化工学报 ›› 2020, Vol. 71 ›› Issue (10): 4760-4772.DOI: 10.11949/0438-1157.20200065
罗雪1( ),黄海军1(
),黄海军1( ),罗自萍1,王治永1,穆小静1,李红茹1,王新潮1,2,张胜涛1,高放1(
),罗自萍1,王治永1,穆小静1,李红茹1,王新潮1,2,张胜涛1,高放1( )
)
收稿日期:2020-01-16
修回日期:2020-04-13
出版日期:2020-10-05
发布日期:2020-10-05
通讯作者:
高放
作者简介:罗雪(1994—),女,硕士研究生,基金资助:
Xue LUO1( ),Haijun HUANG1(
),Haijun HUANG1( ),Ziping LUO1,Zhiyong WANG1,Xiaojing MU1,Hongru LI1,Xinchao WANG1,2,Shengtao ZHANG1,Fang GAO1(
),Ziping LUO1,Zhiyong WANG1,Xiaojing MU1,Hongru LI1,Xinchao WANG1,2,Shengtao ZHANG1,Fang GAO1( )
)
Received:2020-01-16
Revised:2020-04-13
Online:2020-10-05
Published:2020-10-05
Contact:
Fang GAO
摘要:
为寻求绿色、可持续与具备大规模应用潜能的天然植物提取物作为缓蚀剂,选择具有表面活性剂性质的无患子果皮提取物作为研究对象。通过简便乙醇回流萃取获得无患子果皮提取物。在室温条件下,无患子果皮提取物在DMF(N,N-二甲基甲酰胺)/HCl水溶液的混合溶液 (体积比50/50,1.0 mol/L HCl 溶液) 中能够发生自组装产生有序的纳微米聚集材料。傅里叶变换红外光谱 (FT-IR)与X射线光电子能谱 (XPS) 研究表明了无患子果皮提取物聚集体能够对Q235钢表面产生化学吸附作用。利用电化学方法研究了无患子果皮提取物聚集体吸附在钢表面后,在1.0 mol/L盐酸水溶液中的缓蚀性能。结果表明无患子果皮提取物聚集体能够有效抑制钢被盐酸腐蚀,最大缓蚀效率达到90%以上。
中图分类号:
罗雪, 黄海军, 罗自萍, 王治永, 穆小静, 李红茹, 王新潮, 张胜涛, 高放. 无患子果皮提取物的纳微米聚集体对钢的高效缓蚀[J]. 化工学报, 2020, 71(10): 4760-4772.
Xue LUO, Haijun HUANG, Ziping LUO, Zhiyong WANG, Xiaojing MU, Hongru LI, Xinchao WANG, Shengtao ZHANG, Fang GAO. High efficient corrosion inhibition of steel by nano-micro aggregates of Sapindus mukorossi Gaertn peel extracts[J]. CIESC Journal, 2020, 71(10): 4760-4772.
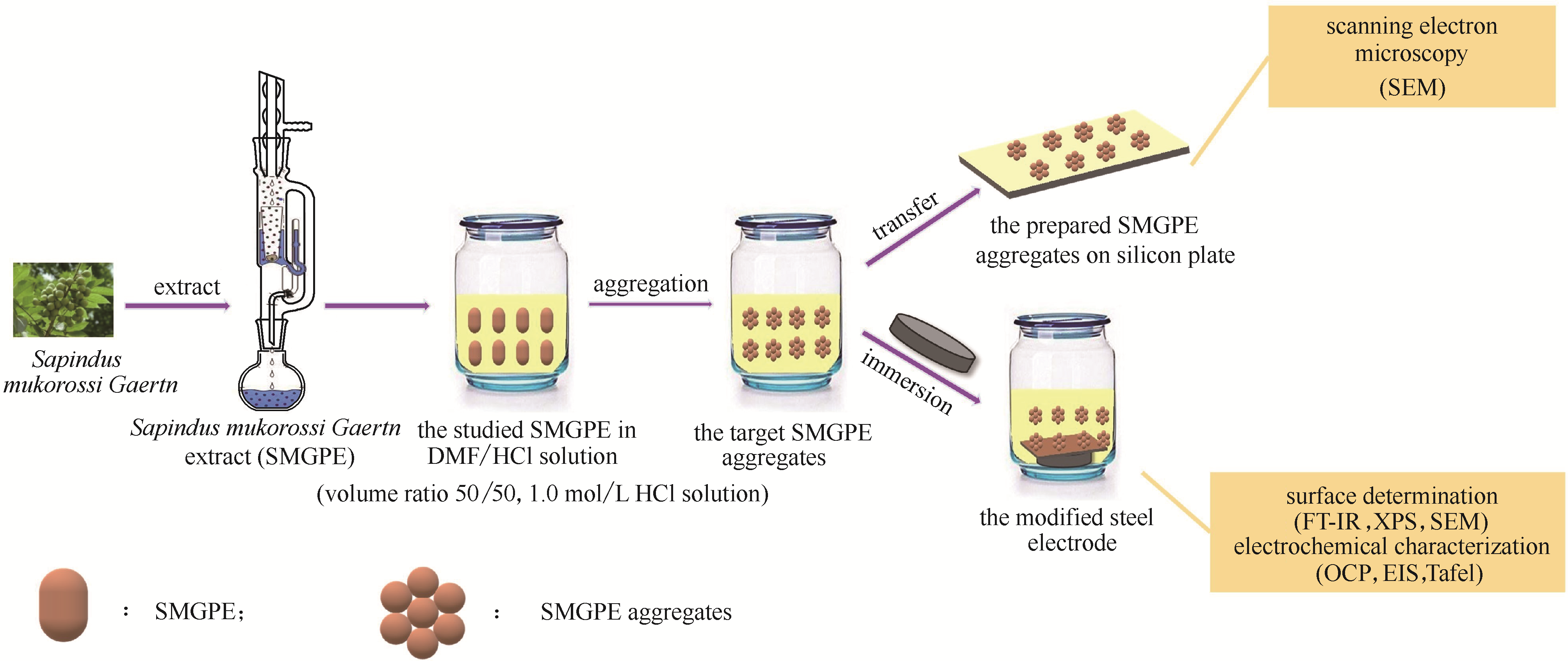
图2 无患子果皮提取物(SMGPE)形成聚集体并在钢表面有效吸附的示意图
Fig.2 Diagrammatic drawing of the formation of SMGPE aggregates and the preparation of the stable SMGPE aggregates protection film on the studied steel specimen surface
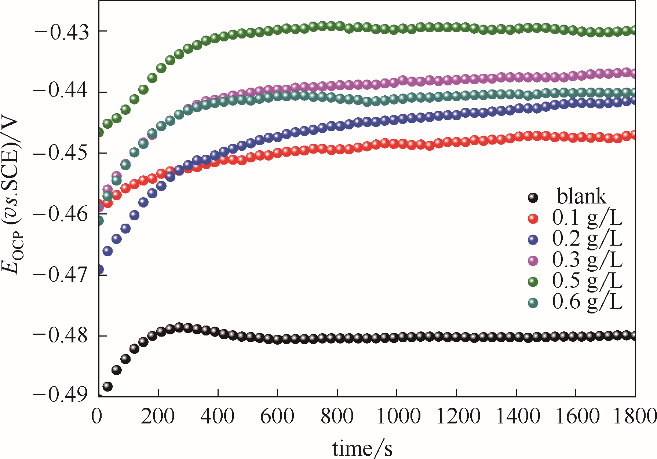
图3 空白钢电极和吸附了不同浓度稳定SMGPE聚集体(聚集时间10 h)的钢电极在1 mol/L HCl溶液中的开路电位 (OCP)曲线
Fig.3 OCP versus time curves in 1.0 mol/L HCl solutions for the investigated unmodified steel electrodes and the stable SMGPE aggregates of different concentrations covered steel electrodes

图4 SMGPE聚集体在浓度为0.5 g/L时,在DMF/HCl(体积比50/50,1.0 mol/L HCl 溶液)混合溶液中聚集一定时间后的扫描电镜图
Fig.4 SEM images of SMGPE aggregates in DMF/HCl (vol ratio 50/50,1.0 mol/L HCl solution) mixed solution at different aggregation time
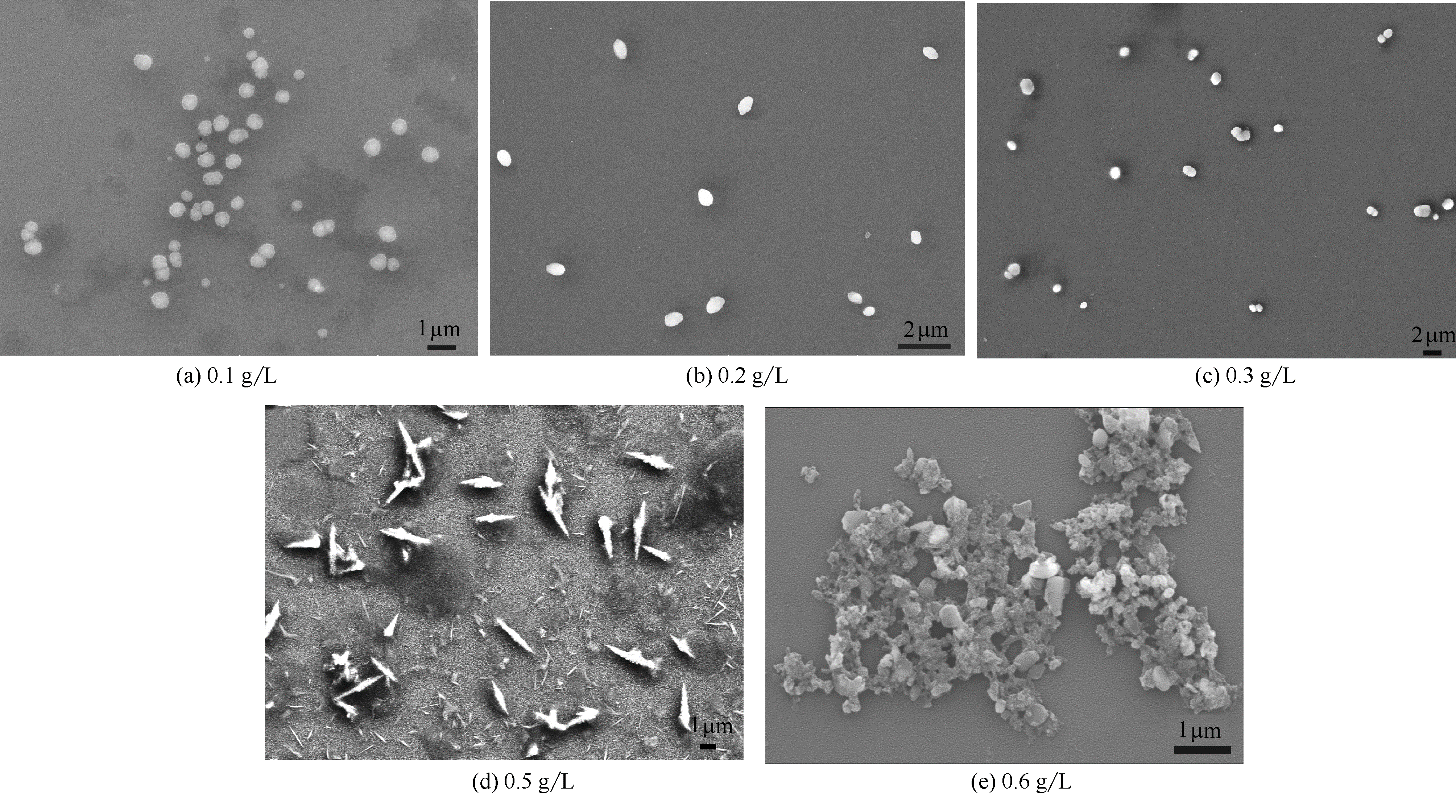
图5 SMGPE在不同浓度时,在DMF/HCl(体积比50/50,1.0 mol/L HCl 溶液)混合溶液中聚集10 h的扫描电镜图
Fig.5 SEM images of SMGPE aggregates in DMF/HCl (vol ratio 50/50,1.0 mol/L HCl solution) mixed solution with different concentrations from 0.1 to 0.6 g/L at 10 h evolution time
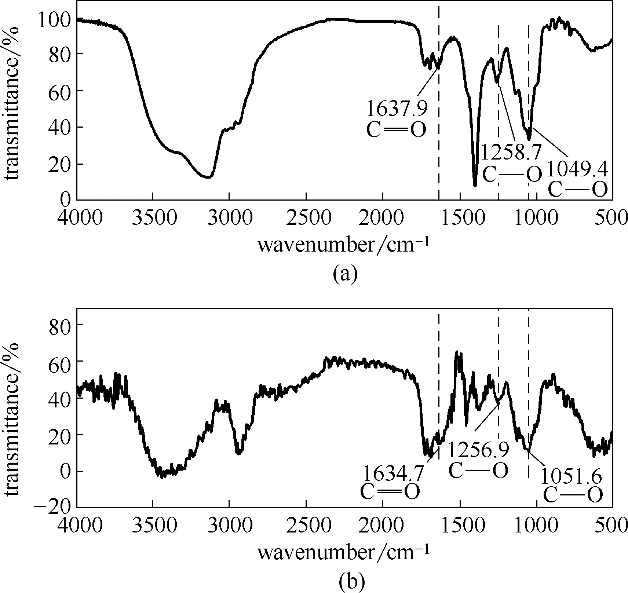
图6 SMGPE固体粉末的红外谱图(a); 稳定SMGPE聚集体 (聚集浓度 0.5 g/L, 聚集时间 10 h) 吸附于钢表面的全反射红外谱图(b)
