CIESC Journal ›› 2025, Vol. 76 ›› Issue (8): 4095-4107.DOI: 10.11949/0438-1157.20250093
• Separation engineering • Previous Articles Next Articles
Huiqin ZHANG1( ), Hongjun ZHAO2, Zhengjun FU3, Li ZHUANG4, Kai DONG5, Tianzhi JIA1(
), Hongjun ZHAO2, Zhengjun FU3, Li ZHUANG4, Kai DONG5, Tianzhi JIA1( ), Xueli CAO2(
), Xueli CAO2( ), Shipeng SUN1,2
), Shipeng SUN1,2
Received:2025-01-22
Revised:2025-03-10
Online:2025-09-17
Published:2025-08-25
Contact:
Tianzhi JIA, Xueli CAO
张荟钦1( ), 赵泓竣2, 付正军3, 庄力4, 董凯5, 贾添智1(
), 赵泓竣2, 付正军3, 庄力4, 董凯5, 贾添智1( ), 曹雪丽2(
), 曹雪丽2( ), 孙世鹏1,2
), 孙世鹏1,2
通讯作者:
贾添智,曹雪丽
作者简介:张荟钦(1984—),男,博士,zhanghuiqin@jsfmtic.com
基金资助:CLC Number:
Huiqin ZHANG, Hongjun ZHAO, Zhengjun FU, Li ZHUANG, Kai DONG, Tianzhi JIA, Xueli CAO, Shipeng SUN. Application of nanofiltration membrane in concentration of ionic rare earth leach solution[J]. CIESC Journal, 2025, 76(8): 4095-4107.
张荟钦, 赵泓竣, 付正军, 庄力, 董凯, 贾添智, 曹雪丽, 孙世鹏. 纳滤膜在离子型稀土浸出液提浓中的应用研究[J]. 化工学报, 2025, 76(8): 4095-4107.
Add to citation manager EndNote|Ris|BibTeX
| 离子 | 离子浓度/ (mg/L) |
|---|---|
| Ca2+ | 158.6 |
| Mg2+ | 1106 |
| Ce3+ | 3.415 |
| La3+ | 56.92 |
Table 1 The main ionic components of the actual rare earth leaching solution
| 离子 | 离子浓度/ (mg/L) |
|---|---|
| Ca2+ | 158.6 |
| Mg2+ | 1106 |
| Ce3+ | 3.415 |
| La3+ | 56.92 |
| 离子 | MNF | MNF-Acid | Duracid | TW-30 | |
|---|---|---|---|---|---|
| Ca2+ | 实验组/(10-2 mg/L) | 4.35 | 5.10 | 3.01 | 6.00 |
| 浸泡组/(10-2 mg/L) | 低于检测限 | ||||
| La3+ | 实验组/(10-2 mg/L) | 0.14 | 0.15 | 0.11 | 0.23 |
| 浸泡组/(10-2 mg/L) | 低于检测限 | ||||
Table 2 Residual ion concentration on the membrane surface
| 离子 | MNF | MNF-Acid | Duracid | TW-30 | |
|---|---|---|---|---|---|
| Ca2+ | 实验组/(10-2 mg/L) | 4.35 | 5.10 | 3.01 | 6.00 |
| 浸泡组/(10-2 mg/L) | 低于检测限 | ||||
| La3+ | 实验组/(10-2 mg/L) | 0.14 | 0.15 | 0.11 | 0.23 |
| 浸泡组/(10-2 mg/L) | 低于检测限 | ||||
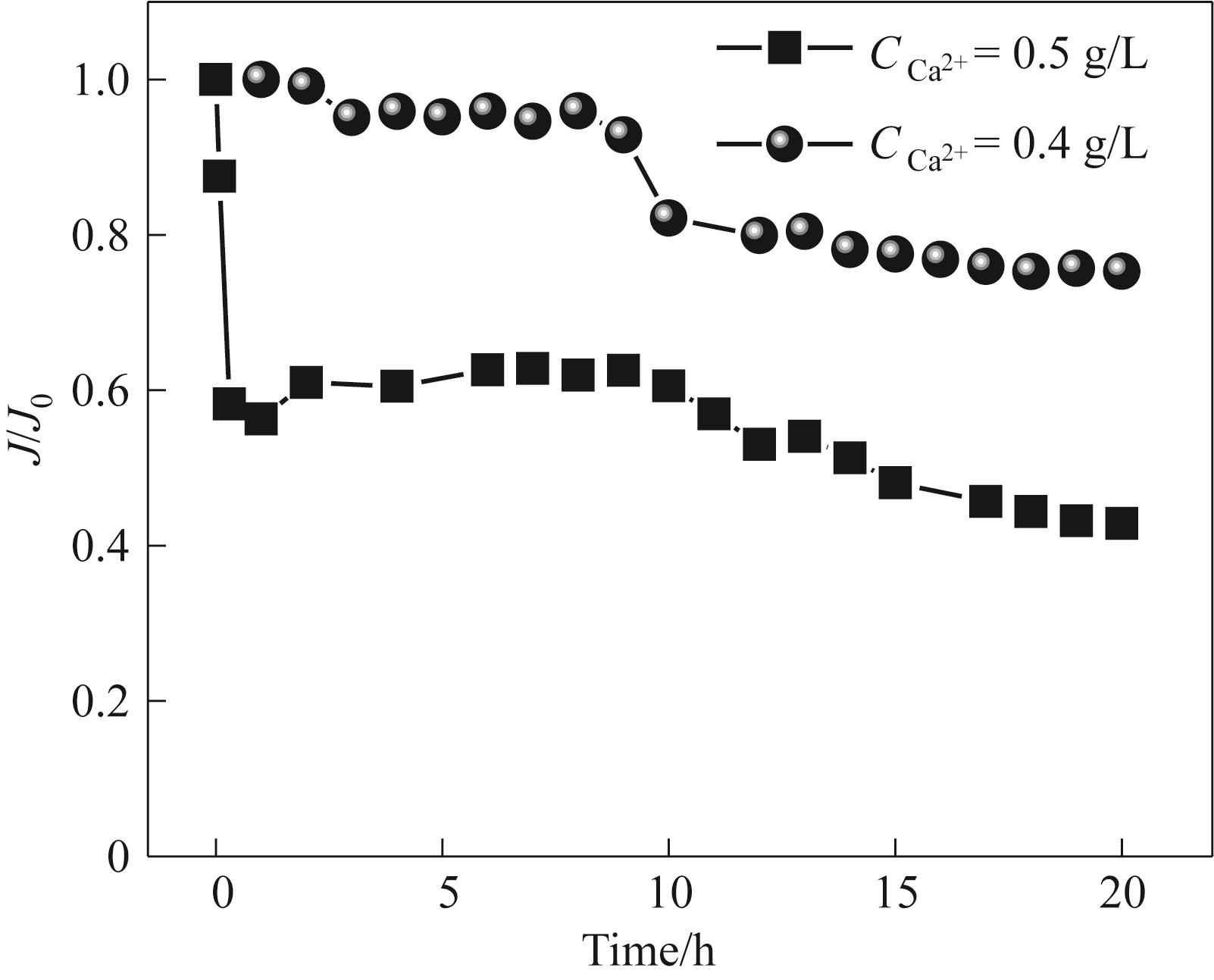
Fig.7 The normalized flux changes of Duracid during the concentration process of simulated rare earth leaching solutions with different concentrations
| 项目 | 浓度/(g/L) | 饱和溶解度/(g/L) | |||
|---|---|---|---|---|---|
| Mg2+ | MgSO4 | La3+ | Ca2+ | CaSO4 | |
