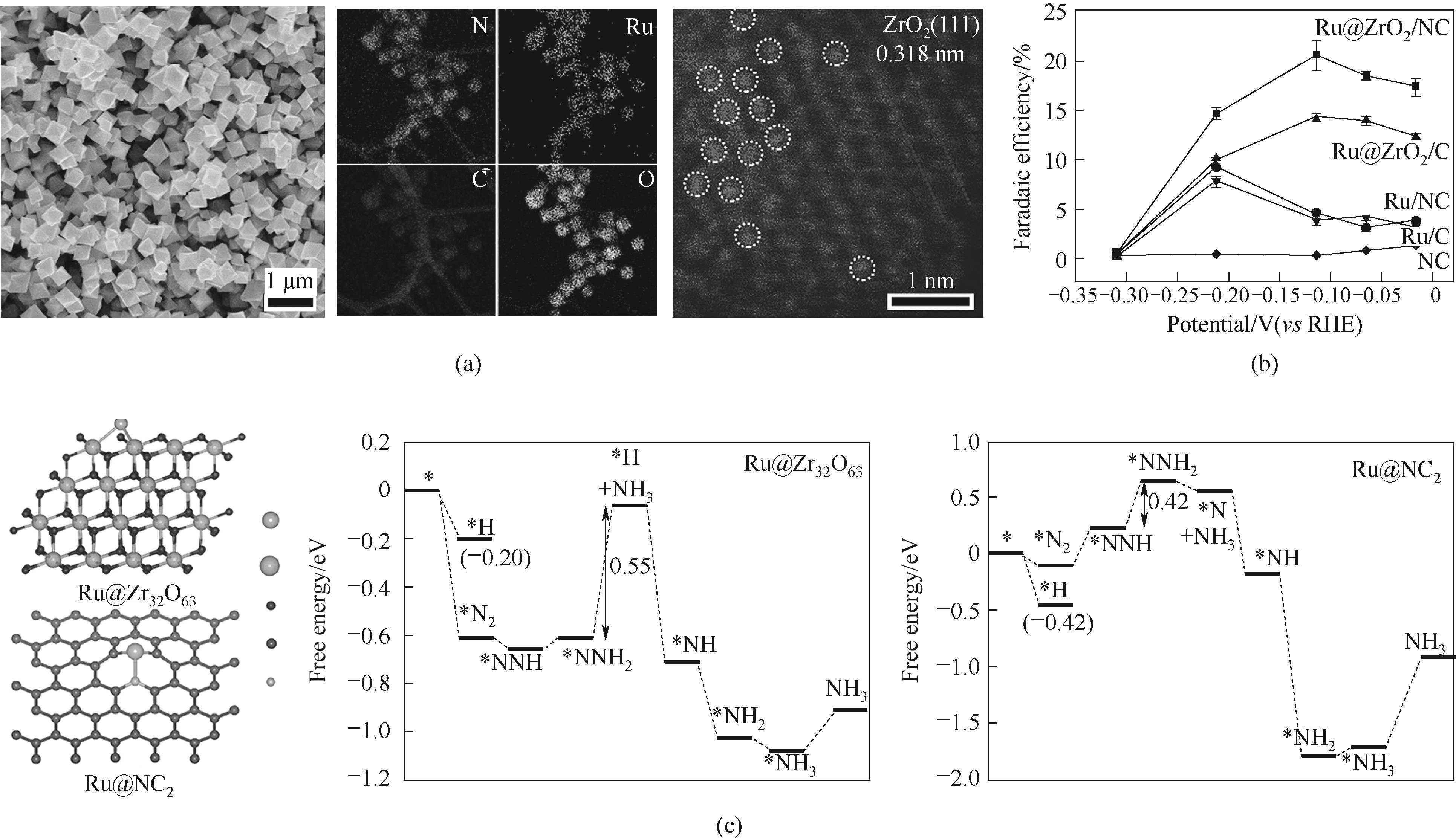
Fig.5 (a) Morphology and structure characterization of Ru@ZrO2/NC; (b) Electrochemical nitrogen reduction activities; (c) Calculation models and free energy diagrams for NRR [44]

Fig.6 (a) The corresponding elemental mapping images of a single PdRu TP; (b) NH3 yield rates and FE of PdRu TPs at selected potentials; (c) NH3 yield rates of different catalysts at -0.2 V(vs RHE); (d) Schematic diagram of electrocatalytic NRR process on the PdRu TPs[80]
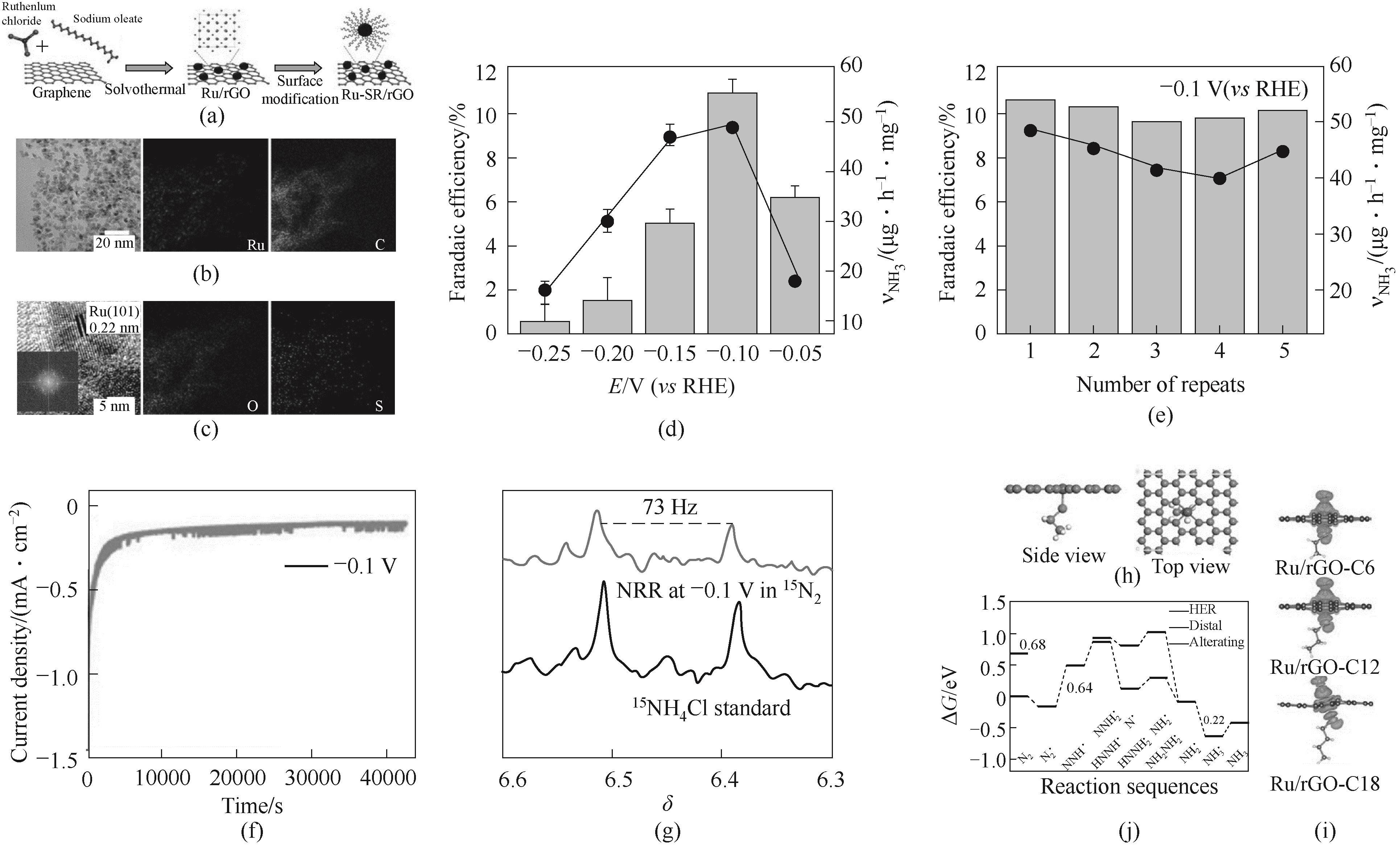
Fig.7 (a) Illustration of synthesis and modification of Ru/rGO with aliphatic thiols; (b) TEM images; (c) HR-TEM images; (d) FE and rate of NH3 production for Ru/rGO-C12; (e) Recyclability and (f) long-term stability of Ru/rGO-C12; (g) NH3 quantification by 1H NMR in 15N2 environment; (h) The optimized atomic configuration; (i) Bader charge distribution; (j) Gibbs free energy profile of NRR on Ru/rGO-C12 [93]

Fig.8 (a) A schematic diagram of the structure of a Ru88Pt12 nanowire; (b) TEM images and (c) HAADF images of Ru88Pt12 nanowires; (d) NH3 production rates and (e) corresponding FE at different potentials; (f) Recyclability and (g) long-term stability of electrocatalysis; (h) Atomic models; (i) Calculated adsorption energies; (j) Projected density of states[111]

Fig.9 (a) Schematic diagram of the structure and fabrication of Ru-Cu NPs; (b) Mapping images of Ru-Cu NPs; (c) FE and NH3 formation rates; (d) 1H NMR spectra; (e) DFT calculations of the NRR process on the electrocatalysts[114]

Fig.10 (a) EPR spectra; (b) XPS spectra of O 1s; (c) Valance bands; (d) Schematic diagram of minimum-energy pathway for electrochemical N2 conversion into NH3 catalyzed by Ru/2H-MoS2 material[125, 127]
| 1 | Fang H H, Liu D, Luo Y, et al. Challenges and opportunities of Ru-based catalysts toward the synthesis and utilization of ammonia[J]. ACS Catalysis, 2022, 12(7): 3938-3954. |
| 2 | Petitpas G. Simulation of boil-off losses during transfer at a LH2 based hydrogen refueling station[J]. International Journal of Hydrogen Energy, 2018, 43(46): 21451-21463. |
| 3 | Metkemeijer R, Achard P. Comparison of ammonia and methanol applied indirectly in a hydrogen fuel cell[J]. International Journal of Hydrogen Energy, 1994, 19(6): 535-542. |
| 4 | Wan Z J, Tao Y K, Shao J, et al. Ammonia as an effective hydrogen carrier and a clean fuel for solid oxide fuel cells[J]. Energy Conversion and Management, 2021, 228: 113729. |
| 5 | Luo Y, Liang S J, Wang X Y, et al. Facile synthesis and high-value utilization of ammonia[J]. Chinese Journal of Chemistry, 2022, 40(8): 953-964. |
| 6 | Yang S, Zhang T, Yang Y Y, et al. Molybdenum-based nitrogen carrier for ammonia production via a chemical looping route[J]. Applied Catalysis B: Environmental, 2022, 312: 121404. |
| 7 | Zhang T, Yu Z L, Yu J Q, et al. Chemical looping ammonia synthesis with high performance supported molybdenum-based nitrogen carrier[J]. Acta Chimica Sinica, 2022, 80(6): 788. |
| 8 | Zhao R B, Xie H T, Chang L, et al. Recent progress in the electrochemical ammonia synthesis under ambient conditions[J]. EnergyChem, 2019, 1(2): 100011. |
| 9 | Wu T T, Fan W J, Zhang Y, et al. Electrochemical synthesis of ammonia: progress and challenges[J]. Materials Today Physics, 2021, 16: 100310. |
| 10 | Rod T H, Logadottir A, Nørskov J K. Ammonia synthesis at low temperatures[J]. The Journal of Chemical Physics, 2000, 112(12): 5343-5347. |
| 11 | Deng J, Iñiguez J A, Liu C. Electrocatalytic nitrogen reduction at low temperature[J]. Joule, 2018, 2(5): 846-856. |
| 12 | Ma W C, He X Y, Wang W, et al. Electrocatalytic reduction of CO2 and CO to multi-carbon compounds over Cu-based catalysts[J]. Chemical Society Reviews, 2021, 50(23): 12897-12914. |
| 13 | Ren Y W, Yu C, Tan X Y, et al. Strategies to suppress hydrogen evolution for highly selective electrocatalytic nitrogen reduction: challenges and perspectives[J]. Energy & Environmental Science, 2021, 14(3): 1176-1193. |