Fig.6 FT-IR spectrum of original SME powder (a); FT-IR spectrum of the stable SMGPE aggregates (aggregation concentration 0.5 g/L, aggregation time 10 h)adsorbed on the studied steel specimen surfaces (b)
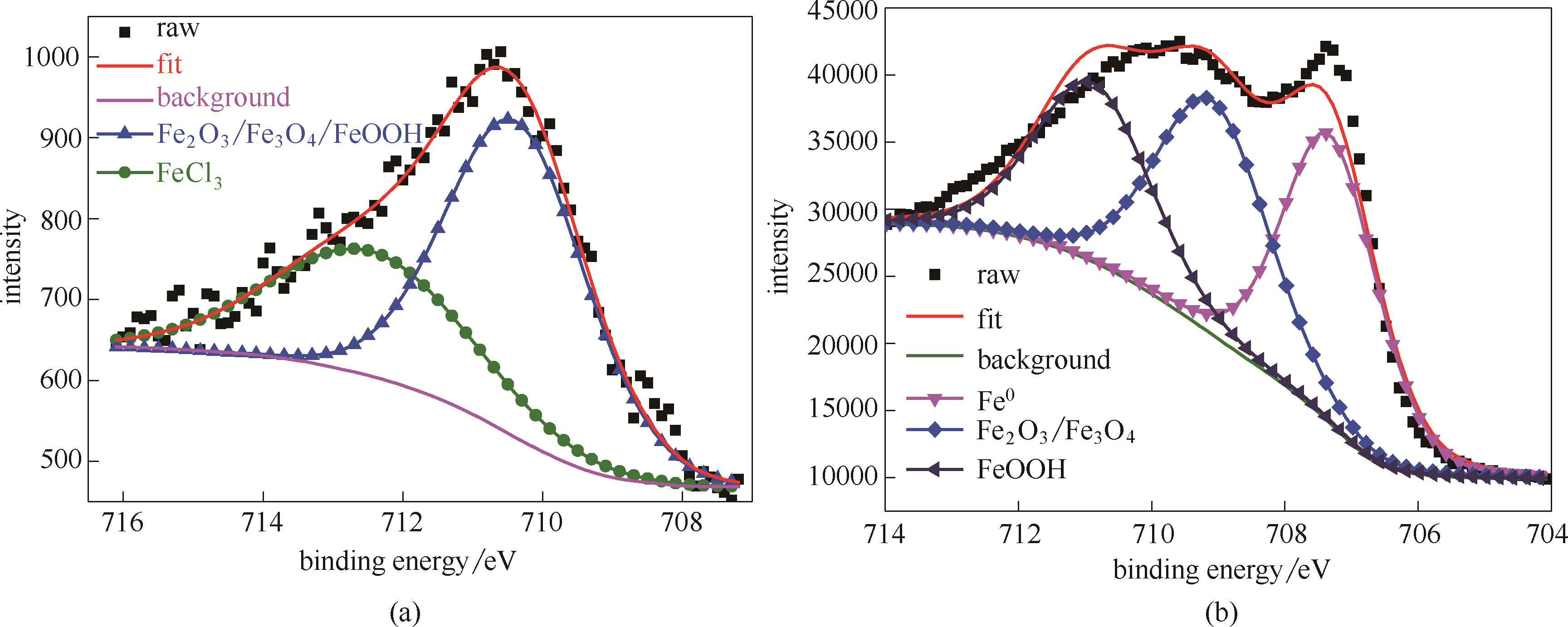
图7 空白钢片在HCl水溶液中浸泡1 h,钢片表面的Fe 2p XPS能级谱图(a); 吸附稳定SMGPE聚集体 (聚集浓度 0.5 g/L, 聚集时间 10 h) 的钢片在HCl水溶液中浸泡1 h后金属表面的Fe 2p XPS能级谱图(b)
Fig.7 Fe 2p XPS spectra from the surfaces of the investigated bare steel immersed in HCl solution for 1 h (a); Fe 2p XPS spectra from the studied steel specimens treated by the stable SMGPE aggregates (aggregation concentration 0.5 g/L, aggregation time 10 h) immersed in HCl solution for 1 h (b)
| 样品 | 化学状态 | 结合能/eV | 半峰宽/eV |
|---|---|---|---|
| 空白钢片 | Fe2O3/Fe3O4/FeOOH | 710.38 | 3.45 |
| FeCl3 | 712.47 | 3.5 | |
| 吸附稳定SMGPE聚集体的钢片 | Fe0 | 707.31 | 1.75 |
| Fe2O3/ Fe3O4 | 709.10 | 2.00 | |
| FeOOH | 710.90 | 2.00 |
Table 1 De-convolution parameters including chemical states, binding energies and FWHMs of Fe 2p XPS spectra peaks obtained from the surfaces of the studied bare steel, the surveyed steel specimens treated by the stable SMGPE aggregates (aggregation concentration 0.5 g/L, aggregation time 10 h) immersed in 1.0 mol/LHCl solution
| 样品 | 化学状态 | 结合能/eV | 半峰宽/eV |
|---|---|---|---|
| 空白钢片 | Fe2O3/Fe3O4/FeOOH | 710.38 | 3.45 |
| FeCl3 | 712.47 | 3.5 | |
| 吸附稳定SMGPE聚集体的钢片 | Fe0 | 707.31 | 1.75 |
| Fe2O3/ Fe3O4 | 709.10 | 2.00 | |
| FeOOH | 710.90 | 2.00 |
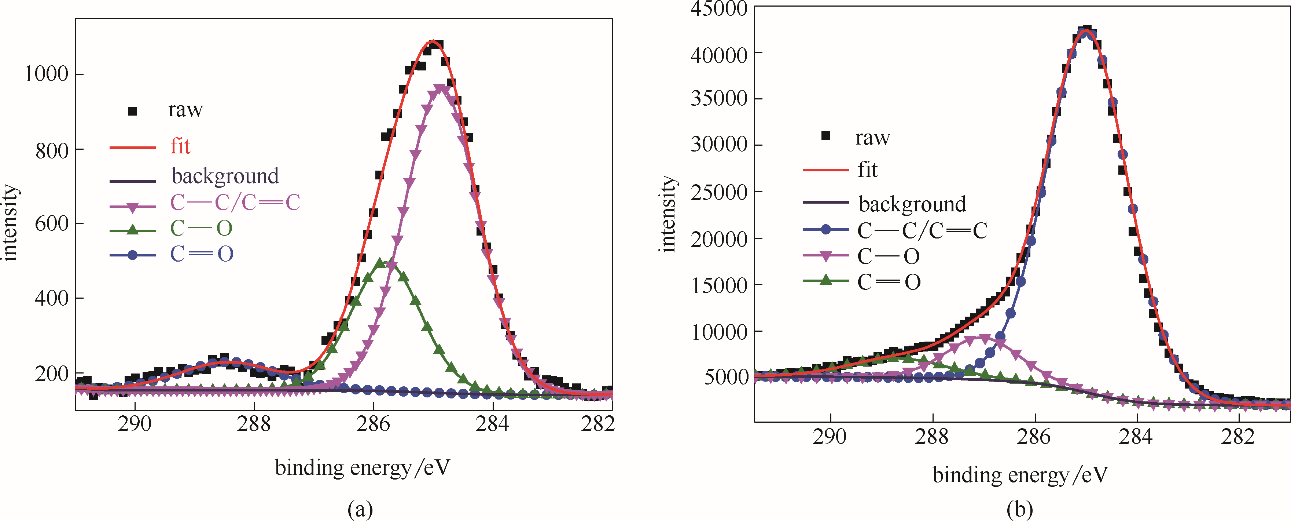
图8 空白钢片在HCl水溶液 (1.0 mol/L)浸泡1 h后,钢片表面的C 1s XPS图谱(a); 吸附稳定SMGPE聚集体 (聚集浓度 0.5 g/L, 聚集时间 10 h) 钢片在HCl水溶液(1.0 mol/L)浸泡1 h后钢片表面的C 1s XPS谱图 (b)表2 在HCl水溶液(1.0 mol/L)浸泡1 h后,空白钢片表面与吸附了稳定SMGPE聚集体的钢片 (聚集浓度 0.5 g/L, 聚集时间 10 h) 表面的C 1s能级分析的化学结构状态、结合能和半峰宽
Fig.8 C 1s XPS spectra from surfaces of the investigated bare steel immersed in HCl solution (1.0 mol/L HCl) for 1 h (a); C 1s XPS spectra from surfaces of the studied steel specimens treated by stable SMGPE aggregates (aggregation concentration 0.5 g/L, aggregation time 10 h) immersed in 1.0 mol/L HCl solution for 1 h (b)
| 样品 | 化学状态 | 结合能/eV | 半峰宽/eV |
|---|---|---|---|
| 空白钢片 | C—C/C C C | 284.90 | 1.62 |
| C—O | 285.79 | 1.55 | |
C O O | 288.43 | 2.00 | |
| 吸附稳定SMGPE聚集体的钢片 | C—C/C C C | 284.98 | 2.06 |
| C—O | 287.03 | 1.90 | |
C O O | 288.68 | 2.30 |
