化工学报 ›› 2023, Vol. 74 ›› Issue (1): 205-223.DOI: 10.11949/0438-1157.20221311
孙嘉辰1,2( ), 裴春雷1,2, 陈赛1,2, 赵志坚1,2, 何盛宝3, 巩金龙1,2(
), 裴春雷1,2, 陈赛1,2, 赵志坚1,2, 何盛宝3, 巩金龙1,2( )
)
收稿日期:2022-09-30
修回日期:2023-02-14
出版日期:2023-01-05
发布日期:2023-03-20
通讯作者:
巩金龙
作者简介:孙嘉辰(1994—),男,博士研究生,Jiachens@tju.edu.cn
基金资助:
Jiachen SUN1,2( ), Chunlei PEI1,2, Sai CHEN1,2, Zhijian ZHAO1,2, Shengbao HE3, Jinlong GONG1,2(
), Chunlei PEI1,2, Sai CHEN1,2, Zhijian ZHAO1,2, Shengbao HE3, Jinlong GONG1,2( )
)
Received:2022-09-30
Revised:2023-02-14
Online:2023-01-05
Published:2023-03-20
Contact:
Jinlong GONG
摘要:
低碳烯烃生产能力是化工行业技术水平的重要标志。随着低碳烯烃市场需求的增加,新型高效的低碳烯烃生产工艺得到了广泛关注。化学链低碳烷烃氧化脱氢技术,通过载氧体中的晶格氧与反应物分子发生反应,实现烷烃分子向烯烃分子的选择性转化,可以提高烯烃产率,有效降低过程能耗和CO2排放。本文针对化学链低碳烷烃氧化脱氢技术,深入分析了载氧体材料的筛选和理论设计、表面活性位和体相氧传输的调控机制、循环稳定性、载氧体的制备、化学链烷烃脱氢反应器和工艺设计优化等方向的研究现状和进展,并系统总结了未来化学链低碳烷烃脱氢技术及相关领域的发展趋势,为化学链烷烃脱氢领域的技术进步提供参考和借鉴。
中图分类号:
孙嘉辰, 裴春雷, 陈赛, 赵志坚, 何盛宝, 巩金龙. 化学链低碳烷烃氧化脱氢技术进展[J]. 化工学报, 2023, 74(1): 205-223.
Jiachen SUN, Chunlei PEI, Sai CHEN, Zhijian ZHAO, Shengbao HE, Jinlong GONG. Advances in chemical-looping oxidative dehydrogenation of light alkanes[J]. CIESC Journal, 2023, 74(1): 205-223.
| 模式 | 载氧体 | 温度/℃ | 气相组成 | 转化率 (H2或C n H2n+2)/% | 选择性/% | 文献 |
|---|---|---|---|---|---|---|
| 选择性氢燃烧 | Na2WO4/CaMnO3 | 850 | 40% H2,40% C2H4,Ar平衡气(100 ml/L) | >85 (H2) | ~90 | [ |
| Na2WO4/Mg6MnO8 | 850 | 80% C2H6,Ar平衡气(4500 h-1) | 81.8(C2H4) | 76.0 | [ | |
| Na2WO4/LaMnO3 | 800 | 40% C2H4,Ar平衡气(3400 h-1) | 70(C2H6) | 85 | [ | |
| Co0.3Mo0.7/Fe2O3 | 825 | 12.5% C2H6,Ar平衡气(40 ml/min) | 56.2(C2H6) | 87.4 | [ | |
| Bi-Ce0.75Zr0.25O2 | 550 | 2.5% C2H4,2.5% H2,N2平衡气(100 ml/min) | 90(H2) | — | [ | |
| Ni-HY | 600 | 10% C2H6, He平衡气(5100 h-1) | 18(C2H6) | 97 | [ | |
| Na2WO4/CuMn2O4 | 720 | 10% C2H6,N2平衡气(50 ml/min) | 58.8(C2H6) | 86.4 | [ | |
| 氧化脱氢 | V-TiO2 | 500 | 19% C3H8,N2平衡气(21 ml/min) | ~17(C3H8) | 90 | [ |
| MoVO x -Al2O3 | 500 | 19% C3H8,N2平衡气(21 ml/min) | 36(C3H8) | 89 | [ | |
| H3PMo12O40/Al2O3 | 450 | 19% C3H8,N2平衡气(21 ml/min) | ~7(C3H8) | >90 | [ | |
| MoO x -Fe2O3 | 600 | 80% C2H6,Ar平衡气(1500 h-1) | 4.9(C2H6) | 62.2 | [ | |
| Mg-La1.6Sr0.4FeCoO6 | 725 | 40% C2H6,N2平衡气(10 ml/min) | 52.9(C2H6) | 89.4 | [ | |
| LiBr-La0.8Sr0.2FeO3 | 500 | 80% C4H10,Ar平衡气(30 ml/min) | 75.6(C4H10) | 56.2 | [ | |
| Li2O-La x Sr2-x FeO4-δ | 700 | 37.5% C2H6,N2平衡气(40 ml/min) | 61(C2H6) | 90 | [ | |
| Li2CO3-La0.8Sr0.2FeO3 | 700 | 80% C2H6,Ar平衡气(40 ml/min) | 50(C2H6) | 91 | [ | |
| Cr-Ce-K/Al2O3 | 630 | 50% C3H8,N2平衡气(17 ml/min) | 57.5(C3H8) | 78 | [ | |
| Ce-SrFeO3 | 725 | 80% C2H6,Ar平衡气(20 ml/min) | 29(C2H6) | 82 | [ |
表1 目前已报道的化学链低碳烷烃脱氢载氧体、反应条件和性能汇总
Table 1 Summary of reported oxygen carriers, reaction conditions, and performance for chemical-looping oxidative dehydrogenation of light alkanes
| 模式 | 载氧体 | 温度/℃ | 气相组成 | 转化率 (H2或C n H2n+2)/% | 选择性/% | 文献 |
|---|---|---|---|---|---|---|
| 选择性氢燃烧 | Na2WO4/CaMnO3 | 850 | 40% H2,40% C2H4,Ar平衡气(100 ml/L) | >85 (H2) | ~90 | [ |
| Na2WO4/Mg6MnO8 | 850 | 80% C2H6,Ar平衡气(4500 h-1) | 81.8(C2H4) | 76.0 | [ | |
| Na2WO4/LaMnO3 | 800 | 40% C2H4,Ar平衡气(3400 h-1) | 70(C2H6) | 85 | [ | |
| Co0.3Mo0.7/Fe2O3 | 825 | 12.5% C2H6,Ar平衡气(40 ml/min) | 56.2(C2H6) | 87.4 | [ | |
| Bi-Ce0.75Zr0.25O2 | 550 | 2.5% C2H4,2.5% H2,N2平衡气(100 ml/min) | 90(H2) | — | [ | |
| Ni-HY | 600 | 10% C2H6, He平衡气(5100 h-1) | 18(C2H6) | 97 | [ | |
| Na2WO4/CuMn2O4 | 720 | 10% C2H6,N2平衡气(50 ml/min) | 58.8(C2H6) | 86.4 | [ | |
| 氧化脱氢 | V-TiO2 | 500 | 19% C3H8,N2平衡气(21 ml/min) | ~17(C3H8) | 90 | [ |
| MoVO x -Al2O3 | 500 | 19% C3H8,N2平衡气(21 ml/min) | 36(C3H8) | 89 | [ | |
| H3PMo12O40/Al2O3 | 450 | 19% C3H8,N2平衡气(21 ml/min) | ~7(C3H8) | >90 | [ | |
| MoO x -Fe2O3 | 600 | 80% C2H6,Ar平衡气(1500 h-1) | 4.9(C2H6) | 62.2 | [ | |
| Mg-La1.6Sr0.4FeCoO6 | 725 | 40% C2H6,N2平衡气(10 ml/min) | 52.9(C2H6) | 89.4 | [ | |
| LiBr-La0.8Sr0.2FeO3 | 500 | 80% C4H10,Ar平衡气(30 ml/min) | 75.6(C4H10) | 56.2 | [ | |
| Li2O-La x Sr2-x FeO4-δ | 700 | 37.5% C2H6,N2平衡气(40 ml/min) | 61(C2H6) | 90 | [ | |
| Li2CO3-La0.8Sr0.2FeO3 | 700 | 80% C2H6,Ar平衡气(40 ml/min) | 50(C2H6) | 91 | [ | |