| 14 | Wang Z C, Zhao H, Liu J, et al. The PdH x metallene with vacancies for synergistically enhancing electrocatalytic N2 fixation[J]. Chemical Engineering Journal, 2022, 450: 137951. |
| 15 | Hu L, Khaniya A, Wang J, et al. Ambient electrochemical ammonia synthesis with high selectivity on Fe/Fe oxide catalyst[J]. ACS Catalysis, 2018, 8(10): 9312-9319. |
| 16 | Xue Z H, Zhang S N, Lin Y X, et al. Electrochemical reduction of N2 into NH3 by donor-acceptor couples of Ni and Au nanoparticles with a 67.8% Faradaic efficiency[J]. Journal of the American Chemical Society, 2019, 141(38): 14976-14980. |
| 17 | Zhu X J, Mou S Y, Peng Q L, et al. Aqueous electrocatalytic N2 reduction for ambient NH3 synthesis: recent advances in catalyst development and performance improvement[J]. Journal of Materials Chemistry A, 2020, 8(4): 1545-1556. |
| 18 | Lv C D, Qian Y M, Yan C S, et al. Defect engineering metal-free polymeric carbon nitride electrocatalyst for effective nitrogen fixation under ambient conditions[J]. Angewandte Chemie International Edition, 2018, 57(32): 10246-10250. |
| 19 | Singh A R, Rohr B A, Schwalbe J A, et al. Electrochemical ammonia synthesis—the selectivity challenge[J]. ACS Catalysis, 2017, 7(1): 706-709. |
| 20 | Skúlason E, Bligaard T, Gudmundsdóttir S, et al. A theoretical evaluation of possible transition metal electro-catalysts for N2 reduction[J]. Phys. Chem. Chem. Phys., 2012, 14(3): 1235-1245. |
| 21 | Wan Y C, Xu J C, Lv R T. Heterogeneous electrocatalysts design for nitrogen reduction reaction under ambient conditions[J]. Materials Today, 2019, 27: 69-90. |
| 22 | Yang B, Ding W L, Zhang H H, et al. Recent progress in electrochemical synthesis of ammonia from nitrogen: strategies to improve the catalytic activity and selectivity[J]. Energy & Environmental Science, 2021, 14(2): 672-687. |
| 23 | Khalil I E, Xue C, Liu W J, et al. The role of defects in metal-organic frameworks for nitrogen reduction reaction: when defects switch to features[J]. Advanced Functional Materials, 2021, 31(17): 2010052. |
| 24 | Wang Z Q, Li C J, Deng K, et al. Ambient nitrogen reduction to ammonia electrocatalyzed by bimetallic PdRu porous nanostructures[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(2): 2400-2405. |
| 25 | Tayyebi E, Abghoui Y, Skúlason E. Elucidating the mechanism of electrochemical N2 reduction at the Ru(0001) electrode[J]. ACS Catalysis, 2019, 9(12): 11137-11145. |
| 26 | Ma X G, Hu J S, Zheng M K, et al. N2 reduction using single transition-metal atom supported on defective WS2 monolayer as promising catalysts: a DFT study[J]. Applied Surface Science, 2019, 489: 684-692. |
| 27 | Fang Q J, Gao Y J, Zhang W, et al. Oxygen groups enhancing the mechanism of nitrogen reduction reaction properties on Ru- or Fe-supported Nb2C MXene[J]. The Journal of Physical Chemistry C, 2021, 125(27): 14636-14645. |
| 28 | Yao Y, Wang H J, Yuan X Z, et al. Electrochemical nitrogen reduction reaction on ruthenium[J]. ACS Energy Letters, 2019, 4(6): 1336-1341. |
| 29 | Peng Y H, Geng Z G, Zhao S T, et al. Pt single atoms embedded in the surface of Ni nanocrystals as highly active catalysts for selective hydrogenation of nitro compounds[J]. Nano Letters, 2018, 18(6): 3785-3791. |
| 30 | Zhu Y Q, Sun W M, Chen W X, et al. Scale-up biomass pathway to cobalt single-site catalysts anchored on N-doped porous carbon nanobelt with ultrahigh surface area[J]. Advanced Functional Materials, 2018, 28(37): 1802167. |
| 31 | Zhao L, Zhao J, Zhao J X, et al. Artificial N2 fixation to NH3 by electrocatalytic Ru NPs at low overpotential[J]. Nanotechnology, 2020, 31(29): 29LT01. |
| 32 | Wang D B, Azofra L M, Harb M, et al. Energy-efficient nitrogen reduction to ammonia at low overpotential in aqueous electrolyte under ambient conditions[J]. ChemSusChem, 2018, 11(19): 3356. |