| pH=5.8 | 1.4 | 7 | 0.4 | 0.51 | 1.73 |
| pH=3 | 0.54 | 1.84 | |||
| pH=1 | 0.82 | 2.79 | |||
| 1%(质量分数)H2SO4 | 0.66 | 2.24 | |||
| pH=5.8 | 1.4 | 7 | 0.4 | 0.51 | 1.73 |
| 2 | 10 | 0.48 | 1.63 | ||
| 2.8 | 14 | 0.46 | 1.56 | ||
| pH=1 | 1.4 | 7 | 0.05 | 0.58 | 1.97 |
| 0.2 | 0.73 | 2.48 | |||
| 0.4 | 0.82 | 2.79 | |||
Table 3 Solubility of CaSO4 under different conditions
| 项目 | 浓度/(g/L) | 饱和溶解度/(g/L) | |||
|---|---|---|---|---|---|
| Mg2+ | MgSO4 | La3+ | Ca2+ | CaSO4 | |
| pH=5.8 | 1.4 | 7 | 0.4 | 0.51 | 1.73 |
| pH=3 | 0.54 | 1.84 | |||
| pH=1 | 0.82 | 2.79 | |||
| 1%(质量分数)H2SO4 | 0.66 | 2.24 | |||
| pH=5.8 | 1.4 | 7 | 0.4 | 0.51 | 1.73 |
| 2 | 10 | 0.48 | 1.63 | ||
| 2.8 | 14 | 0.46 | 1.56 | ||
| pH=1 | 1.4 | 7 | 0.05 | 0.58 | 1.97 |
| 0.2 | 0.73 | 2.48 | |||
| 0.4 | 0.82 | 2.79 | |||
| pH | Hermia模型拟合精度 | ||
|---|---|---|---|
| 完全孔堵塞 | 中间孔堵塞 | 标准孔堵塞 | |
| 5.8 | 0.9169 | 0.8806 | 0.9472 |
| 3 | 0.9779 | 0.9702 | 0.9360 |
| 1 | 0.9648 | 0.9640 | 0.9284 |
Table 4 The fitting results of Hermia model for membrane fouling process under different pH conditions
| pH | Hermia模型拟合精度 | ||
|---|---|---|---|
| 完全孔堵塞 | 中间孔堵塞 | 标准孔堵塞 | |
| 5.8 | 0.9169 | 0.8806 | 0.9472 |
| 3 | 0.9779 | 0.9702 | 0.9360 |
| 1 | 0.9648 | 0.9640 | 0.9284 |
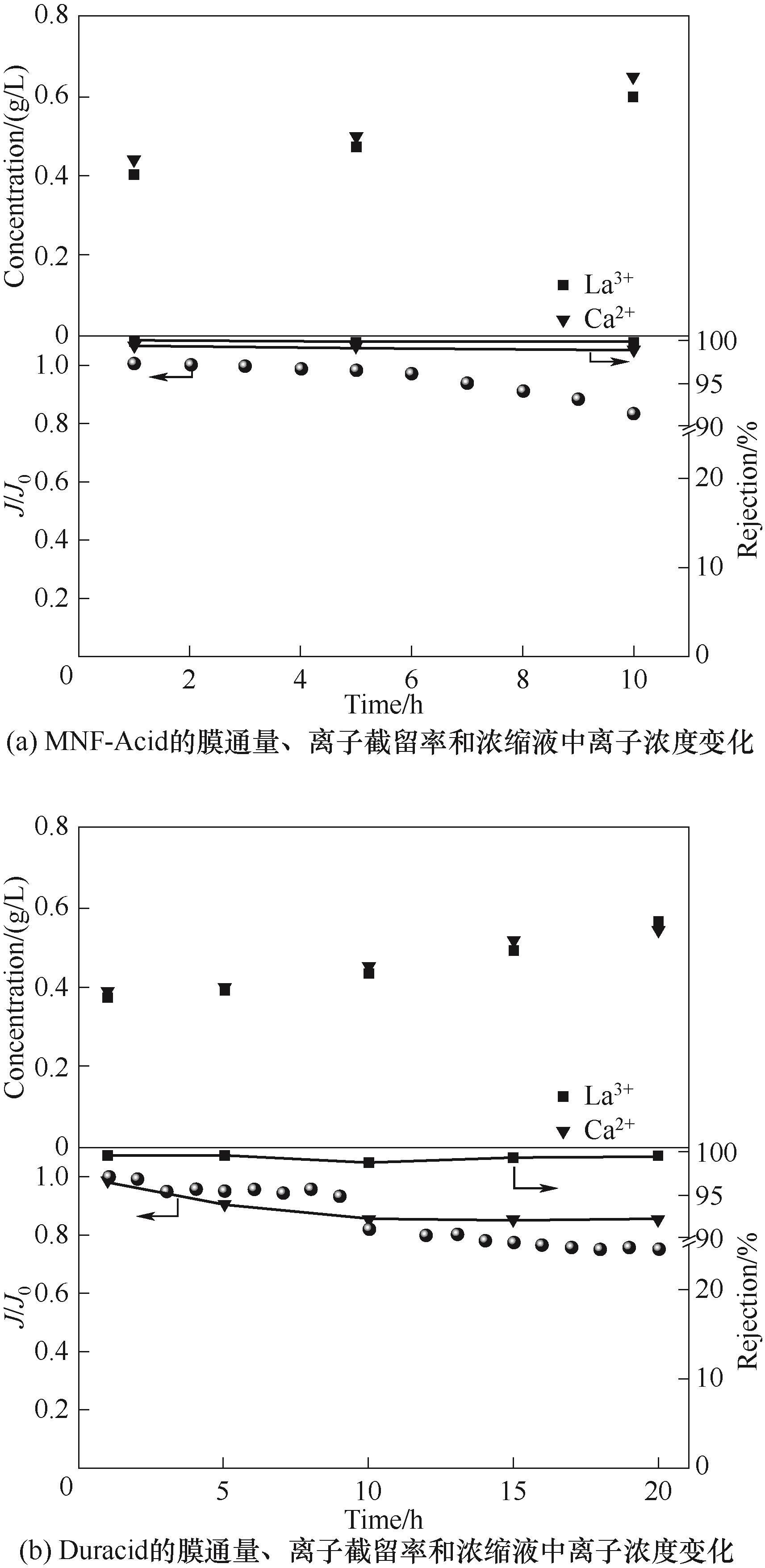
Fig.10 The performance changes of MNF-Acid and Duracid membranes during the concentration process of rare earth leaching solution under the condition of pH=1
| pH | 浓度/(g/L) | 饱和溶解度/(g/L) | |||
|---|---|---|---|---|---|
| Mg2+ | MgSO4 | La3+ | Ca2+ | CaSO4 | |
| 5.8 | 1.4 | 7 | 0.4 | 0.51 | 1.73 |
| 5.8 | 1.75 | 8.75 | 0.5 | 0.50 | 1.7 |
| 5.8 | 2.1 | 10.5 | 0.6 | 0.48 | 1.63 |
| 5.8 | 2 | 10 | 0.57 | 0.50 | 1.7 |
| 5.8 | 2.8 | 14 | 0.8 | 0.49 | 1.67 |
| 3 | 1.4 | 7 | 0.4 | 0.54 | 1.84 |
| 3 | 2 | 10 | 0.57 | 0.51 | 1.73 |
| 3 | 2.8 | 14 | 0.8 | 0.5 | 1.7 |
| 1 | 1.4 | 7 | 0.4 | 0.82 | 2.79 |
| 1 | 2 | 10 | 0.57 | 0.75 | 2.55 |
| 1 | 2.8 | 14 | 0.8 | 0.75 | 2.55 |
Table 5 Solubility of CaSO4 under different recovery rates
| pH | 浓度/(g/L) | 饱和溶解度/(g/L) | |||
|---|---|---|---|---|---|
| Mg2+ | MgSO4 | La3+ | Ca2+ | CaSO4 | |