| Cr-Ce-K/Al2O3 | 630 | 50% C3H8,N2平衡气(17 ml/min) | 57.5(C3H8) | 78 | [ | |
| Ce-SrFeO3 | 725 | 80% C2H6,Ar平衡气(20 ml/min) | 29(C2H6) | 82 | [ |
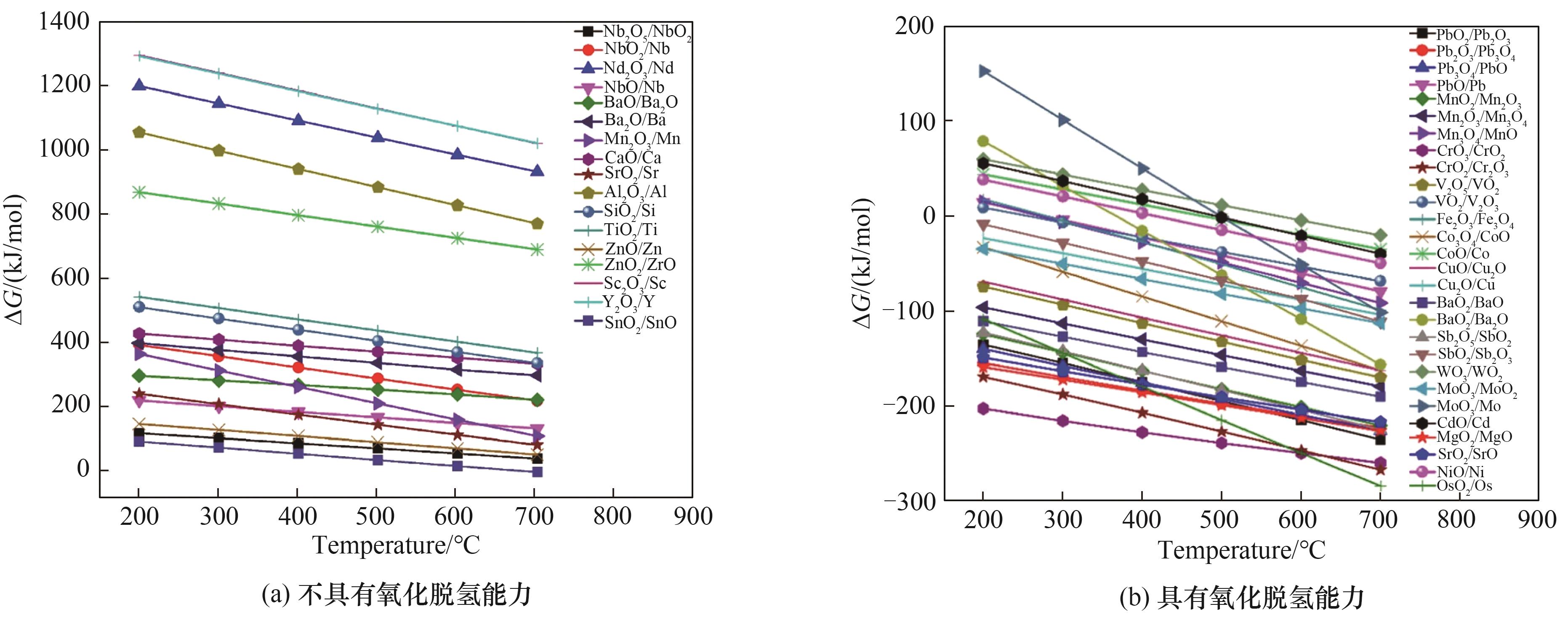
图3 45种金属氧化物在不同温度下参与丙烷厌氧氧化脱氢反应的Gibbs自由能(∆G)[46]
Fig.3 The ΔG for 45 different metal oxides in propane oxidative dehydrogenation reaction systems as a function of temperature[46]
| 名称 | 反应模型 | 微分形式f(x)=1/kapp×dx/dt | 积分形式g(x)=kapp dt |
|---|---|---|---|
| F1 | First-order or Avrami–Erofe’ev (n=1) | (1-x) | -ln(1-x) |
| F1.5 | Three-halves order | (1-x)3/2 | 2[(1-x)-1/2-1] |
| F2 | Second-order | (1-x)2 | 1/(1-x)-1 |
| F3 | Third-order | (1-x)3 | (1/2)[(1-x)-2-1] |
| R1 | Zero-order | 1 | x |
| R2 | Contracting area | 2(1-x)1/2 | 1-(1-x)1/2 |
| R3 | Contracting volume | 3(1-x)2/3 | 1-(1-x)1/3 |
| D1 | 1-D diffusion | 1/(2x) | x2 |
| D2 | 2-D diffusion, Valensi equation | 1/[-ln(1-x)] | (1-x)ln(1-x)+x |
| D3 | 3-D diffusion, Jander equation | 3(1-x)1/3/[2(1-x)-1/3-1] | [1-(1-x)1/3]2 |
| D4 | Ginstling-Brounshtein equation | 3/[2(1-x)-1/3-1] | 1-2x/3-(1-x)2/3 |
| AE0.5 | Avrami-Erofe’ev (n=0.5) | (1/2)(1-x)[-ln(1-x)]-1 | [-ln(1-x)]2 |
| AE1.5 | Avrami-Erofe’ev (n=1.5) | (3/2)(1-x)[-ln(1-x)]1/3 | [-ln(1-x)]2/3 |
| AE2 | Avrami-Erofe’ev (n=2) | 2(1-x)[-ln(1-x)]1/2 | [-ln(1-x)]1/2 |
| AE3 | Avrami-Erofe’ev (n=3) | 3(1-x)[-ln(1-x)]2/3 | [-ln(1-x)]1/3 |
表2 不同动力学模型微分和积分表达式[87]
Table 2 Rate and integral expressions for different solid-state kinetic models[87]
| 名称 | 反应模型 | 微分形式f(x)=1/kapp×dx/dt | 积分形式g(x)=kapp dt |
|---|---|---|---|
| F1 | First-order or Avrami–Erofe’ev (n=1) | (1-x) | -ln(1-x) |
| F1.5 | Three-halves order | (1-x)3/2 | 2[(1-x)-1/2-1] |
| F2 | Second-order | (1-x)2 | 1/(1-x)-1 |
| F3 | Third-order | (1-x)3 | (1/2)[(1-x)-2-1] |
| R1 | Zero-order | 1 | x |
| R2 | Contracting area | 2(1-x)1/2 | 1-(1-x)1/2 |
| R3 | Contracting volume | 3(1-x)2/3 | 1-(1-x)1/3 |
| D1 | 1-D diffusion | 1/(2x) | x2 |
| D2 | 2-D diffusion, Valensi equation | 1/[-ln(1-x)] | (1-x)ln(1-x)+x |
| D3 | 3-D diffusion, Jander equation | 3(1-x)1/3/[2(1-x)-1/3-1] | [1-(1-x)1/3]2 |
| D4 | Ginstling-Brounshtein equation | 3/[2(1-x)-1/3-1] | 1-2x/3-(1-x)2/3 |
| AE0.5 | Avrami-Erofe’ev (n=0.5) | (1/2)(1-x)[-ln(1-x)]-1 | [-ln(1-x)]2 |
| AE1.5 | Avrami-Erofe’ev (n=1.5) | (3/2)(1-x)[-ln(1-x)]1/3 | [-ln(1-x)]2/3 |
| AE2 | Avrami-Erofe’ev (n=2) | 2(1-x)[-ln(1-x)]1/2 | [-ln(1-x)]1/2 |
| AE3 | Avrami-Erofe’ev (n=3) | 3(1-x)[-ln(1-x)]2/3 | [-ln(1-x)]1/3 |
| 1 | McCoy M. The case for saltigo[J]. Chemical & Engineering News, 2006, 84(11): 28. |
| 2 | Sattler J J H B, Ruiz-Martinez J, Santillan-Jimenez E, et al. Catalytic dehydrogenation of light alkanes on metals and metal oxides[J]. Chemical Reviews, 2014, 114(20): 10613-10653. |
| 3 | Monai M, Gambino M, Wannakao S, et al. Propane to olefins tandem catalysis: a selective route towards light olefins production[J]. Chemical Society Reviews, 2021, 50(20): 11503-11529. |
| 4 | Chen S, Chang X, Sun G D, et al. Propane dehydrogenation: catalyst development, new chemistry, and emerging technologies[J]. Chemical Society Reviews, 2021, 50(5): 3315-3354. |
| 5 | Kung H H. Oxidative dehydrogenation of light (C2 to C4) alkanes[M]//Advances in Catalysis. Amsterdam: Elsevier, 1994: 1-38. |
| 6 | Zhu X, Imtiaz Q, Donat F, et al. Chemical looping beyond combustion—a perspective[J]. Energy & Environmental Science, 2020, 13(3): 772-804. |
| 7 | Adanez J, Abad A, Garcia-Labiano F, et al. Progress in chemical-looping combustion and reforming technologies[J]. Progress in Energy and Combustion Science, 2012, 38(2): 215-282. |
| 8 | Tang M C, Xu L, Fan M H. Progress in oxygen carrier development of methane-based chemical-looping reforming: a review[J]. Applied Energy, 2015, 151(1): 143-156. |
| 9 | He F, Huang Z, Wei G Q, et al. Biomass chemical-looping gasification coupled with water/CO2-splitting using NiFe2O4 as an oxygen carrier[J]. Energy Conversion and Management, 2019, 201(1): 112157. |