| 33 | Zhang S, Zhao Y X, Shi R, et al. Sub-3 nm ultrafine Cu2O for visible light driven nitrogen fixation[J]. Angewandte Chemie International Edition, 2021, 60(5): 2554-2560. |
| 34 | Jiang M H, Tao A Y, Hu Y, et al. Crystalline modulation engineering of Ru nanoclusters for boosting ammonia electrosynthesis from dinitrogen or nitrate[J]. ACS Applied Materials & Interfaces, 2022, 14(15): 17470-17478. |
| 35 | Shi M M, Bao D, Wulan B R, et al. Au sub-nanoclusters on TiO2 toward highly efficient and selective electrocatalyst for N2 conversion to NH3 at ambient conditions[J]. Advanced Materials, 2017, 29(17): 1606550. |
| 36 | Han Z Y, Tranca D, Rodríguez-Hernández F, et al. Embedding Ru clusters and single atoms into perovskite oxide boosts nitrogen fixation and affords ultrahigh ammonia yield rate[J]. Small, 2023, 19(17): 2208102. |
| 37 | Han J X, Meng X Y, Lu L, et al. Single-atom Fe-N x -C as an efficient electrocatalyst for zinc-air batteries[J]. Advanced Functional Materials, 2019, 29(41): 1808872. |
| 38 | Zhuang Z C, Li Y, Li Z L, et al. MoB/g-C3N4 interface materials as a Schottky catalyst to boost hydrogen evolution[J]. Angewandte Chemie International Edition, 2018, 57(2): 496-500. |
| 39 | Zheng Y, Jiao Y, Zhu Y H, et al. Molecule-level g-C3N4 coordinated transition metals as a new class of electrocatalysts for oxygen electrode reactions[J]. Journal of the American Chemical Society, 2017, 139(9): 3336-3339. |
| 40 | Yu B, Li H, White J, et al. Tuning the catalytic preference of ruthenium catalysts for nitrogen reduction by atomic dispersion[J]. Advanced Functional Materials, 2020, 30(6): 1905665. |
| 41 | Chen X Z, Zhao X J, Kong Z Z, et al. Unravelling the electrochemical mechanisms for nitrogen fixation on single transition metal atoms embedded in defective graphitic carbon nitride[J]. Journal of Materials Chemistry A, 2018, 6(44): 21941-21948. |
| 42 | Liu X, Jiao Y, Zheng Y, et al. Building up a picture of the electrocatalytic nitrogen reduction activity of transition metal single-atom catalysts[J]. Journal of the American Chemical Society, 2019, 141(24): 9664-9672. |
| 43 | Zhang L F, Zhao W H, Zhang W H, et al. gt-C3N4 coordinated single atom as an efficient electrocatalyst for nitrogen reduction reaction[J]. Nano Research, 2019, 12(5): 1181-1186. |
| 44 | Tao H C, Choi C, Ding L X, et al. Nitrogen fixation by Ru single-atom electrocatalytic reduction[J]. Chem, 2019, 5(1): 204-214. |
| 45 | Geng Z G, Liu Y, Kong X D, et al. N2 electrochemical reduction: achieving a record-high yield rate of 120.9 μ g N H 3 ·mgcat. -1·h-1 for N2 electrochemical reduction over Ru single-atom catalysts[J]. Advanced Materials, 2018, 30(40): 1870301. |
| 46 | Ji Y J, Li Y F, Dong H L, et al. Ruthenium single-atom catalysis for electrocatalytic nitrogen reduction unveiled by grand canonical density functional theory[J]. Journal of Materials Chemistry A, 2020, 8(39): 20402-20407. |
| 47 | Naguib M, Kurtoglu M, Presser V, et al. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2 [J]. Advanced Materials, 2011, 23(37): 4248-4253. |
| 48 | Zhao J X, Zhang L, Xie X Y, et al. Ti3C2T x (T = F, OH) MXene nanosheets: conductive 2D catalysts for ambient electrohydrogenation of N2 to NH3 [J]. Journal of Materials Chemistry A, 2018, 6(47): 24031-24035. |
| 49 | Luo Y R, Chen G F, Ding L, et al. Efficient electrocatalytic N2 fixation with MXene under ambient conditions[J]. Joule, 2019, 3(1): 279-289. |
| 50 | Zhang J Q, Zhao Y F, Guo X, et al. Single platinum atoms immobilized on an MXene as an efficient catalyst for the hydrogen evolution reaction[J]. Nature Catalysis, 2018, 1(12): 985-992. |
| 51 | Li Z, Yu L, Milligan C, et al. Two-dimensional transition metal carbides as supports for tuning the chemistry of catalytic nanoparticles[J]. Nature Communications, 2018, 9(1): 1-8. |
| 52 | Gao Y J, Cao Y Y, Zhuo H, et al. Mo2TiC2 MXene: a promising catalyst for electrocatalytic ammonia synthesis[J]. Catalysis Today, 2020, 339: 120-126. |