| 5.8 | 1.4 | 7 | 0.4 | 0.51 | 1.73 |
| 5.8 | 1.75 | 8.75 | 0.5 | 0.50 | 1.7 |
| 5.8 | 2.1 | 10.5 | 0.6 | 0.48 | 1.63 |
| 5.8 | 2 | 10 | 0.57 | 0.50 | 1.7 |
| 5.8 | 2.8 | 14 | 0.8 | 0.49 | 1.67 |
| 3 | 1.4 | 7 | 0.4 | 0.54 | 1.84 |
| 3 | 2 | 10 | 0.57 | 0.51 | 1.73 |
| 3 | 2.8 | 14 | 0.8 | 0.5 | 1.7 |
| 1 | 1.4 | 7 | 0.4 | 0.82 | 2.79 |
| 1 | 2 | 10 | 0.57 | 0.75 | 2.55 |
| 1 | 2.8 | 14 | 0.8 | 0.75 | 2.55 |
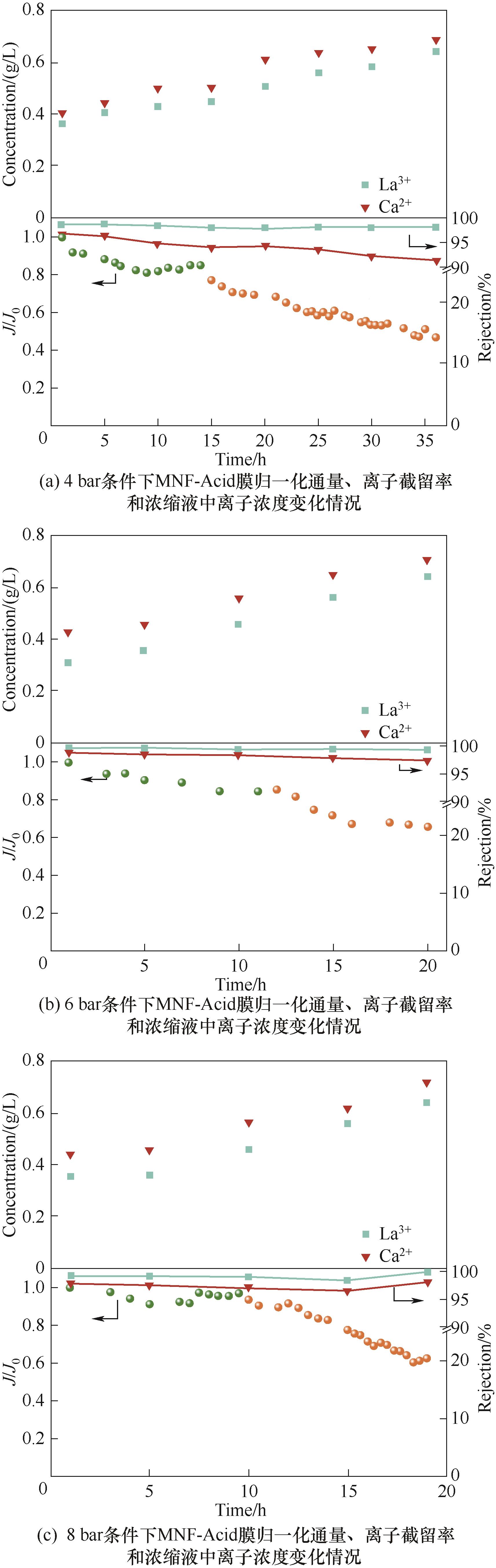
Fig.11 The performance changes of MNF-Acid membrane during the concentration process of rare earth leaching solution under different pressure conditions
| 压力/bar | Hermia模型拟合精度 | ||
|---|---|---|---|
| 完全孔堵塞 | 中间孔堵塞 | 标准孔堵塞 | |
| 4 | 0.9423 | 0.9336 | 0.9464 |
| 6 | 0.9648 | 0.9640 | 0.9284 |
| 8 | 0.9601 | 0.9488 | 0.9554 |
Table 6 The performance changes of MNF-Acid membrane during the concentration process of rare earth leaching solution under different pressure conditions
| 压力/bar | Hermia模型拟合精度 | ||
|---|---|---|---|
| 完全孔堵塞 | 中间孔堵塞 | 标准孔堵塞 | |
| 4 | 0.9423 | 0.9336 | 0.9464 |
| 6 | 0.9648 | 0.9640 | 0.9284 |
| 8 | 0.9601 | 0.9488 | 0.9554 |
| 流量/(L/h) | Hermia模型拟合精度 | ||
|---|---|---|---|
| 完全孔堵塞 | 中间孔堵塞 | 标准孔堵塞 | |
| 30 | 不适用 | 0.9154 | 0.9893 |
| 60 | 0.9864 | 0.9681 | 0.9740 |
| 90 | 0.9626 | 0.9562 | 0.7371 |
Table 7 The performance changes of MNF-Acid membrane during the concentration process of rare earth leaching solution under different feed flow rate
| 流量/(L/h) | Hermia模型拟合精度 | ||
|---|---|---|---|
| 完全孔堵塞 | 中间孔堵塞 | 标准孔堵塞 | |
| 30 | 不适用 | 0.9154 | 0.9893 |
| 60 | 0.9864 | 0.9681 | 0.9740 |
| 90 | 0.9626 | 0.9562 | 0.7371 |
| pH | 处理效率/(L/h) | 最大通量衰减 程度/% | 不可逆通量 衰减程度/% |
|---|---|---|---|
| 5.8 | 2.22 | 39 | 9.50 |
| 1 | 3.56 | 34 | 1.10 |
Table 8 Component concentration efficiency and flux attenuation degree
| pH | 处理效率/(L/h) | 最大通量衰减 程度/% | 不可逆通量 衰减程度/% |
|---|---|---|---|
| 5.8 | 2.22 | 39 | 9.50 |
| 1 | 3.56 | 34 | 1.10 |
| pH | 离子 | 时间/(min) | 浓度/(g/L) | |
|---|---|---|---|---|
| 真实 | 理论 | |||
| 5.8 | Ca2+ | 35 | 0.797 | 0.791 |
| 65 | 1.247 | 1.4 | ||
| La3+ | 35 | 0.528 | 0.525 | |
| 65 | 1.062 | 0.96 | ||
| 1 | Ca2+ | 55 | 1.359 | 1.3 |