| 10 | Sun Z, Chen S Y, Hu J, et al. Ca2Fe2O5: a promising oxygen carrier for CO/CH4 conversion and almost-pure H2 production with inherent CO2 capture over a two-step chemical looping hydrogen generation process[J]. Applied Energy, 2018, 211(1): 431-442. |
| 11 | Gao W B, Guo J P, Wang P K, et al. Production of ammonia via a chemical looping process based on metal imides as nitrogen carriers[J]. Nature Energy, 2018, 3(12): 1067-1075. |
| 12 | Zeng L, Cheng Z, Fan J A, et al. Metal oxide redox chemistry for chemical looping processes[J]. Nature Reviews Chemistry, 2018, 2(11): 349-364. |
| 13 | Haribal V P, Neal L M, Li F X. Oxidative dehydrogenation of ethane under a cyclic redox scheme—process simulations and analysis[J]. Energy, 2017, 119(15): 1024-1035. |
| 14 | Dai Y H, Gao X, Wang Q J, et al. Recent progress in heterogeneous metal and metal oxide catalysts for direct dehydrogenation of ethane and propane[J]. Chemical Society Reviews, 2021, 50(9): 5590-5630. |
| 15 | Gomez E, Yan B H, Kattel S, et al. Carbon dioxide reduction in tandem with light-alkane dehydrogenation[J]. Nature Reviews Chemistry, 2019, 3(11): 638-649. |
| 16 | Wang Y L, Hu P, Yang J, et al. C—H bond activation in light alkanes: a theoretical perspective[J]. Chemical Society Reviews, 2021, 50(7): 4299-4358. |
| 17 | Cavani F, Ballarini N, Cericola A. Oxidative dehydrogenation of ethane and propane: how far from commercial implementation?[J]. Catalysis Today, 2007, 127(1/2/3/4): 113-131. |
| 18 | Callahan J L, Grasselli R K. A selectivity factor in vapor-phase hydrocarbon oxidation catalysis[J]. AIChE Journal, 1963, 9(6): 755-760. |
| 19 | Eastman A D, Kolts J H. Oxidative dehydrogenation catalyst: US4370259[P]. 1983-01-25. |
| 20 | Creaser D, Andersson B, Hudgins R R, et al. Cyclic operation of the oxidative dehydrogenation of propane[J]. Chemical Engineering Science, 1999, 54(20): 4437-4448. |
| 21 | Vrieland G E, Khazai B, Murchison C B. Anaerobic oxidation of butane to butadiene over magnesium molybdate catalysts (Ⅱ): Magnesia alumina supported catalysts[J]. Applied Catalysis A: General, 1996, 134(1): 123-145. |
| 22 | Grasselli R K, Stern D L, Tsikoyiannis J G. Catalytic dehydrogenation (DH) of light paraffins combined with selective hydrogen combustion (SHC)[J]. Applied Catalysis A: General, 1999, 189(1): 9-14. |
| 23 | Neal L M, Yusuf S, Sofranko J A, et al. Oxidative dehydrogenation of ethane: a chemical looping approach[J]. Energy Technology, 2016, 4(10): 1200-1208. |
| 24 | Dudek R B, Gao Y F, Zhang J S, et al. Manganese‐containing redox catalysts for selective hydrogen combustion under a cyclic redox scheme[J]. AIChE Journal, 2018, 64(8): 3141-3150. |
| 25 | Yusuf S, Neal L, Bao Z H, et al. Effects of sodium and tungsten promoters on Mg6MnO8-based core-shell redox catalysts for chemical looping-oxidative dehydrogenation of ethane[J]. ACS Catalysis, 2019, 9(4): 3174-3186. |
| 26 | Ding W X, Zhao K, Jiang S C, et al. Alkali-metal enhanced LaMnO3 perovskite oxides for chemical looping oxidative dehydrogenation of ethane[J]. Applied Catalysis A: General, 2021, 609(5): 117910-117918. |
| 27 | Tian X, Zheng C H, Li F X, et al. Co and Mo co-doped Fe2O3 for selective ethylene production via chemical looping oxidative dehydrogenation[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(23): 8002-8011. |
| 28 | Chan M S C, Baldovi H G, Dennis J S. Enhancing the capacity of oxygen carriers for selective oxidations through phase cooperation: bismuth oxide and ceria-zirconia[J]. Catalysis Science & Technology, 2018, 8(3): 887-897. |
| 29 | Wang C J, Yang B, Gu Q Q, et al. Near 100% ethene selectivity achieved by tailoring dual active sites to isolate dehydrogenation and oxidation[J]. Nature Communications, 2021, 12: 5447. |
| 30 | Wang T, Gao Y, Liu Y, et al. Core-shell Na2WO4/CuMn2O4 oxygen carrier with high oxygen capacity for chemical looping oxidative dehydrogenation of ethane[J]. Fuel, 2021, 303(1): 121286. |