| 53 | Azofra L M, Li N, MacFarlane D R, et al. Promising prospects for 2D d2—d4 M3C2 transition metal carbides (MXenes) in N2 capture and conversion into ammonia[J]. Energy & Environmental Science, 2016, 9(8): 2545-2549. |
| 54 | Ni J, Shi S Y, Zhang C F, et al. Enhanced catalytic performance of the carbon-supported Ru ammonia synthesis catalyst by an introduction of oxygen functional groups via gas-phase oxidation[J]. Journal of Catalysis, 2022, 409: 78-86. |
| 55 | Peng W, Luo M, Xu X D, et al. Spontaneous atomic ruthenium doping in Mo2CT x MXene defects enhances electrocatalytic activity for the nitrogen reduction reaction[J]. Advanced Energy Materials, 2020, 10(25): 2001364. |
| 56 | Chen G, Ding M M, Zhang K, et al. Single-atomic ruthenium active sites on Ti3C2 MXene with oxygen-terminated surface synchronize enhanced activity and selectivity for electrocatalytic nitrogen reduction to ammonia[J]. ChemSusChem, 2022, 15(3): e202102352. |
| 57 | Er S, de Wijs G A, Brocks G. DFT study of planar boron sheets: a new template for hydrogen storage[J]. The Journal of Physical Chemistry C, 2009, 113(43): 18962-18967. |
| 58 | Liu C W, Li Q Y, Zhang J, et al. Conversion of dinitrogen to ammonia on Ru atoms supported on boron sheets: a DFT study[J]. Journal of Materials Chemistry A, 2019, 7(9): 4771-4776. |
| 59 | Dinh K N, Zhang Y, Zhu J X, et al. Phosphorene-based electrocatalysts[J]. Chemistry: A European Journal, 2020, 26(29): 6437-6446. |
| 60 | Zhang L L, Ding L X, Chen G F, et al. Ammonia synthesis under ambient conditions: selective electroreduction of dinitrogen to ammonia on black phosphorus nanosheets[J]. Angewandte Chemie International Edition, 2019, 58(9): 2612-2616. |
| 61 | Wang M Y, Xu Z W, Zhang X Z, et al. B-modified phosphorene for N2 fixation: a highly efficient metal-free photocatalyst[J]. Applied Surface Science, 2021, 554: 149614. |
| 62 | Liu J D, Wei Z X, Dou Y H, et al. Ru-doped phosphorene for electrochemical ammonia synthesis[J]. Rare Metals, 2020, 39(8): 874-880. |
| 63 | Xu G R, Li H, Bati A S R, et al. Nitrogen-doped phosphorene for electrocatalytic ammonia synthesis[J]. Journal of Materials Chemistry A, 2020, 8(31): 15875-15883. |
| 64 | Zhang B, Asakura H, Zhang J, et al. Stabilizing a Platinum1 single-atom catalyst on supported phosphomolybdic acid without compromising hydrogenation activity[J]. Angewandte Chemie International Edition, 2016, 55(29): 8319-8323. |
| 65 | Liao W R, Liu H X, Qi L, et al. Lithium/bismuth co-functionalized phosphotungstic acid catalyst for promoting dinitrogen electroreduction with high Faradaic efficiency[J]. Cell Reports Physical Science, 2021, 2(9): 100557. |
| 66 | Gao L Y, Wang F T, Yu M G, et al. A novel phosphotungstic acid-supported single metal atom catalyst with high activity and selectivity for the synthesis of NH3 from electrochemical N2 reduction: a DFT prediction[J]. Journal of Materials Chemistry A, 2019, 7(34): 19838-19845. |
| 67 | Ying Y R, Luo X, Qiao J L, et al. “More is different”: synergistic effect and structural engineering in double-atom catalysts[J]. Advanced Functional Materials, 2021, 31(3): 2007423. |
| 68 | Deng T, Cen C, Shen H J, et al. Atom-pair catalysts supported by N-doped graphene for the nitrogen reduction reaction: d-band center-based descriptor[J]. The Journal of Physical Chemistry Letters, 2020, 11(15): 6320-6329. |
| 69 | Wang M Y, Song R F, Zhang Q, et al. Synergy effect of Cu-Ru dual atoms anchored to N-doped phosphorene for nitrogen reduction reaction[J]. Fuel, 2022, 321: 124101. |
| 70 | He T W, Santiago A R P, Du A J. Atomically embedded asymmetrical dual-metal dimers on N-doped graphene for ultra-efficient nitrogen reduction reaction[J]. Journal of Catalysis, 2020, 388: 77-83. |
| 71 | Schlögl R. Catalytic synthesis of ammonia—a “never-ending story”?[J]. Angewandte Chemie International Edition, 2003, 42(18): 2004-2008. |