| La3+ | 0.925 | 0.893 | ||
Table 9 Real ion concentration and theoretical concentration during the concentration process of components
| pH | 离子 | 时间/(min) | 浓度/(g/L) | |
|---|---|---|---|---|
| 真实 | 理论 | |||
| 5.8 | Ca2+ | 35 | 0.797 | 0.791 |
| 65 | 1.247 | 1.4 | ||
| La3+ | 35 | 0.528 | 0.525 | |
| 65 | 1.062 | 0.96 | ||
| 1 | Ca2+ | 55 | 1.359 | 1.3 |
| La3+ | 0.925 | 0.893 | ||
| [1] | Xu Z G, Li G, Yang H F, et al. Development review on leaching technology and leaching agents of weathered crust elution-deposited rare earth ores[J]. Minerals, 2023, 13(9): 1223. |
| [2] | Xiao Y F, Feng Z Y, Huang X W, et al. Recovery of rare earth from the ion-adsorption type rare earths ore(Ⅱ): Compound leaching[J]. Hydrometallurgy, 2016, 163: 83-90. |
| [3] | He Q, Qiu J, Chen J F, et al. Progress in green and efficient enrichment of rare earth from leaching liquor of ion adsorption type rare earth ores[J]. Journal of Rare Earths, 2022, 40(3): 353-364. |
| [4] | 王道广, 王均凤, 张香平, 等. 离子液体在稀土萃取分离中的应用[J]. 化工学报, 2020, 71(10): 4379-4394. |
| Wang D G, Wang J F, Zhang X P, et al. Application of ionic liquid in extraction and separation of rare earth[J]. CIESC Journal, 2020, 71(10): 4379-4394. | |
| [5] | Liu L L, Liu Y X, Luo J Q, et al. Membrane pre-concentration as an efficient strategy to enhance the enrichment of rare earth by MgO precipitation[J]. Chemical Engineering Journal, 2024, 491: 152083. |
| [6] | Zhu D M, Qiu T S, Zhong J F, et al. Molecular dynamics simulation of aluminum inhibited leaching during ion-adsorbed type rare earth ore leaching process[J]. Journal of Rare Earths, 2019, 37(12): 1334-1340. |
| [7] | 刘宁, 褚昌辉, 王乾, 等. 用于混合一价盐分离的纳滤膜的制备及性能研究[J]. 化工学报, 2021, 72(1): 578-588. |
| Liu N, Chu C H, Wang Q, et al. Preparation of nanofiltration membrane for separation of mixed monovalent salts[J]. CIESC Journal, 2021, 72(1): 578-588. | |
| [8] | Shirazi S, Lin C J, Chen D. Inorganic fouling of pressure-driven membrane processes: a critical review[J]. Desalination, 2010, 250(1): 236-248. |
| [9] | Zhu X B, Jassby D. Electroactive membranes for water treatment: enhanced treatment functionalities, energy considerations, and future challenges[J]. Accounts of Chemical Research, 2019, 52(5): 1177-1186. |