| 31 | Chen S, Pei C L, Chang X, et al. Coverage-dependent behaviors of vanadium oxides for chemical looping oxidative dehydrogenation[J]. Angewandte Chemie International Edition, 2020, 59(49): 22072-22079. |
| 32 | Chen S, Zeng L, Mu R T, et al. Modulating lattice oxygen in dual-functional Mo-V-O mixed oxides for chemical looping oxidative dehydrogenation[J]. Journal of the American Chemical Society, 2019, 141(47): 18653-18657. |
| 33 | Jiang C G, Chang X, Wang X H, et al. Enhanced C—H bond activation by tuning the local environment of surface lattice oxygen of MoO3 [J]. Chemical Science, 2022, 13(25): 7468-7474. |
| 34 | Novotný P, Yusuf S, Li F X, et al. Oxidative dehydrogenation of ethane using MoO3/Fe2O3 catalysts in a cyclic redox mode[J]. Catalysis Today, 2018, 317(1): 50-55. |
| 35 | Li M, Gao Y F, Zhao K, et al. Mg-doped La1.6Sr0.4FeCoO6 for anaerobic oxidative dehydrogenation of ethane using surface-absorbed oxygen with tuned electronic structure[J]. Fuel Processing Technology, 2021, 216(1): 106771-106780. |
| 36 | Gao Y F, Wang X J, Corolla N, et al. Alkali metal halide-coated perovskite redox catalysts for anaerobic oxidative dehydrogenation of n-butane[J]. Science Advances, 2022, 8(30): eabo7343. |
| 37 | Gao Y F, Neal L M, Li F X. Li-promoted La x Sr2– x FeO4- δ core-shell redox catalysts for oxidative dehydrogenation of ethane under a cyclic redox scheme[J]. ACS Catalysis, 2016, 6(11): 7293-7302. |
| 38 | Gao Y F, Haeri F, He F, et al. Alkali metal-promoted La x Sr2- x FeO4- δ redox catalysts for chemical looping oxidative dehydrogenation of ethane[J]. ACS Catalysis, 2018, 8(3): 1757-1766. |
| 39 | Gao Y F, Wang X J, Liu J C, et al. A molten carbonate shell modified perovskite redox catalyst for anaerobic oxidative dehydrogenation of ethane[J]. Science Advances, 2020, 6(17): eaaz9339. |
| 40 | Kang K H, Kim T H, Choi W C, et al. Dehydrogenation of propane to propylene over CrO y -CeO2-K2O/γ-Al2O3 catalysts: effect of cerium content[J]. Catalysis Communications, 2015, 72(5): 68-72. |
| 41 | Tian X, Zheng C H, Zhao H B. Ce-modified SrFeO3- δ for ethane oxidative dehydrogenation coupled with CO2 splitting via a chemical looping scheme[J]. Applied Catalysis B: Environmental, 2022, 303: 120894. |
| 42 | Dudek R B, Li F X. Selective hydrogen combustion as an effective approach for intensified chemical production via the chemical looping strategy[J]. Fuel Processing Technology, 2021, 218: 106827. |
| 43 | Wang X J, Gao Y F, Krzystowczyk E, et al. High-throughput oxygen chemical potential engineering of perovskite oxides for chemical looping applications[J]. Energy & Environmental Science, 2022, 15(4): 1512-1528. |
| 44 | Fan L S. Chemical Looping Partial Oxidation: Gasification, Reforming, and Chemical Syntheses[M].Cambridge: Cambridge University Press, 2017: 57-60. |
| 45 | Liu H Y, Wang B J, Fan M H, et al. Study on carbon deposition associated with catalytic CH4 reforming by using density functional theory[J]. Fuel, 2013, 113: 712-718. |
| 46 | Wu T W, Yu Q B, Hou L M, et al. Selecting suitable oxygen carriers for chemical looping oxidative dehydrogenation of propane by thermodynamic method[J]. Journal of Thermal Analysis and Calorimetry, 2020, 140(4): 1837-1843. |
| 47 | Carrero C A, Schlögl R, Wachs I E, et al. Critical literature review of the kinetics for the oxidative dehydrogenation of propane over well-defined supported vanadium oxide catalysts[J]. ACS Catalysis, 2014, 4(10): 3357-3380. |
| 48 | Zhao Z J, Chiu C C, Gong J L. Molecular understandings on the activation of light hydrocarbons over heterogeneous catalysts[J]. Chemical Science, 2015, 6(8): 4403-4425. |
| 49 | Zhao Z J, Liu S H, Zha S J, et al. Theory-guided design of catalytic materials using scaling relationships and reactivity descriptors[J]. Nature Reviews Materials, 2019, 4(12): 792-804. |
| 50 | Latimer A A, Kulkarni A R, Aljama H, et al. Understanding trends in C—H bond activation in heterogeneous catalysis[J]. Nature Materials, 2017, 16(2): 225-229. |