| 72 | Li J, Gao X, Zhu L, et al. Graphdiyne for crucial gas involved catalytic reactions in energy conversion applications[J]. Energy & Environmental Science, 2020, 13(5): 1326-1346. |
| 73 | Zhang L L, Cong M Y, Ding X, et al. A Janus Fe-SnO2 catalyst that enables bifunctional electrochemical nitrogen fixation[J]. Angewandte Chemie International Edition, 2020, 59(27): 10888-10893. |
| 74 | Cheng N C, Zhang L, Doyle-Davis K, et al. Single-atom catalysts: from design to application[J]. Electrochemical Energy Reviews, 2019, 2(4): 539-573. |
| 75 | Sato K, Imamura K, Kawano Y, et al. A low-crystalline ruthenium nano-layer supported on praseodymium oxide as an active catalyst for ammonia synthesis[J]. Chemical Science, 2017, 8(1): 674-679. |
| 76 | Wan J W, Chen W X, Jia C Y, et al. Defect effects on TiO2 nanosheets: stabilizing single atomic site Au and promoting catalytic properties[J]. Advanced Materials, 2018, 30(11): 1705369. |
| 77 | Cai X Y, Yang F, An L, et al. Evaluation of electrocatalytic activity of noble metal catalysts toward nitrogen reduction reaction in aqueous solutions under ambient conditions[J]. ChemSusChem, 2022, 15(1): e202102234. |
| 78 | Andersen S Z, Čolić V, Yang S, et al. A rigorous electrochemical ammonia synthesis protocol with quantitative isotope measurements[J]. Nature, 2019, 570(7762): 504-508. |
| 79 | Shi L, Yin Y, Wang S B, et al. Rational catalyst design for N2 reduction under ambient conditions: strategies toward enhanced conversion efficiency[J]. ACS Catalysis, 2020, 10(12): 6870-6899. |
| 80 | Wang H J, Li Y H, Li C J, et al. One-pot synthesis of bi-metallic PdRu tripods as an efficient catalyst for electrocatalytic nitrogen reduction to ammonia[J]. Journal of Materials Chemistry A, 2019, 7(2): 801-805. |
| 81 | Bu L Z, Shao Q, Pi Y C, et al. Coupled s-p-d exchange in facet-controlled Pd3Pb tripods enhances oxygen reduction catalysis[J]. Chem, 2018, 4(2): 359-371. |
| 82 | Wang H J, Li Y H, Yang D D, et al. Direct fabrication of bi-metallic PdRu nanorod assemblies for electrochemical ammonia synthesis[J]. Nanoscale, 2019, 11(12): 5499-5505. |
| 83 | Lu Q, Hutchings G S, Yu W T, et al. Highly porous non-precious bimetallic electrocatalysts for efficient hydrogen evolution[J]. Nature Communications, 2015, 6(1): 1-8. |
| 84 | Wang Z Q, Tian W J, Dai Z C, et al. Bimetallic mesoporous RhRu film for electrocatalytic nitrogen reduction to ammonia[J]. Inorganic Chemistry Frontiers, 2021, 8(18): 4276-4281. |
| 85 | Li Y H, Yu H J, Wang Z Q, et al. One-step synthesis of self-standing porous palladium-ruthenium nanosheet array on Ni foam for ambient electrosynthesis of ammonia[J]. International Journal of Hydrogen Energy, 2020, 45(11): 5997-6005. |
| 86 | Liu A M, Gao M F, Ren X F, et al. A two-dimensional Ru@MXene catalyst for highly selective ambient electrocatalytic nitrogen reduction[J]. Nanoscale, 2020, 12(20): 10933-10938. |
| 87 | Smith R D L, Prévot M S, Fagan R D, et al. Photochemical route for accessing amorphous metal oxide materials for water oxidation catalysis[J]. Science, 2013, 340(6128): 60-63. |
| 88 | Lv C D, Yan C S, Chen G, et al. An amorphous noble-metal-free electrocatalyst that enables nitrogen fixation under ambient conditions[J]. Angewandte Chemie International Edition, 2018, 57(21): 6073-6076. |
| 89 | Fang Z W, Wu P, Qian Y M, et al. Gel-derived amorphous bismuth-nickel alloy promotes electrocatalytic nitrogen fixation via optimizing nitrogen adsorption and activation[J]. Angewandte Chemie International Edition, 2021, 60(8): 4275-4281. |
| 90 | Liao W R, Qi L, Wang Y L, et al. Interfacial engineering promoting electrosynthesis of ammonia over Mo/phosphotungstic acid with high performance[J]. Advanced Functional Materials, 2021, 31(22): 2009151. |
| 91 | Back S, Jung Y. On the mechanism of electrochemical ammonia synthesis on the Ru catalyst[J]. Physical Chemistry Chemical Physics, 2016, 18(13): 9161-9166. |