Table 2 De-convolution parameters including chemical states, binding energies and FWHMs of C 1s XPS spectra peaks obtained from surfaces of the studied bare steel specimen and the studied steel specimens treated by the stable SMGPE aggregates (aggregation concentration 0.5 g/L, aggregation time 10 h) immersed in 1.0 mol/L HCl solution for 1 h
| 样品 | 化学状态 | 结合能/eV | 半峰宽/eV |
|---|---|---|---|
| 空白钢片 | C—C/C C C | 284.90 | 1.62 |
| C—O | 285.79 | 1.55 | |
C O O | 288.43 | 2.00 | |
| 吸附稳定SMGPE聚集体的钢片 | C—C/C C C | 284.98 | 2.06 |
| C—O | 287.03 | 1.90 | |
C O O | 288.68 | 2.30 |
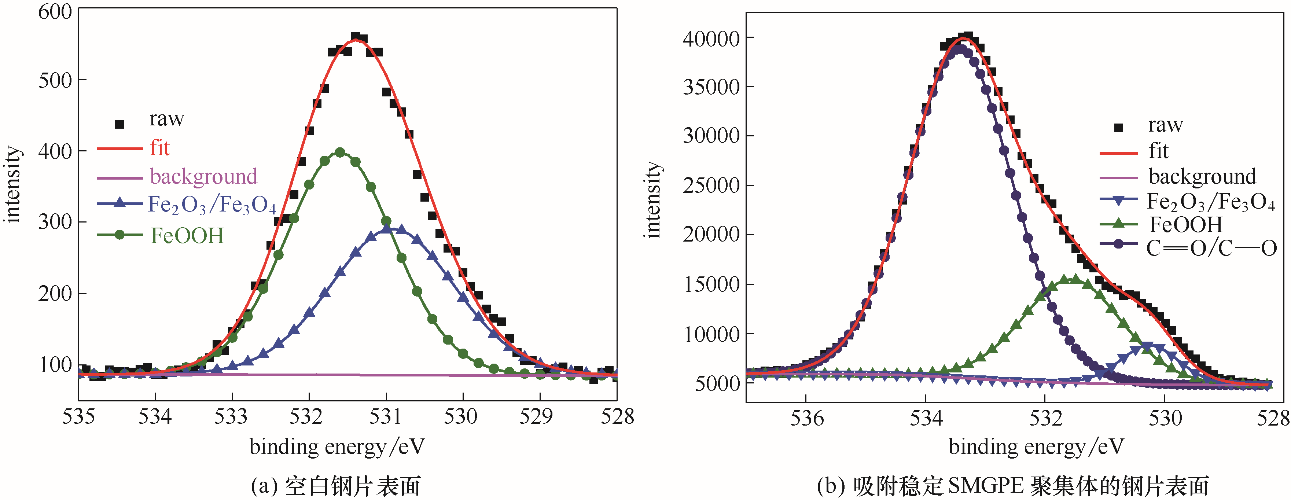
图9 未吸附和吸附了稳定SMGPE聚集体 (聚集浓度 0.5 g/L, 聚集时间 10 h) 的钢片在1.0 mol/L盐酸溶液中浸泡1 h后,钢片表面的O 1s XPS谱图
Fig.9 O 1s XPS spectra from surfaces of the investigated bare steel immersed in 1.0 mol/L HCl solution for 3 h; O 1s XPS spectra from surfaces of the studied steel specimens treated by stable SMGPE aggregates (aggregation concentration 0.5 g/L, aggregation time 10 h) immersed in HCl solution for 1 h
| 样品 | 化学状态 | 结合能/eV | 半峰宽/eV |
|---|---|---|---|
| 空白钢片 | Fe2O3/ Fe3O4 | 530.92 | 2.00 |
| FeOOH | 531.61 | 1.80 | |
| 吸附稳定SMGPE聚集体的钢片 | Fe2O3/ Fe3O4 | 530.24 | 1.90 |
| FeOOH | 531.55 | 1.75 | |
C O/C—O O/C—O | 533.41 | 1.60 |
表3 空白钢片和吸附了SMGPE聚集体的钢片 (聚集浓度 0.5 g/L, 聚集时间 10 h) 在1.0 mol/L盐酸溶液中浸泡1 h后的O 1s能级分析的化学结构状态、结合能和半峰宽
Table 3 De-convolution parameters including chemical states, binding energies and FWHMs of O 1s XPS spectra peaks obtained from surfaces of the studied bare steel specimen and the studied steel specimens treated by stable SMGPE aggregates immersed in 1.0 mol/L HCl solution for 1 h (aggregation concentration, 0.5 g/L, aggregation time, 10 h)
| 样品 | 化学状态 | 结合能/eV | 半峰宽/eV |
|---|---|---|---|
| 空白钢片 | Fe2O3/ Fe3O4 | 530.92 | 2.00 |
| FeOOH | 531.61 | 1.80 | |
| 吸附稳定SMGPE聚集体的钢片 | Fe2O3/ Fe3O4 | 530.24 | 1.90 |
| FeOOH | 531.55 | 1.75 | |
C O/C—O O/C—O | 533.41 | 1.60 |
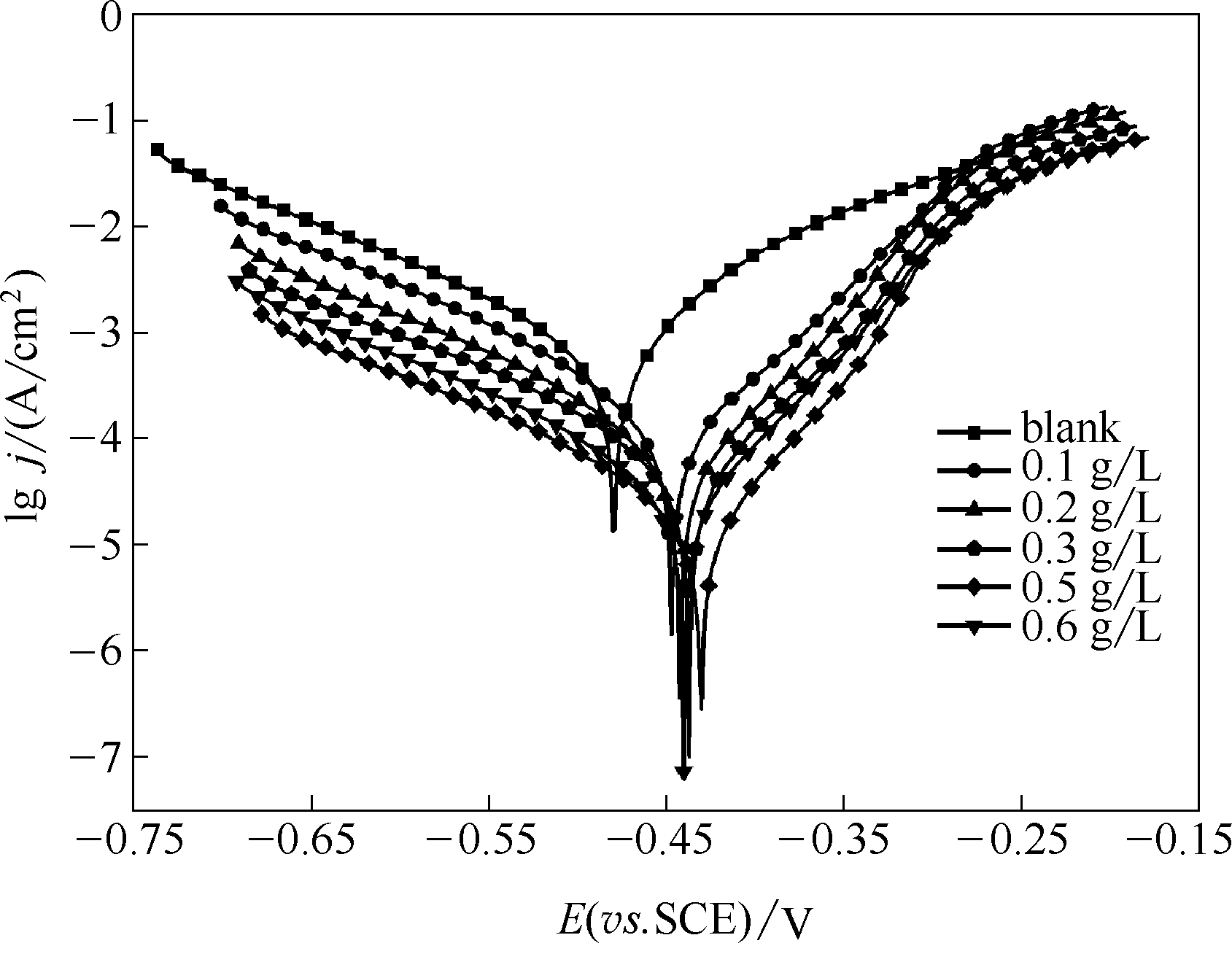
图11 空白钢电极和吸附了不同浓度稳定SMGPE聚集体(聚集时间10 h)的钢电极在1 mol/L HCl溶液中的动电位极化曲线
Fig.11 Potentiodynamic polarization curves in 1 mol/L HCl solutions for the investigated unmodified steel electrodes and the stable SMGPE aggregates of different concentrations (aggregation time 10 h) covered steel electrodes
| 电极 | 极化参数 | |||||
|---|---|---|---|---|---|---|
| C/(g/L) | Ecorr (vs. SCE) /V | Icorr/(A/cm2) | βc/(V/dec) | βa/(V/dec) | ηj/% | |
| 空白钢电极 | — | -0.480 | 4.387×10-7 | -0.1278 | 0.1068 | — |
| 稳定SMGPE聚集体吸附的钢电极 | 0.1 | -0.447 | 1.004×10-7 | -0.1146 | 0.0585 | 77.11 |
| 0.2 | -0.442 | 4.957×10-8 | -0.1175 | 0.0508 | 88.7 | |
| 0.3 | -0.437 | 4.549×10-8 | -0.1233 | 0.0515 | 89.63 | |