| [10] | Ashfaq M Y, Al-Ghouti M A, Da'na D A, et al. Investigating the effect of temperature on calcium sulfate scaling of reverse osmosis membranes using FTIR, SEM-EDX and multivariate analysis[J]. Science of the Total Environment, 2020, 703: 134726. |
| [11] | Wang Y B, Mao X Y, Chen C, et al. Effect of sulfuric acid concentration on morphology of calcium sulfate hemihydrate crystals[J]. Materials Research Express, 2020, 7(10): 105501. |
| [12] | Clampett J B, Fowler R T. Equilibrium solubility of calcium sulphate hemihydrate in sodium chloride: magnesium sulphate solutions at elevated temperatures[J]. Journal of Applied Chemistry, 1964, 14(2): 81-86. |
| [13] | Zhang T, Wang Q Y, Yang Y, et al. Revealing the contradiction between DLVO/XDLVO theory and membrane fouling propensity for oil-in-water emulsion separation[J]. Journal of Hazardous Materials, 2024, 466: 133594. |
| [14] | 唐和礼, 张冰, 黄冬梅, 等. XDLVO理论在膜污染解析中的应用研究[J]. 化工学报, 2021, 72(3): 1230-1241. |
| Tang H L, Zhang B, Huang D M, et al. Advances in membrane fouling analysis based on XDLVO theory[J]. CIESC Journal, 2021, 72(3): 1230-1241. | |
| [15] | Wang L, Li Z H, Fan J H, et al. The intelligent prediction of membrane fouling during membrane filtration by mathematical models and artificial intelligence models[J]. Chemosphere, 2024, 349: 141031. |
| [16] | Zheng W J, Chen Y, Xu X H, et al. Research on the factors influencing nanofiltration membrane fouling and the prediction of membrane fouling[J]. Journal of Water Process Engineering, 2024, 59: 104876. |
| [17] | Gao M F, He Q L, Dong H Z, et al. Identification of the coupled fouling mechanism involved in microfiltration of tobacco extracts liquid by multistage Hermia model[J]. Journal of Food Process Engineering, 2022, 45(2): e13961. |
| [18] | Ahmad A, Raish M, Alkharfy K M, et al. Solubility, solubility parameters and solution thermodynamics of thymoquinone in different mono solvents[J]. Journal of Molecular Liquids, 2018, 272: 912-918. |