| 51 | Dickens C F, Montoya J H, Kulkarni A R, et al. An electronic structure descriptor for oxygen reactivity at metal and metal-oxide surfaces[J]. Surface Science, 2019, 681: 122-129. |
| 52 | Xiong C Y, Chen S, Yang P P, et al. Structure-performance relationships for propane dehydrogenation over aluminum supported vanadium oxide[J]. ACS Catalysis, 2019, 9(7): 5816-5827. |
| 53 | Jiang C G, Song H B, Sun G, et al. Data-driven interpretable descriptors for the structure-activity relationship of surface lattice oxygen on doped vanadium oxides[J]. Angewandte Chemie International Edition, 2022, 134(35): e202206758. |
| 54 | Idriss H, Barteau M A. Active sites on oxides: from single crystals to catalysts[M]// Advances in Catalysis. New York: Academic Press, 2000: 261-331. |
| 55 | Hao F, Gao Y F, Neal L, et al. Sodium tungstate-promoted CaMnO3 as an effective, phase-transition redox catalyst for redox oxidative cracking of cyclohexane[J]. Journal of Catalysis, 2020, 385: 213-223. |
| 56 | Tian X, Dudek R B, Gao Y F, et al. Redox oxidative cracking of n-hexane with Fe-substituted barium hexaaluminates as redox catalysts[J]. Catalysis Science & Technology, 2019, 9(9): 2211-2220. |
| 57 | Tsikoyiannis J G, Stern D L, Grasselli R K. Metal oxides as selective hydrogen combustion (SHC) catalysts and their potential in light paraffin dehydrogenation[J]. Journal of Catalysis, 1999, 184(1): 77-86. |
| 58 | Wang J, Song Y H, Liu Z T, et al. Active and selective nature of supported CrO x for the oxidative dehydrogenation of propane with carbon dioxide[J]. Applied Catalysis B: Environmental, 2021, 297(15): 120400. |
| 59 | Abello M C, Gomez M F, Ferretti O. Mo/γ-Al2O3 catalysts for the oxidative dehydrogenation of propane: effect of Mo loading[J]. Applied Catalysis A: General, 2001, 207(1/2): 421-431. |
| 60 | Khodakov A, Yang J, Su S, et al. Structure and properties of vanadium oxide-zirconia catalysts for propane oxidative dehydrogenation[J]. Journal of Catalysis, 1998, 177(2): 343-351. |
| 61 | Fukudome K, Ikenaga N O, Miyake T, et al. Oxidative dehydrogenation of propane using lattice oxygen of vanadium oxides on silica[J]. Catalysis Science & Technology, 2011, 1(6): 987-998. |
| 62 | Wu T W, Yu Q B, Roghair I, et al. Chemical looping oxidative dehydrogenation of propane: a comparative study of Ga-based, Mo-based, V-based oxygen carriers[J]. Chemical Engineering and Processing-Process Intensification, 2020, 157: 108137. |
| 63 | Novotný P, Yusuf S, Li F X, et al. MoO3/Al2O3 catalysts for chemical-looping oxidative dehydrogenation of ethane[J]. The Journal of Chemical Physics, 2020, 152(4): 044713. |
| 64 | Wu T W, Yu Q B, Wang K, et al. Development of V-based oxygen carriers for chemical looping oxidative dehydrogenation of propane[J]. Catalysts, 2021, 11(1): 119. |
| 65 | Sim S, Gong S J, Bae J, et al. Chromium oxide supported on Zr modified alumina for stable and selective propane dehydrogenation in oxygen free moving bed process[J]. Molecular Catalysis, 2017, 436: 164-173. |
| 66 | Yu Z L, Yang Y Y, Yang S, et al. Iron-based oxygen carriers in chemical looping conversions: a review[J]. Carbon Resources Conversion, 2019, 2(1): 23-34. |
| 67 | Grant J T, Venegas J M, McDermott W P, et al. Aerobic oxidations of light alkanes over solid metal oxide catalysts[J]. Chemical Reviews, 2018, 118(5): 2769-2815. |
| 68 | Zheng Y S, Zhang M, Li Q, et al. Electronic origin of oxygen transport behavior in La-based perovskites: a density functional theory study[J]. The Journal of Physical Chemistry C, 2019, 123(1): 275-290. |
| 69 | Wang H F, Gong X Q, Guo Y L, et al. A model to understand the oxygen vacancy formation in Zr-doped CeO2: electrostatic interaction and structural relaxation[J]. The Journal of Physical Chemistry C, 2009, 113(23): 10229-10232. |
| 70 | Jiang X, Sharma L, Fung V, et al. Oxidative dehydrogenation of propane to propylene with soft oxidants via heterogeneous catalysis[J]. ACS Catalysis, 2021, 11(4): 2182-2234. |
| 71 | Chang H, Bjørgum E, Mihai O, et al. Effects of oxygen mobility in La-Fe-based perovskites on the catalytic activity and selectivity of methane oxidation[J]. ACS Catalysis, 2020, 10(6): 3707-3719. |