| 92 | Wakerley D, Lamaison S, Ozanam F, et al. Bio-inspired hydrophobicity promotes CO2 reduction on a Cu surface[J]. Nature Materials, 2019, 18(11): 1222-1227. |
| 93 | Ahmed M I, Liu C W, Zhao Y, et al. Metal-sulfur linkages achieved by organic tethering of ruthenium nanocrystals for enhanced electrochemical nitrogen reduction[J]. Angewandte Chemie International Edition, 2020, 59(48): 21465-21469. |
| 94 | Yang Y J, Wang S Q, Wen H M, et al. Nanoporous gold embedded ZIF composite for enhanced electrochemical nitrogen fixation[J]. Angewandte Chemie International Edition, 2019, 58(43): 15362-15366. |
| 95 | Li F W, Thevenon A, Rosas-Hernández A, et al. Molecular tuning of CO2-to-ethylene conversion[J]. Nature, 2020, 577(7791): 509-513. |
| 96 | Xu G R, Batmunkh M, Donne S, et al. Ruthenium(Ⅲ) polyethyleneimine complexes for bifunctional ammonia production and biomass upgrading[J]. Journal of Materials Chemistry A, 2019, 7(44): 25433-25440. |
| 97 | Li R Y, He K Y, Xu P W, et al. Synthesis of a ruthenium-graphene quantum dot-graphene hybrid as a promising single-atom catalyst for electrochemical nitrogen reduction with ultrahigh yield rate and selectivity[J]. Journal of Materials Chemistry A, 2021, 9(43): 24582-24589. |
| 98 | Zhao S L, Wang Y, Dong J C, et al. Ultrathin metal-organic framework nanosheets for electrocatalytic oxygen evolution[J]. Nature Energy, 2016, 1(12): 16184. |
| 99 | Zhang Z Q, Yao K D, Cong L C, et al. Facile synthesis of a Ru-dispersed N-doped carbon framework catalyst for electrochemical nitrogen reduction[J]. Catalysis Science & Technology, 2020, 10(5): 1336-1342. |
| 100 | Zhao J, Liu L J, Yang Y, et al. Insights into electrocatalytic nitrate reduction to ammonia via Cu-based bimetallic catalysts[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(6): 2468-2475. |
| 101 | Zhang T H, Zhu J, Wang J J, et al. Ru alloying with La or Y for ammonia synthesis via integrated dissociative and associative mechanism with superior operational stability[J]. Chemical Engineering Science, 2022, 252: 117255. |
| 102 | Rao X F, Liu M M, Chien M F, et al. Recent progress in noble metal electrocatalysts for nitrogen-to-ammonia conversion[J]. Renewable and Sustainable Energy Reviews, 2022, 168: 112845. |
| 103 | Jin Y, Ding X, Zhang L L, et al. Boosting electrocatalytic reduction of nitrogen to ammonia under ambient conditions by alloy engineering[J]. Chemical Communications, 2020, 56(77): 11477-11480. |
| 104 | Liu A M, Liang X Y, Gao M F, et al. Ru and Fe alloying on a two-dimensional MXene support for enhanced electrochemical synthesis of ammonia[J]. ChemCatChem, 2022, 14(7): e202101775. |
| 105 | Kour G, Mao X, Du A J. Computational screening of single-atom alloys TM@Ru(0001) for enhanced electrochemical nitrogen reduction reaction[J]. Journal of Materials Chemistry A, 2022, 10(11): 6204-6215. |
| 106 | Montoya J H, Tsai C, Vojvodic A, et al. The challenge of electrochemical ammonia synthesis: a new perspective on the role of nitrogen scaling relations[J]. ChemSusChem, 2015, 8(13): 2180-2186. |
| 107 | Zhang J M, Tao H B, Kuang M, et al. Advances in thermodynamic-kinetic model for analyzing the oxygen evolution reaction[J]. ACS Catalysis, 2020, 10(15): 8597-8610. |
| 108 | Mahmood J, Li F, Jung S M, et al. An efficient and pH-universal ruthenium-based catalyst for the hydrogen evolution reaction[J]. Nature Nanotechnology, 2017, 12(5): 441-446. |
| 109 | Tong Y Y, Guo H P, Liu D L, et al. Vacancy engineering of iron-doped W18O49 nanoreactors for low-barrier electrochemical nitrogen reduction[J]. Angewandte Chemie International Edition, 2020, 59(19): 7356-7361. |
| 110 | Mavrikakis M, Hammer B, Nørskov J K. Effect of strain on the reactivity of metal surfaces[J]. Physical Review Letters, 1998, 81(13): 2819-2822. |