| [19] | Fan C Y, Yan J M, Liu H D, et al. Performance and membrane fouling characteristics of a drinking water multistage NF system based on membrane autopsy from a full-scale system[J]. Journal of Water Process Engineering, 2024, 58: 104909. |
| [20] | Field R W, Wu D, Howell J A, et al. Critical flux concept for microfiltration fouling[J]. Journal of Membrane Science, 1995, 100(3): 259-272. |
| [21] | Xie D Y, Li J, Zhang H, et al. A novel electrochemical method for the removal of aluminum from ionic rare earth leachate[J]. Separation and Purification Technology, 2024, 345: 127296. |
| [22] | 鹿钦礼, 刘德俊, 马贵阳, 等. 注汽锅炉高含盐回用水引发爆管分析[J]. 辽宁石油化工大学学报, 2010, 30(2): 19-22. |
| Lu Q L, Liu D J, Ma G Y, et al. Analysis of explosion of tube with high salt recycling water in steam injection boiler[J]. Journal of Liaoning Shihua University, 2010, 30(2): 19-22. | |
| [23] | Zhang Z X, Bright V M, Greenberg A R. Use of capacitive microsensors and ultrasonic time-domain reflectometry for in-situ quantification of concentration polarization and membrane fouling in pressure-driven membrane filtration[J]. Sensors and Actuators B: Chemical, 2006, 117(2): 323-331. |
| [24] | Cui C, Luo C, Tian T, et al. Comparative evaluation of acid-resistant nanofiltration membranes for heavy metal removal in acidic wastewater[J]. Desalination, 2024, 576: 117349. |
| [25] | Lee J, Shin Y, Boo C, et al. Performance, limitation, and opportunities of acid-resistant nanofiltration membranes for industrial wastewater treatment[J]. Journal of Membrane Science, 2023, 666: 121142. |
| [26] | Bai Y, Gao P, Fang R, et al. Constructing positively charged acid-resistant nanofiltration membranes via surface postgrafting for efficient removal of metal ions from electroplating rinse wastewater[J]. Separation and Purification Technology, 2022, 297: 121500. |
| [27] | Hoang T A, Ang H M, Rohl A L. Effects of temperature on the scaling of calcium sulphate in pipes[J]. Powder Technology, 2007, 179(1/2): 31-37. |
| [28] | Li J F, Liu P P, Wu C L, et al. Common ion effect in the hydrolysis reaction of MgCa alloy hydride-salt composites[J]. International Journal of Hydrogen Energy, 2017, 42(2): 1429-1435. |