| 0.5 | -0.430 | 2.820×10-8 | -0.1226 | 0.0424 | 93.57 | |
| 0.6 | -0.440 | 3.767×10-4 | -0.1212 | 0.0524 | 91.41 | |
表4 空白钢电极和吸附了不同浓度稳定SMGPE聚集体(聚集时间10 h)的钢电极在1 mol/L HCl溶液中的动电位极化曲线参数
Table 4 Polarization parameters for the studied unmodified and modified steel specimens by different concentrations of stable SMGPE aggregates (aggregation time 10 h) in 1.0 mol/L HCl solution
| 电极 | 极化参数 | |||||
|---|---|---|---|---|---|---|
| C/(g/L) | Ecorr (vs. SCE) /V | Icorr/(A/cm2) | βc/(V/dec) | βa/(V/dec) | ηj/% | |
| 空白钢电极 | — | -0.480 | 4.387×10-7 | -0.1278 | 0.1068 | — |
| 稳定SMGPE聚集体吸附的钢电极 | 0.1 | -0.447 | 1.004×10-7 | -0.1146 | 0.0585 | 77.11 |
| 0.2 | -0.442 | 4.957×10-8 | -0.1175 | 0.0508 | 88.7 | |
| 0.3 | -0.437 | 4.549×10-8 | -0.1233 | 0.0515 | 89.63 | |
| 0.5 | -0.430 | 2.820×10-8 | -0.1226 | 0.0424 | 93.57 | |
| 0.6 | -0.440 | 3.767×10-4 | -0.1212 | 0.0524 | 91.41 | |
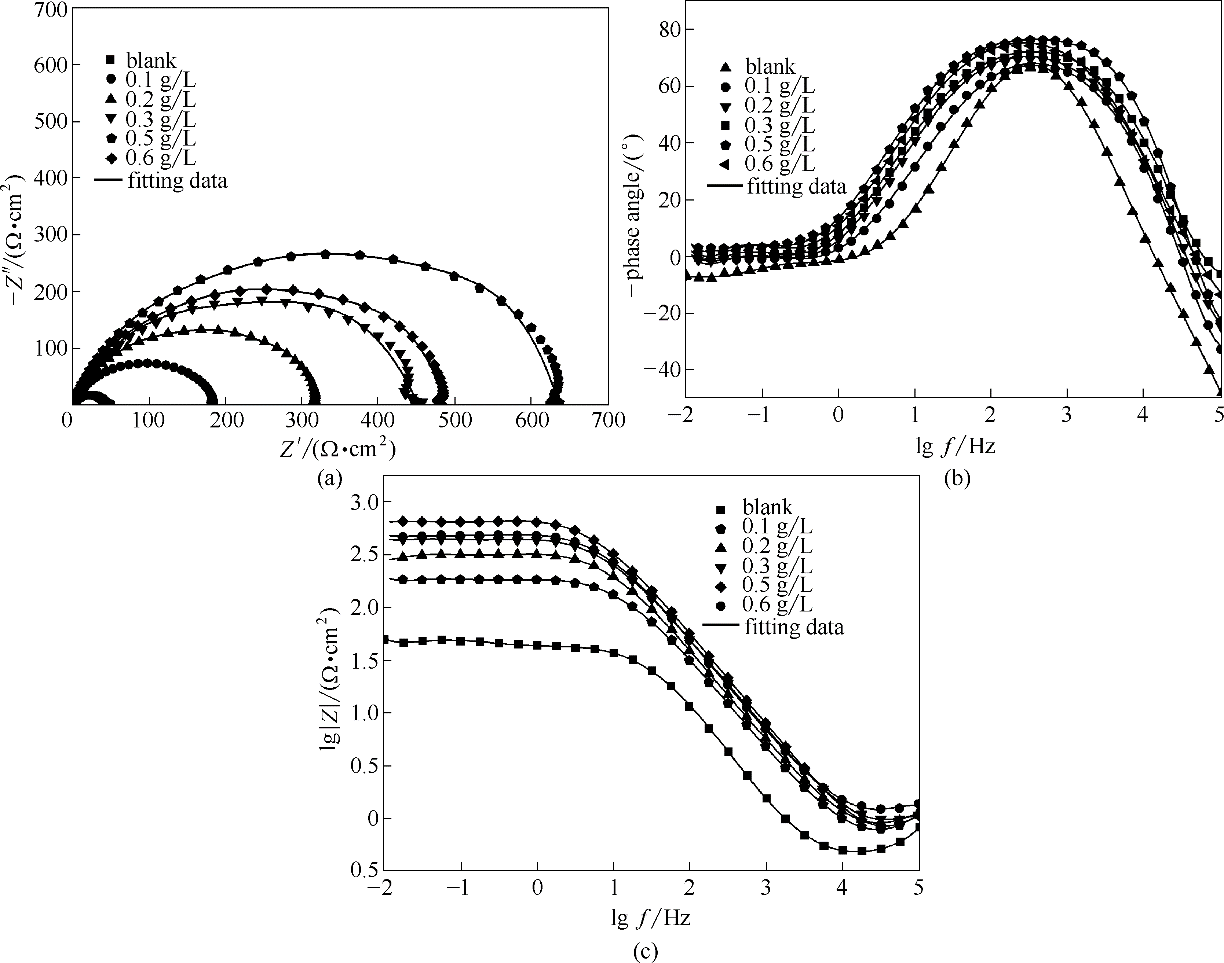
图12 空白钢电极和吸附了不同浓度的稳定SMGPE聚集体(聚集时间10 h)的钢电极在1.0 mol/L HCl溶液中的Nyquist图(a); 空白钢电极(b)和吸附了不同浓度SMGPE聚集体的钢电极(c)在1.0 mol/L HCl溶液中的Bode图
Fig.12 Nyquist curves for the studied naked steel electrodes and SMGPE aggregates (aggregation time 10 h) of various concentrations covered steel electrodes(a); Bode plots for the studied naked steel electrodes (b) and SMGPE aggregates (aggregation time 10 h) of various concentrations covered steel electrodes (c)
| 电极 | C/ (g/L) | 电化学参数 | χ2 | ||||
|---|---|---|---|---|---|---|---|
| Rs/ (Ω·cm2) | Rct / (Ω·cm2) | Cdl / (F/cm2) | n | ηE /% | |||
| 空白钢电极 | — | 0.6096 | 44.36 | 1.408×10-7 | 0.9551 | — | 4.81×103 |
| SMGPE聚集体吸附的钢电极 | 0.1 | 0.8946 | 182.0 | 6.34×10-8 | 0.8832 | 75.63 | 2.41×103 |
| 0.2 | 0.9725 | 315.4 | 5.48×10-8 | 0.8829 | 85.94 | 1.52×103 | |
| 0.3 | 0.9667 | 451.2 | 4.49×10-8 | 0.8707 | 90.17 | 4.65×103 | |
| 0.5 | 0.8991 | 635.0 | 3.98×10-8 | 0.8928 | 93.01 | 1.95×103 | |
| 0.6 | 1.2320 | 484.4 | 4.46×10-8 | 0.8867 | 90.92 | 7.48×103 | |
表5 空白钢电极和吸附了不同浓度稳定SMGPE聚集体(聚集时间10 h)的钢电极在1.0 mol/L HCl溶液中的交流阻抗参数
Table 5 Electrochemical impedance parameters for the studied unmodified and different concentrations of stable SMGPE aggregates (aggregation time 10 h) modified steel specimens in 1.0 mol/L HCl solution
| 电极 | C/ (g/L) | 电化学参数 | χ2 | ||||
|---|---|---|---|---|---|---|---|
| Rs/ (Ω·cm2) | Rct / (Ω·cm2) | Cdl / (F/cm2) | n | ηE /% | |||
| 空白钢电极 | — | 0.6096 | 44.36 | 1.408×10-7 | 0.9551 | — | 4.81×103 |
| SMGPE聚集体吸附的钢电极 | 0.1 | 0.8946 | 182.0 | 6.34×10-8 | 0.8832 | 75.63 | 2.41×103 |