| 111 | Zhang W Q, Yang L T, An C H, et al. Enhancing electrochemical nitrogen reduction with Ru nanowires via the atomic decoration of Pt[J]. Journal of Materials Chemistry A, 2020, 8(47): 25142-25147. |
| 112 | Zhao L, Liu X J, Zhang S, et al. Rational design of bimetallic Rh0.6Ru0.4 nanoalloys for enhanced nitrogen reduction electrocatalysis under mild conditions[J]. Journal of Materials Chemistry A, 2021, 9(1): 259-263. |
| 113 | Manjunatha R, Schechter A. Electrochemical synthesis of ammonia using ruthenium-platinum alloy at ambient pressure and low temperature[J]. Electrochemistry Communications, 2018, 90: 96-100. |
| 114 | Kim C, Song J Y, Choi C, et al. Atomic-scale homogeneous Ru-Cu alloy nanoparticles for highly efficient electrocatalytic nitrogen reduction[J]. Advanced Materials, 2022, 34(40): 2205270. |
| 115 | Du X H, Li W P, Chang H T, et al. Dual heterogeneous structures lead to ultrahigh strength and uniform ductility in a Co-Cr-Ni medium-entropy alloy[J]. Nature Communications, 2020, 11: 2390. |
| 116 | Yin H Q, Du A J. Revealing the potential of ternary medium-entropy alloys as exceptional electrocatalysts toward nitrogen reduction: an example of heusler alloys[J]. ACS Applied Materials & Interfaces, 2022, 14(13): 15235-15242. |
| 117 | Zhao R, Liu C, Zhang X, et al. An ultrasmall Ru2P nanoparticles-reduced graphene oxide hybrid: an efficient electrocatalyst for NH3 synthesis under ambient conditions[J]. Journal of Materials Chemistry A, 2020, 8(1): 77-81. |
| 118 | Abghoui Y, Skúlason E. Computational predictions of catalytic activity of zincblende (110) surfaces of metal nitrides for electrochemical ammonia synthesis[J]. The Journal of Physical Chemistry C, 2017, 121(11): 6141-6151. |
| 119 | Gao Q, Huang C Q, Ju Y M, et al. Phase-selective syntheses of cobalt telluride nanofleeces for efficient oxygen evolution catalysts[J]. Angewandte Chemie International Edition, 2017, 56(27): 7769-7773. |
| 120 | Wang J, Huang B L, Ji Y J, et al. A general strategy to glassy M-Te (M = Ru, Rh, Ir) porous nanorods for efficient electrochemical N2 fixation[J]. Advanced Materials, 2020, 32(11): 1907112. |
| 121 | Liao W R, Xie K, Liu L J, et al. Triggering in-plane defect cluster on MoS2 for accelerated dinitrogen electroreduction to ammonia[J]. Journal of Energy Chemistry, 2021, 62: 359-366. |
| 122 | Guo D X, Wang S, Xu J, et al. Defect and interface engineering for electrochemical nitrogen reduction reaction under ambient conditions[J]. Journal of Energy Chemistry, 2022, 65: 448-468. |
| 123 | Liu Y T, Li D, Yu J Y, et al. Stable confinement of black phosphorus quantum dots on black tin oxide nanotubes: a robust, double-active electrocatalyst toward efficient nitrogen fixation[J]. Angewandte Chemie, 2019, 131(46): 16591-16596. |
| 124 | Montini T, Melchionna M, Monai M, et al. Fundamentals and catalytic applications of CeO2-based materials[J]. Chemical Reviews, 2016, 116(10): 5987-6041. |
| 125 | Ding Y, Huang L S, Zhang J B, et al. Ru-doped, oxygen-vacancy-containing CeO2 nanorods toward N2 electroreduction[J]. Journal of Materials Chemistry A, 2020, 8(15): 7229-7234. |
| 126 | Cheng S, Gao Y J, Yan Y L, et al. Oxygen vacancy enhancing mechanism of nitrogen reduction reaction property in Ru/TiO2 [J]. Journal of Energy Chemistry, 2019, 39: 144-151. |
| 127 | Suryanto B H R, Wang D B, Azofra L M, et al. MoS2 polymorphic engineering enhances selectivity in the electrochemical reduction of nitrogen to ammonia[J]. ACS Energy Letters, 2019, 4(2): 430-435. |
| 128 | Li X, Li T, Ma Y, et al. Boosted electrocatalytic N2 reduction to NH3 by defect-rich MoS2 nanoflower[J]. Advanced Energy Materials, 2018, 8(30): 1801357. |
| 129 | Zhao J, Wang X, Xu Z C, et al. Hybrid catalysts for photoelectrochemical reduction of carbon dioxide: a prospective review on semiconductor/metal complex co-catalyst systems[J]. Journal of Materials Chemistry A, 2014, 2(37): 15228. |