| [29] | Dutrizac J E. The behaviour of the rare earth elements during gypsum (CaSO4·2H2O) precipitation[J]. Hydrometallurgy, 2017, 174: 38-46. |
| [30] | Barati N, Husein M M, Azaiez J. Iron oxide doped ceramic membranes for combined organic-inorganic colloidal fouling mitigation[J]. Separation and Purification Technology, 2023, 323: 124498. |
| [31] | Hoek E M V, Kim A S, Elimelech M. Influence of crossflow membrane filter geometry and shear rate on colloidal fouling in reverse osmosis and nanofiltration separations[J]. Environmental Engineering Science, 2002, 19(6): 357-372. |
| [32] | Chong Y K, Fletcher D F, Liang Y Y. CFD simulation of hydrodynamics and concentration polarization in osmotically assisted reverse osmosis membrane systems[J]. Journal of Water Process Engineering, 2024, 57: 104535. |
| [1] | Fang WANG, Suxia MA, Ying TIAN, Zhongyuan LIU. NO x emission prediction method of CFB unit based on 1D mechanism model dynamicly corrected with LSTM [J]. CIESC Journal, 2025, 76(7): 3416-3425. |
| [2] | Yanqiu LU, Yang DI, Wenbo SHI, Congcong YIN, Yong WANG. Research progress of smart responsive membranes based on novel porous organic polymers [J]. CIESC Journal, 2025, 76(5): 2101-2118. |
| [3] | Xinchen XIANG, Dan LU, Ying ZHAO, Zhikan YAO, Ruiqiang KOU, Danjun ZHENG, Zhijun ZHOU, Lin ZHANG. Preparation of highly positively charged NF membranes with surface quaternization modification and Li+/Mg2+ separation performance [J]. CIESC Journal, 2025, 76(5): 2377-2386. |
| [4] | Fangping XU, Hui YANG, Jun CHEN, Jianyong ZHU, Rongxiu LU. Soft sensor of rare earth element content with transfer learning and residual attention convolutional neural network [J]. CIESC Journal, 2025, 76(4): 1647-1660. |
| [5] | Lingyu LI, Xin HU, Huaigang CHENG, Yun ZHAO, Dong AN, Yujun MA, Jiahao JIN, Xudong YU, Weidong ZHANG. Isothermal evaporation salt-forming regions of the ternary water-salt systems K+(Mg2+), Ca2+//Cl--H2O [J]. CIESC Journal, 2025, 76(1): 120-130. |
| [6] | Xinyue WANG, Xiaohu XU, Haiyang ZHANG, Chunhua YIN. Study on encapsulation and properties vitamin A acetate/cyclodextrin [J]. CIESC Journal, 2024, 75(S1): 321-328. |