| 0.2 | 0.9725 | 315.4 | 5.48×10-8 | 0.8829 | 85.94 | 1.52×103 | |
| 0.3 | 0.9667 | 451.2 | 4.49×10-8 | 0.8707 | 90.17 | 4.65×103 | |
| 0.5 | 0.8991 | 635.0 | 3.98×10-8 | 0.8928 | 93.01 | 1.95×103 | |
| 0.6 | 1.2320 | 484.4 | 4.46×10-8 | 0.8867 | 90.92 | 7.48×103 | |
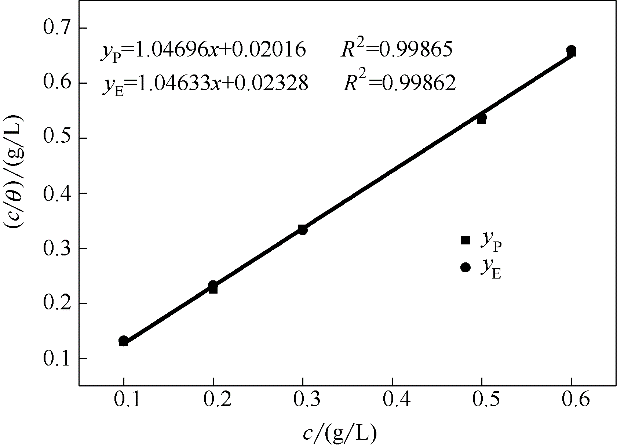
图14 在298 K下,吸附了不同浓度稳定SMGPE聚集体的钢在1.0 mol/L HCl溶液中的Langmuir吸附等温线
Fig.14 Langmuir adsorption isotherm of stable SMGPE aggregates on the studied steel specimen surfaces in 1.0 mol/L HCl solution at 298 K (yP to Tafel curves, yE to electrochemical impedance spectroscopy)
| 测试方法 | 吸附能 |
|---|---|
| Polarization | -26790 |
| EIS | -26430 |
表6 在298 K下,稳定SMGPE聚集体对钢的吸附热力学参数
Table 6 Thermodynamic parameters for the adsorption of SMGPE aggregates at 298 K
| 测试方法 | 吸附能 |
|---|---|
| Polarization | -26790 |
| EIS | -26430 |
| 1 | Wang H, Akid R. Encapsulated cerium nitrate inhibitors to provide high-performance anti-corrosion sol-gel coatings on mild steel[J]. Corrosion Science, 2008, 50: 1142-1148. |
| 2 | Yu J, Zhang Y, Jin X, et al. Fabrication and optical emission spectroscopy of enhanced corrosion-resistant CPEO films on Q235 low carbon steel[J]. Surface and Coatings Technology, 2019, 363: 411-418. |
| 3 | Yi C. Corrosion inhibition effect of 2-hydroxy phosphonoacetic acid and pyrophosfate on Q235 steel, electrochemical noise and EIS analysis[J]. International Journal of Electrochemical Science, 2019, 14: 6759-6772. |
| 4 | Wang C. Synthesis, crystal structure and corrosion inhibition effect of s-benzyl-O,O'-bis(p-tert-butyl phenyl)dithiophosphate for Q235 steel in 1.0 M HCl[J]. International Journal of Electrochemical Science, 2019, 31(6): 3443-3454. |
| 5 | Guo L, Obot I B, Zheng X W, et al. Theoretical insight into an empirical rule about organic corrosion inhibitors containing nitrogen, oxygen, and sulfur atoms[J]. Applied Surface Science, 2017, 406: 301-306. |
| 6 | Qiang Y J, Fu S L, Zhang S T, et al. Designing and fabricating of single and double alkyl-chain indazole derivatives self-assembled monolayer for corrosion inhibition of copper[J]. Corrosion Science, 20181, 40: 111-121. |
| 7 | Qiang Y J, Zhang S T, Tan B, et al. Evaluation of Ginkgo leaf extract as an eco-friendly corrosion inhibitor of X70 steel in HCl solution[J]. Corrosion Science, 2018, 133: 6-16. |
| 8 | Umoren S A. Biomaterials for corrosion protection: evaluation of mustard seed extract as eco-friendly corrosion inhibitor for X60 steel in acid media[J]. Journal of Adhesion Science and Technology, 2016, 30: 1858-1879. |