| 72 | Dai X P, Li R J, Yu C C, et al. Unsteady-state direct partial oxidation of methane to synthesis gas in a fixed-bed reactor using AFeO3 (A= La, Nd, Eu) perovskite-type oxides as oxygen storage[J]. The Journal of Physical Chemistry B, 2006, 110(45): 22525-22531. |
| 73 | Qin L, Cheng Z, Fan J A, et al. Nanostructure formation mechanism and ion diffusion in iron-titanium composite materials with chemical looping redox reactions[J]. Journal of Materials Chemistry A, 2015, 3(21): 11302-11312. |
| 74 | Shafiefarhood A, Galinsky N, Huang Y, et al. Fe2O3@La x Sr1- x FeO3 core-shell redox catalyst for methane partial oxidation[J]. ChemCatChem, 2014, 6(3): 790-799. |
| 75 | Hu J W, Galvita V V, Poelman H, et al. A core-shell structured Fe2O3/ZrO2@ZrO2 nanomaterial with enhanced redox activity and stability for CO2 conversion[J]. Journal of CO2 Utilization, 2017, 17: 20-31. |
| 76 | Liu L, Zachariah M R. Enhanced performance of alkali metal doped Fe2O3 and Fe2O3/Al2O3 composites as oxygen carrier material in chemical looping combustion[J]. Energy & Fuels, 2013, 27(8): 4977-4983. |
| 77 | Imtiaz Q, Kurlov A, Rupp J L M, et al. Highly efficient oxygen-storage material with intrinsic coke resistance for chemical looping combustion-based CO2 capture[J]. ChemSusChem, 2015, 8(12): 2055-2065. |
| 78 | Hossain M M, de Lasa H I. Reactivity and stability of Co-Ni/Al2O3 oxygen carrier in multicycle CLC[J]. AIChE Journal, 2007, 53(7): 1817-1829. |
| 79 | Zhao H B, Liu L M, Wang B W, et al. Sol-gel-derived NiO/NiAl2O4 oxygen carriers for chemical-looping combustion by coal char[J]. Energy & Fuels, 2008, 22(2): 898-905. |
| 80 | Lambert A, Delquié C, Clémeneçon I, et al. Synthesis and characterization of bimetallic Fe/Mn oxides for chemical looping combustion[J]. Energy Procedia, 2009, 1(1): 375-381. |
| 81 | Wang S Z, Wang G X, Jiang F, et al. Chemical looping combustion of coke oven gas by using Fe2O3/CuO with MgAl2O4 as oxygen carrier[J]. Energy & Environmental Science, 2010, 3(9): 1353-1360. |
| 82 | de Diego L F, Garcı́a-Labiano F, Adánez J, et al. Development of Cu-based oxygen carriers for chemical-looping combustion[J]. Fuel, 2004, 83(13): 1749-1757. |
| 83 | Zhao H B, Mei D F, Ma J, et al. Comparison of preparation methods for iron-alumina oxygen carrier and its reduction kinetics with hydrogen in chemical looping combustion[J]. Asia-Pacific Journal of Chemical Engineering, 2014, 9(4): 610-622. |
| 84 | Liu Y K, Long Y H, Tang Y Q, et al. Effect of preparation method on the structural characteristics of NiO-ZrO2 oxygen carriers for chemical-looping combustion[J]. Chemical Research in Chinese Universities, 2019, 35(6): 1024-1031. |
| 85 | Marin G B, Galvita V V, Yablonsky G S. Kinetics of chemical processes: from molecular to industrial scale[J]. Journal of Catalysis, 2021, 404: 745-759. |
| 86 | Iliuta I, Tahoces R, Patience G S, et al. Chemical-looping combustion process: kinetics and mathematical modeling[J]. AIChE Journal, 2010, 56(4): 1063-1079. |
| 87 | Khawam A, Flanagan D R. Solid-state kinetic models: basics and mathematical fundamentals[J]. The Journal of Physical Chemistry B, 2006, 110(35): 17315-17328. |
| 88 | Hancock J D, Sharp J H. Method of comparing solid‐state kinetic data and its application to the decomposition of kaolinite, brucite, and BaCO3 [J]. Journal of the American Ceramic Society, 1972, 55(2): 74-77. |
| 89 | Tian Y, Dudek R B, Westmoreland P R, et al. Effect of sodium tungstate promoter on the reduction kinetics of CaMn0.9Fe0.1O3 for chemical looping-oxidative dehydrogenation of ethane[J]. Chemical Engineering Journal, 2020, 398(15): 125583. |
| 90 | Chen Y Y, Nadgouda S, Shah V, et al. Oxidation kinetic modelling of Fe-based oxygen carriers for chemical looping applications: impact of the topochemical effect[J]. Applied Energy, 2020, 279(1): 115701. |