| 130 | Tang X R, Tian X Q, Zhou L, et al. Connection of Ru nanoparticles with rich defects enables the enhanced electrochemical reduction of nitrogen[J]. Physical Chemistry Chemical Physics, 2022, 24(19): 11491-11495. |
| 131 | 刘恒源, 王海辉, 徐建鸿. 电催化氮还原合成氨电化学系统研究进展[J]. 化工学报, 2022, 73(1): 32-45. |
| Liu H Y, Wang H H, Xu J H. Advances in electrochemical systems for ammonia synthesis by electrocatalytic reduction of nitrogen[J]. CIESC Journal, 2022, 73(1): 32-45. | |
| 132 | 郑沐云, 万宇驰, 吕瑞涛. 电催化氮气还原合成氨催化材料研究进展[J]. 化工学报, 2020, 71(6): 2481-2491. |
| Zheng M Y, Wan Y C, Lyu R T. Research progress on electrocatalytic nitrogen reduction reaction catalysts for ammonia synthesis[J]. CIESC Journal, 2020, 71(6): 2481-2491. |
| [1] | Yitong LI, Hang GUO, Hao CHEN, Fang YE. Study on operating conditions of proton exchange membrane fuel cells with non-uniform catalyst distributions [J]. CIESC Journal, 2023, 74(9): 3831-3840. |
| [2] | Yepin CHENG, Daqing HU, Yisha XU, Huayan LIU, Hanfeng LU, Guokai CUI. Application of ionic liquid-based deep eutectic solvents for CO2 conversion [J]. CIESC Journal, 2023, 74(9): 3640-3653. |
| [3] | Jie CHEN, Yongsheng LIN, Kai XIAO, Chen YANG, Ting QIU. Study on catalytic synthesis of sec-butanol by tunable choline-based basic ionic liquids [J]. CIESC Journal, 2023, 74(9): 3716-3730. |
| [4] | Xuejin YANG, Jintao YANG, Ping NING, Fang WANG, Xiaoshuang SONG, Lijuan JIA, Jiayu FENG. Research progress in dry purification technology of highly toxic gas PH3 [J]. CIESC Journal, 2023, 74(9): 3742-3755. |
| [5] | Yali HU, Junyong HU, Suxia MA, Yukun SUN, Xueyi TAN, Jiaxin HUANG, Fengyuan YANG. Development of novel working fluid and study on electrochemical characteristics of reverse electrodialysis heat engine [J]. CIESC Journal, 2023, 74(8): 3513-3521. |
| [6] | Feifei YANG, Shixi ZHAO, Wei ZHOU, Zhonghai NI. Sn doped In2O3 catalyst for selective hydrogenation of CO2 to methanol [J]. CIESC Journal, 2023, 74(8): 3366-3374. |
| [7] | Kaixuan LI, Wei TAN, Manyu ZHANG, Zhihao XU, Xuyu WANG, Hongbing JI. Design of cobalt-nitrogen-carbon/activated carbon rich in zero valent cobalt active site and application of catalytic oxidation of formaldehyde [J]. CIESC Journal, 2023, 74(8): 3342-3352. |
| [8] | Xin YANG, Xiao PENG, Kairu XUE, Mengwei SU, Yan WU. Preparation of molecularly imprinted-TiO2 and its properties of photoelectrocatalytic degradation of solubilized PHE [J]. CIESC Journal, 2023, 74(8): 3564-3571. |
| [9] | Mengmeng ZHANG, Dong YAN, Yongfeng SHEN, Wencui LI. Effect of electrolyte types on the storage behaviors of anions and cations for dual-ion batteries [J]. CIESC Journal, 2023, 74(7): 3116-3126. |
| [10] | Yaxin CHEN, Hang YUAN, Guanzhang LIU, Lei MAO, Chun YANG, Ruifang ZHANG, Guangya ZHANG. Advances in enzyme self-immobilization mediated by protein nanocages [J]. CIESC Journal, 2023, 74(7): 2773-2782. |
| [11] | Xiaoling TANG, Jiarui WANG, Xuanye ZHU, Renchao ZHENG. Biosynthesis of chiral epichlorohydrin by halohydrin dehalogenase based on Pickering emulsion system [J]. CIESC Journal, 2023, 74(7): 2926-2934. |
| [12] | Yajie YU, Jingru LI, Shufeng ZHOU, Qingbiao LI, Guowu ZHAN. Construction of nanomaterial and integrated catalyst based on biological template: a review [J]. CIESC Journal, 2023, 74(7): 2735-2752. |
| [13] | Jiali GE, Tuxiang GUAN, Xinmin QIU, Jian WU, Liming SHEN, Ningzhong BAO. Synthesis of FeF3 nanoparticles covered by vertical porous carbon for high performance Li-ion battery cathode [J]. CIESC Journal, 2023, 74(7): 3058-3067. |
| [14] | Yuanhao QU, Wenyi DENG, Xiaodan XIE, Yaxin SU. Study on electro-osmotic dewatering of sludge assisted by activated carbon/graphite [J]. CIESC Journal, 2023, 74(7): 3038-3050. |
| [15] | Pan LI, Junyang MA, Zhihao CHEN, Li WANG, Yun GUO. Effect of the morphology of Ru/α-MnO2 on NH3-SCO performance [J]. CIESC Journal, 2023, 74(7): 2908-2918. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||