| 9 | Li L, Xu W, Lei J, et al. Experimental and theoretical investigations of Michelia alba leaves extract as a green highly-effective corrosion inhibitor for different steel materials in acidic solution[J]. RSC Advances, 2015, 5: 93724-93732. |
| 10 | Mobin M, Aslam R, Zehra S, et al. Bio-/environment-friendly cationic gemini surfactant as novel corrosion inhibitor for mild steel in 1 M HCl solution[J]. Journal of Surfactants and Detergents, 2016, 20: 57-74. |
| 11 | El Achouri M, Kertit S, Gouttaya H M, et al. Corrosion inhibition of iron in 1 M HCl by some gemini surfactants in the series of alkanediyl-α, ω-bis-(dimethyl tetradecyl ammonium bromide) [J]. Progress in Organic Coatings, 2001, 43: 267-273. |
| 12 | Huang H C, Liao S C, Chang F R, et al. Molluscicidal saponins from apindus mukorossi, inhibitory agents of golden apple snails, pomacea canaliculata[J]. Journal of Food Agriculture and Environment, 2003, 51: 4916-4919. |
| 13 | 魏敏平. 无患子抑菌成分的分离纯化及其应用研究[D]. 无锡: 江南大学, 2018. |
| Wei M P. Study on the separation and purification of antibacterial components from Sapindus mukurossiand its application[D]. Wuxi: Jiangnan University, 2018. | |
| 14 | 徐圆圆, 贾黎明, 陈仲, 等. 无患子三萜皂苷研究进展[J]. 化学通报, 2018, 12(81): 1078-1088. |
| Xu Y Y, Jia L M, Chen Z, et al. Advances on Triterpenoid Saponin of Sapindus mukorossi[J]. Chemistry, 2018, 12(81): 1078-1088. | |
| 15 | 周礼彬. 川滇无患子总皂苷化学成分及表面活性的应用性能研究[D]. 昆明: 云南中医学院, 2017. |
| Zhou L B. Study on the chemical components and the application of surface active properties of the total saponins from sapindus delavayi[D]. Kunming: Yunnan University of Traditional Chinese Medicine, 2017. | |
| 16 | Li X, Deng S, Fu H. Inhibition of the corrosion of steel in HCl, H2SO4 solutions by bamboo leaf extract[J]. Corrosion Science, 2012, 62: 163-175. |
| 17 | Chidiebere M A, Oguzie E E, Liu L, et al. Ascorbic acid as corrosion inhibitor for Q235 mild steel in acidic environments[J]. Journal of Industrial and Engineering Chemistry, 2015, 26: 182-192. |
| 18 | Mallaiya K, Subramaniam R, Srikandan S S, et al. Electrochemical characterization of the protective film formed by the unsymmetrical Schiff's base on the mild steel surface in acid media[J]. Electrochimica. Acta, 2011, 56: 3857-3863. |
| 19 | Zarrouk A, Hammouti B, Lakhlifi T, et al. New 1 H -pyrrole-2, 5-dione derivatives as efficient organic inhibitors of carbon steel corrosion in hydrochloric acid medium: electrochemical, XPS and DFT studies[J]. Corrosion Science, 2015, 90: 572-584. |