| 91 | Riley J, Siriwardane R, Tian H J, et al. Experimental and kinetic analysis for particle scale modeling of a CuO-Fe2O3-Al2O3 oxygen carrier during reduction with H2 in chemical looping combustion applications[J]. Applied Energy, 2018, 228(15): 1515-1530. |
| 92 | Riley J, Siriwardane R, Tian H J, et al. Particle scale modeling of CuFeAlO4 during reduction with CO in chemical looping applications[J]. Applied Energy, 2019, 251(1): 113178. |
| 93 | Li Z, Cai J, Liu L. A first-principles microkinetic rate equation theory for heterogeneous reactions: application to reduction of Fe2O3 in chemical looping[J]. Industrial & Engineering Chemistry Research, 2021, 60(43): 15514-15524. |
| 94 | Sokolov S, Bychkov V Y, Stoyanova M, et al. Effect of VO x species and support on coke formation and catalyst stability in nonoxidative propane dehydrogenation[J]. ChemCatChem, 2015, 7(11): 1691-1700. |
| 95 | Al-Ghamdi S A, Hossain M M, de Lasa H I. Kinetic modeling of ethane oxidative dehydrogenation over VO x /Al2O3 catalyst in a fluidized-bed riser simulator[J]. Industrial & Engineering Chemistry Research, 2013, 52(14): 5235-5244. |
| 96 | Hossain M M. Kinetics of oxidative dehydrogenation of propane to propylene using lattice oxygen of VO x /CaO/γ-Al2O3 catalysts[J]. Industrial & Engineering Chemistry Research, 2017, 56(15): 4309-4318. |
| 97 | Fan L S, Zeng L, Wang W, et al. Chemical looping processes for CO2 capture and carbonaceous fuel conversion-prospect and opportunity[J]. Energy & Environmental Science, 2012, 5(6): 7254-7280. |
| 98 | Joshi A, Shah V, Mohapatra P, et al. Chemical looping—a perspective on the next-gen technology for efficient fossil fuel utilization[J]. Advances in Applied Energy, 2021, 3(25): 100044. |
| 99 | Song T, Shen L H. Review of reactor for chemical looping combustion of solid fuels[J]. International Journal of Greenhouse Gas Control, 2018, 76: 92-110. |
| 100 | 刘一君, 陈时熠, 胡骏, 等. 化学链反应器研究进展[J]. 化工学报, 2021, 72(5): 2392-2412. |
| Liu Y J, Chen S Y, Hu J, et al. Review on reactors for chemical looping process[J]. CIESC Journal, 2021, 72(5): 2392-2412. | |
| 101 | Darvishi A, Davand R, Khorasheh F, et al. Modeling-based optimization of a fixed-bed industrial reactor for oxidative dehydrogenation of propane[J]. Chinese Journal of Chemical Engineering, 2016, 24(5): 612-622. |
| 102 | Rostom S, de Lasa H. Propane oxidative dehydrogenation on vanadium-based catalysts under oxygen-free atmospheres[J]. Catalysts, 2020, 10(4): 418. |
| 103 | Fattahi M, Kazemeini M, Khorasheh F, et al. Fixed‐bed multi‐tubular reactors for oxidative dehydrogenation in ethylene process[J]. Chemical Engineering & Technology, 2013, 36(10): 1691-1700. |
| 104 | Kotanjac Ž S, van Sint Annaland M, Kuipers J A M. A packed bed membrane reactor for the oxidative dehydrogenation of propane on a Ga2O3/MoO3 based catalyst[J]. Chemical Engineering Science, 2010, 65(1): 441-445. |
| 105 | Che-Galicia G, Ruiz-Martínez R S, López-Isunza F, et al. Modeling of oxidative dehydrogenation of ethane to ethylene on a MoVTeNbO/TiO2 catalyst in an industrial-scale packed bed catalytic reactor[J]. Chemical Engineering Journal, 2015, 280(15): 682-694. |
| 106 | Chu B Z, Truter L, Alexander Nijhuis T A, et al. Oxidative dehydrogenation of ethane to ethylene over phase-pure M1MoVNbTeO x catalysts in a micro-channel reactor[J]. Catalysis Science & Technology, 2015, 5(5): 2807-2813. |
| 107 | Rostom S, de Lasa H I. Propane oxidative dehydrogenation using consecutive feed injections and fluidizable VO x /γ-Al2O3 and VO x /ZrO2-γAl2O3 catalysts[J]. Industrial & Engineering Chemistry Research, 2017, 56(45): 13109-13124. |
| 108 | 曾亮, 巩金龙. 化学链重整直接制氢技术进展[J]. 化工学报, 2015, 66(8): 2854-2862. |
| Zeng L, Gong J L, Advances in chemical looping reforming for direct hydrogen production[J]. CIESC Journal, 2015, 66(8): 2854-2862. | |
| 109 | Zaynali Y, Alavi-Amleshi S M. Comparative study of propane oxidative dehydrogenation in fluidized and fixed bed reactor[J]. Particulate Science and Technology, 2017, 35(6): 667-673. |