| [7] | Zhixing ZHAO, Zhihao YAO, Xuefeng YU, Yousheng YANG, Ying ZENG, Xudong YU. Multi-temperature phase diagram of lithium-sodium-magnesium coexistence sulfate system and its application [J]. CIESC Journal, 2024, 75(6): 2123-2133. |
| [8] | Youming SI, Lingfeng ZHENG, Pengzhong CHEN, Jiangli FAN, Xiaojun PENG. Performance and mechanism of novel antimony oxo cluster photoresist [J]. CIESC Journal, 2024, 75(4): 1705-1717. |
| [9] | Yuxiang CHEN, Chuanlei LIU, Zijun GONG, Qiyue ZHAO, Guanchu GUO, Hao JIANG, Hui SUN, Benxian SHEN. Machine learning-assisted solvent molecule design for efficient absorption of ethanethiol [J]. CIESC Journal, 2024, 75(3): 914-923. |
| [10] | Yong ZHANG, Jingbo ZHAO, Limin QUAN. A prediction method for effluent ammonia nitrogen concentration based on convolutional layer and attention mechanism long short-term memory network [J]. CIESC Journal, 2024, 75(12): 4679-4688. |
| [11] | Yuxi WU, Yuanhui TANG, Qiang GUO, Yakai LIN, Lixin YU, Xiaolin WANG. Experimental study and simulation on nanofiltration separation of lithium and magnesium from sulfate desorption solution [J]. CIESC Journal, 2024, 75(12): 4563-4575. |
| [12] | Han TANG, Jin CAI, Haihang QIN, Guangjin CHEN, Changyu SUN. Predictive model on gas solubility in water-rich phase coexisted with gas hydrates [J]. CIESC Journal, 2024, 75(11): 4348-4358. |
| [13] | Chao HU, Yuming DONG, Wei ZHANG, Hongling ZHANG, Peng ZHOU, Hongbin XU. Preparation of high-concentration positive electrolyte of vanadium redox flow battery by activating vanadium pentoxide with highly concentrated sulfuric acid [J]. CIESC Journal, 2023, 74(S1): 338-345. |
| [14] | Xudong YU, Qi LI, Niancu CHEN, Li DU, Siying REN, Ying ZENG. Phase equilibria and calculation of aqueous ternary system KCl + CaCl2 + H2O at 298.2, 323.2, and 348.2 K [J]. CIESC Journal, 2023, 74(8): 3256-3265. |
| [15] | Xiqing ZHANG, Yanting WANG, Yanhong XU, Shuling CHANG, Tingting SUN, Ding XUE, Lihong ZHANG. Effect of Mg content on isobutane dehydrogenation properties over nanosheets supported Pt-In catalysts [J]. CIESC Journal, 2023, 74(6): 2427-2435. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||