| 20 | Kharbach Y, Qachchachi F Z, Haoudi A, et al. Anticorrosion performance of three newly synthesized isatin derivatives on carbon steel in hydrochloric acid pickling environment: electrochemical, surface and theoretical studies[J]. Journal of Molecular Liquids, 2017, 246: 302-316. |
| 21 | Bouanis M, Tourabi M, Nyassi A, et al. Corrosion inhibition performance of 2, 5-bis(4-dimethylaminophenyl)-1, 3, 4-oxadiazole for carbon steel in HCl solution: gravimetric, electrochemical and XPS studies[J]. Applied Surface Science, 2016, 389: 952-966. |
| 22 | Boumhara K, Tabyaoui M, Jama C, et al. Artemisia Mesatlantica essential oil as green inhibitor for carbon steel corrosion in 1 M HCl solution: electrochemical and XPS investigations[J]. Journal of Industrial and Engineering Chemistry, 2015, 29: 146-155. |
| 23 | Ji G, Anjum S, Sundaram S, et al. Musa paradisica peel extract as green corrosion inhibitor for mild steel in HCl solution[J]. Corrosion Science, 2015, 90: 107-117. |
| 24 | Hussin M H, Jain K M, Razali N N, et al.The effect of Tinospora crispa extracts as a natural mild steel corrosion inhibitor in 1 M HCl solution[J]. Arabian Journal of Chemistry, 2016, 9: 616-624. |
| 25 | Singh A K, Quraishi M A. Investigation of adsorption of isoniazid derivatives at mild steel/hydrochloric acid interface: electrochemical and weight loss methods[J]. Materials Chemistry and Physics, 2010, 123: 666–677. |
| 26 | Sudheer, Quraishi M A. Electrochemical and theoretical investigation of triazole derivatives on corrosion inhibition behavior of copper in hydrochloric acid medium[J]. Corrosion Science, 2013, 70: 161-169. |
| 27 | Huang H J, Fu Y, Li F, et al.Orderly self-assembly of new ionic copolymers for efficiently protecting copper in aggressive sulfuric acid solution[J]. Chemical Engineering Journal, 2020, 384: 123293. |
| 28 | Wang Z Q, Gong Y L, Zhang L, et al. Self-assembly of new dendrimers basing on strong π-π intermolecular interaction for application to protect copper[J]. Chemical Engineering Journal, 2018, 342: 238-250. |
| 29 | Ostovari A, Hoseinieh S M, Peikari M, et al. Corrosion inhibition of mild steel in 1 M HCl solution by henna extract: a comparative study of the inhibition by henna and its constituents (Lawsone, Gallic acid, α-d-Glucose and Tannic acid) [J]. Corrosion Science, 2009, 51: 1935-1949. |