| 110 | Argyle M, Bartholomew C. Heterogeneous catalyst deactivation and regeneration: a review[J]. Catalysts, 2015, 5(1): 145-269. |
| 111 | Shen Q, Huang F, Tian M, et al. Effect of regeneration period on the selectivity of synthesis gas of Ba-hexaaluminates in chemical looping partial oxidation of methane[J]. ACS Catalysis, 2018, 9(1): 722-731. |
| 112 | Park C, Hsieh T L, Pottimurthy Y, et al. Design and operations of a 15 kWth subpilot unit for the methane-to-syngas chemical looping process with CO2 utilization[J]. Industrial & Engineering Chemistry Research, 2019, 59(15): 6886-6899. |
| 113 | 韦迪, 喻俊杰, 邵媛媛, 等. 丙烷化学链氧化脱氢过程模拟与能耗分析[J]. 石油学报 (石油加工), 2020, 36(6): 1361-1369. |
| Wei D, Yu J J, Shao Y Y, et al. Process simulation and energy consumption analysis of chemical looping oxidative dehydrogenation of propane[J]. Acta Petrolei Sinica(Petroleum Processing Section), 2020, 36(6): 1361-1369. | |
| 114 | Rostom S, de Lasa H. Downer fluidized bed reactor modeling for catalytic propane oxidative dehydrogenation with high propylene selectivity[J]. Chemical Engineering and Processing-Process Intensification, 2019, 137: 87-99. |
| 115 | Brody L, Neal L, Liu J C, et al. Autothermal chemical looping oxidative dehydrogenation of ethane: redox catalyst performance, longevity, and process analysis[J]. Energy & Fuels, 2022, 36(17): 9736-9744. |
| 116 | Yusuf S, Haribal V, Jackson D, et al. Mixed iron-manganese oxides as redox catalysts for chemical looping-oxidative dehydrogenation of ethane with tailorable heat of reactions[J]. Applied Catalysis B: Environmental, 2019, 257(15): 117885. |
| 117 | Yüzbasi N S, Abdala P M, Imtiaz Q, et al. The effect of copper on the redox behaviour of iron oxide for chemical-looping hydrogen production probed by in situ X-ray absorption spectroscopy[J]. Physical Chemistry Chemical Physics, 2018, 20(18): 12736-12745. |
| 118 | Huang Z, Gao N, Lin Y, et al. Exploring the migration and transformation of lattice oxygen during chemical looping with NiFe2O4 oxygen carrier[J]. Chemical Engineering Journal, 2022, 429(1): 132064. |
| 119 | Zichittella G, Polyhach Y, Tschaggelar R, et al. Quantification of redox sites during catalytic propane oxychlorination by operando EPR spectroscopy[J]. Angewandte Chemie International Edition, 2021, 60(7): 3596-3602. |
| 120 | Docherty S R, Rochlitz L, Payard P A, et al. Heterogeneous alkane dehydrogenation catalysts investigated via a surface organometallic chemistry approach[J]. Chemical Society Reviews, 2021, 50(9): 5806-5822. |
| [1] | 周小文, 杜杰, 张战国, 许光文. 基于甲烷脉冲法的Fe2O3-Al2O3载氧体还原特性研究[J]. 化工学报, 2023, 74(6): 2611-2623. |
| [2] | 袁妮妮, 郭拓, 白红存, 何育荣, 袁永宁, 马晶晶, 郭庆杰. 化学链燃烧过程Fe2O3/Al2O3载氧体表面CH4反应:ReaxFF-MD模拟[J]. 化工学报, 2022, 73(9): 4054-4061. |
| [3] | 郭丹, 方雨洁, 许一寒, 李致远, 黄守莹, 王胜平, 马新宾. 乙烷和二氧化碳催化转化的研究进展[J]. 化工学报, 2022, 73(8): 3406-3416. |
| [4] | 王保文, 张港, 刘同庆, 李炜光, 王梦家, 林德顺, 马晶晶. CeO2/CuFe2O4氧载体CH4化学链重整耦合CO2热催化还原研究[J]. 化工学报, 2022, 73(12): 5414-5426. |
| [5] | 赵旭, 卜昌盛, 王昕晔, 张鑫, 程晓磊, 王乃继, 朴桂林. 铁基载氧体辅助无烟煤焦富氧燃烧动力学分析[J]. 化工学报, 2022, 73(1): 384-392. |
| [6] | 赵林洲, 郑燕娥, 李孔斋, 王亚明, 蒋丽红, 范浩熙, 王雅静, 祝星, 魏永刚. Ce1-xNixOy氧载体在化学链甲烷重整耦合CO2还原中的应用[J]. 化工学报, 2021, 72(8): 4371-4380. |
| [7] | 刘一君, 陈时熠, 胡骏, 周威, 向文国. 化学链反应器研究进展[J]. 化工学报, 2021, 72(5): 2392-2412. |
| [8] | 蔡润夏, 李凡星. 复杂氧化物载氧体的调变策略及在过程强化中的应用[J]. 化工学报, 2021, 72(12): 6122-6130. |
| [9] | 孙焱, 沈晓文, 许细薇, 蒋恩臣, 刘雪聪. 化学链耦合催化重整热解生物油制备合成气[J]. 化工学报, 2021, 72(11): 5607-5619. |
| [10] | 丁鼎, 陆文多, 侯璐, 陆安慧. 纤维状BPO4/SiO2催化剂的制备及其丙烷氧化脱氢性能[J]. 化工学报, 2021, 72(11): 5590-5597. |
| [11] | 仉利, 姚宗路, 赵立欣, 李志合, 易维明, 付鹏, 袁超. 生物质热化学转化提质及其催化剂研究进展[J]. 化工学报, 2020, 71(8): 3416-3427. |
| [12] | 袁妮妮,白红存,安梅,胡修德,郭庆杰. 化学链过程中Cu低浓度掺杂改性Fe-基载氧体反应性能:实验与理论模拟[J]. 化工学报, 2020, 71(11): 5294-5302. |
| [13] | 苏迎辉,郑浩,张磊,曾亮. LaMn1-x-yFexCoyO3-δ钙钛矿载氧体用于化学链部分氧化[J]. 化工学报, 2020, 71(11): 5265-5277. |
| [14] | 吴志强, 张博, 杨伯伦. 生物质化学链转化技术研究进展[J]. 化工学报, 2019, 70(8): 2835-2853. |
| [15] | 王璐璐, 宋涛, 张将, 段媛媛, 沈来宏. 10MWth高硫石油焦化学链气化制合成气耦合硫磺回收新系统模拟研究[J]. 化工学报, 2019, 70(6): 2279-2288. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号