| 30 | Sığırcık G, Yildirim D, Tüken T. Synthesis and inhibitory effect of N, N'-bis(1-phenylethanol)ethylenediamine against steel corrosion in HCl media[J]. Corrosion Science, 2017, 120: 184-193. |
| 31 | Li L, Zhang X, Lei J, et al. Adsorption and corrosion inhibition of Osmanthus fragran leaves extract on carbon steel[J]. Corrosion Science, 2012, 63: 82-90. |
| 32 | Biswas A, Pal S, Udayabhanu G. Experimental and theoretical studies of Xanthan gum and its graft co-polymer as corrosion inhibitor for mild steel in 15% HCl[J]. Applied Surface Science, 2015, 353: 173-183. |
| 33 | Chauhan D S, Ansari K R, Sorour A A, et al. Thiosemicarbazide and thiocarbohydrazide functionalized chitosan as ecofriendly corrosion inhibitors for carbon steel in hydrochloric acid solution[J]. International Journal of Biological Macromolecules, 2018, 107: 1747-1757. |
| 34 | Hu Z, Meng Y, Ma X, et al. Experimental and theoretical studies of benzothiazole derivatives as corrosion inhibitors for carbon steel in 1 M HCl[J]. Corrosion Science, 2016, 112: 563-575. |
| 35 | Obot I B, Obi-Egbedi N O. Adsorption properties and inhibition of mild steel corrosion in sulphuric acid solution by ketoconazole: experimental and theoretical investigation[J]. Corrosion Science, 2010, 52: 198-204. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 康飞, 吕伟光, 巨锋, 孙峙. 废锂离子电池放电路径与评价研究[J]. 化工学报, 2023, 74(9): 3903-3911. |
| [3] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [4] | 陈佳起, 赵万玉, 姚睿充, 侯道林, 董社英. 开心果壳基碳点的合成及其对Q235碳钢的缓蚀行为研究[J]. 化工学报, 2023, 74(8): 3446-3456. |
| [5] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [6] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [7] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [8] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [9] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [10] | 李艳辉, 丁邵明, 白周央, 张一楠, 于智红, 邢利梅, 高鹏飞, 王永贞. 非常规服役超临界锅炉的微纳尺度腐蚀动力学模型建立及应用[J]. 化工学报, 2023, 74(6): 2436-2446. |
| [11] | 王新悦, 王俊杰, 曹思贤, 王翠, 李灵坤, 吴宏宇, 韩静, 吴昊. 玻璃内包材界面修饰对机械应力诱导的单克隆抗体聚集体形成的影响[J]. 化工学报, 2023, 74(6): 2580-2588. |
| [12] | 陈韶云, 徐东, 陈龙, 张禹, 张远方, 尤庆亮, 胡成龙, 陈建. 单层聚苯胺微球阵列结构的制备及其吸附性能[J]. 化工学报, 2023, 74(5): 2228-2238. |
| [13] | 王蕾, 王磊, 白云龙, 何柳柳. SA膜状锂离子筛的制备及其锂吸附性能[J]. 化工学报, 2023, 74(5): 2046-2056. |
| [14] | 蔺彩虹, 王丽, 吴瑜, 刘鹏, 杨江峰, 李晋平. 沸石中碱金属阳离子对CO2/N2O吸附分离性能的影响[J]. 化工学报, 2023, 74(5): 2013-2021. |
| [15] | 李辰鑫, 潘艳秋, 何流, 牛亚宾, 俞路. 基于碳微晶结构的炭膜模型及其气体分离模拟[J]. 化工学报, 2023, 74(5): 2057-2066. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号