化工学报 ›› 2021, Vol. 72 ›› Issue (8): 3997-4008.DOI: 10.11949/0438-1157.20201933
郭盛争1( ),吴送姑1(
),吴送姑1( ),苏鑫1,高伟2,牛志平2,龚俊波1
),苏鑫1,高伟2,牛志平2,龚俊波1
收稿日期:2020-12-29
修回日期:2021-04-16
出版日期:2021-08-05
发布日期:2021-08-05
通讯作者:
吴送姑
作者简介:郭盛争(1994—),男,硕士研究生,基金资助:
Shengzheng GUO1( ),Songgu WU1(
),Songgu WU1( ),Xin SU1,Wei GAO2,Zhiping NIU2,Junbo GONG1
),Xin SU1,Wei GAO2,Zhiping NIU2,Junbo GONG1
Received:2020-12-29
Revised:2021-04-16
Online:2021-08-05
Published:2021-08-05
Contact:
Songgu WU
摘要:
莱鲍迪苷A是极为重要的新型甜味剂,由于缺乏合理的溶解度数据且其成核机理不明确,导致现存的结晶工艺得到的结晶产品存在细晶多、粒度分布不均等问题。为此,首先利用激光动态法测定了莱鲍迪苷A在甲醇-水、乙醇-水、正丙醇-水、丙酮-水中的溶解度及在甲醇-水中的介稳区宽度,并利用Wilson方程对溶解度进行模型验证;研究了溶剂组成、饱和温度、搅拌速率与冷却速率对介稳区宽度的影响,研究表明,莱鲍迪苷A的介稳区宽度随溶剂中甲醇含量与冷却速率的增加而变宽,随饱和温度与搅拌速率的增加而变窄;基于经典成核理论与相关模型,计算了莱鲍迪苷A的固液界面能γ与临界Gibbs自由能
中图分类号:
郭盛争, 吴送姑, 苏鑫, 高伟, 牛志平, 龚俊波. 莱鲍迪苷A溶解度与介稳区宽度的测定及其结晶过程研究[J]. 化工学报, 2021, 72(8): 3997-4008.
Shengzheng GUO, Songgu WU, Xin SU, Wei GAO, Zhiping NIU, Junbo GONG. Determination of solubility and metastable zone width of rebaudioside A and study on its crystallization process[J]. CIESC Journal, 2021, 72(8): 3997-4008.
| 药品名称 | 质量分数 | 摩尔质量/ (g·mol-1) | 来源 |
|---|---|---|---|
| 莱鲍迪苷A | ≥0.980 | 967.01 | 晨光生物科技股份有限公司 |
| 甲醇 | ≥0.998 | 32.04 | 天津市康科德科技有限公司 |
| 乙醇 | ≥0.997 | 46.07 | 天津市康科德科技有限公司 |
| 正丙醇 | ≥0.995 | 60.1 | 天津市康科德科技有限公司 |
| 丙酮 | ≥0.995 | 58.08 | 天津福晨化学试剂有限公司 |
| 去离子水 | ≥0.995 | 18.02 | 实验室自制 |
表1 实验试剂
Table 1 The list of chemicals
| 药品名称 | 质量分数 | 摩尔质量/ (g·mol-1) | 来源 |
|---|---|---|---|
| 莱鲍迪苷A | ≥0.980 | 967.01 | 晨光生物科技股份有限公司 |
| 甲醇 | ≥0.998 | 32.04 | 天津市康科德科技有限公司 |
| 乙醇 | ≥0.997 | 46.07 | 天津市康科德科技有限公司 |
| 正丙醇 | ≥0.995 | 60.1 | 天津市康科德科技有限公司 |
| 丙酮 | ≥0.995 | 58.08 | 天津福晨化学试剂有限公司 |
| 去离子水 | ≥0.995 | 18.02 | 实验室自制 |
| 仪器名称 | 型号/规格 | 生产厂家 |
|---|---|---|
| 分析天平 | AL204 | 瑞士Mettler-Toledo公司 |
| 水浴恒温槽 | CF41 | 优莱博仪器有限公司 |
| 夹套结晶器 | 200 ml | 天津易普佳玻璃仪器有限 公司 |
| 数显机械搅拌 | ZNCL-BS140 | 天津星科仪器有限公司 |
| Pixact在线颗粒成像系统 | PCM | 北京海菲尔格科技有限公司 |
| 激光发射仪 | JDW3-200 | 北京大学物理系 |
| 激光测量仪 | JG2 | 北京大学物理系 |
表2 实验设备
Table 2 The list of experimental facilities
| 仪器名称 | 型号/规格 | 生产厂家 |
|---|---|---|
| 分析天平 | AL204 | 瑞士Mettler-Toledo公司 |
| 水浴恒温槽 | CF41 | 优莱博仪器有限公司 |
| 夹套结晶器 | 200 ml | 天津易普佳玻璃仪器有限 公司 |
| 数显机械搅拌 | ZNCL-BS140 | 天津星科仪器有限公司 |
| Pixact在线颗粒成像系统 | PCM | 北京海菲尔格科技有限公司 |
| 激光发射仪 | JDW3-200 | 北京大学物理系 |
| 激光测量仪 | JG2 | 北京大学物理系 |
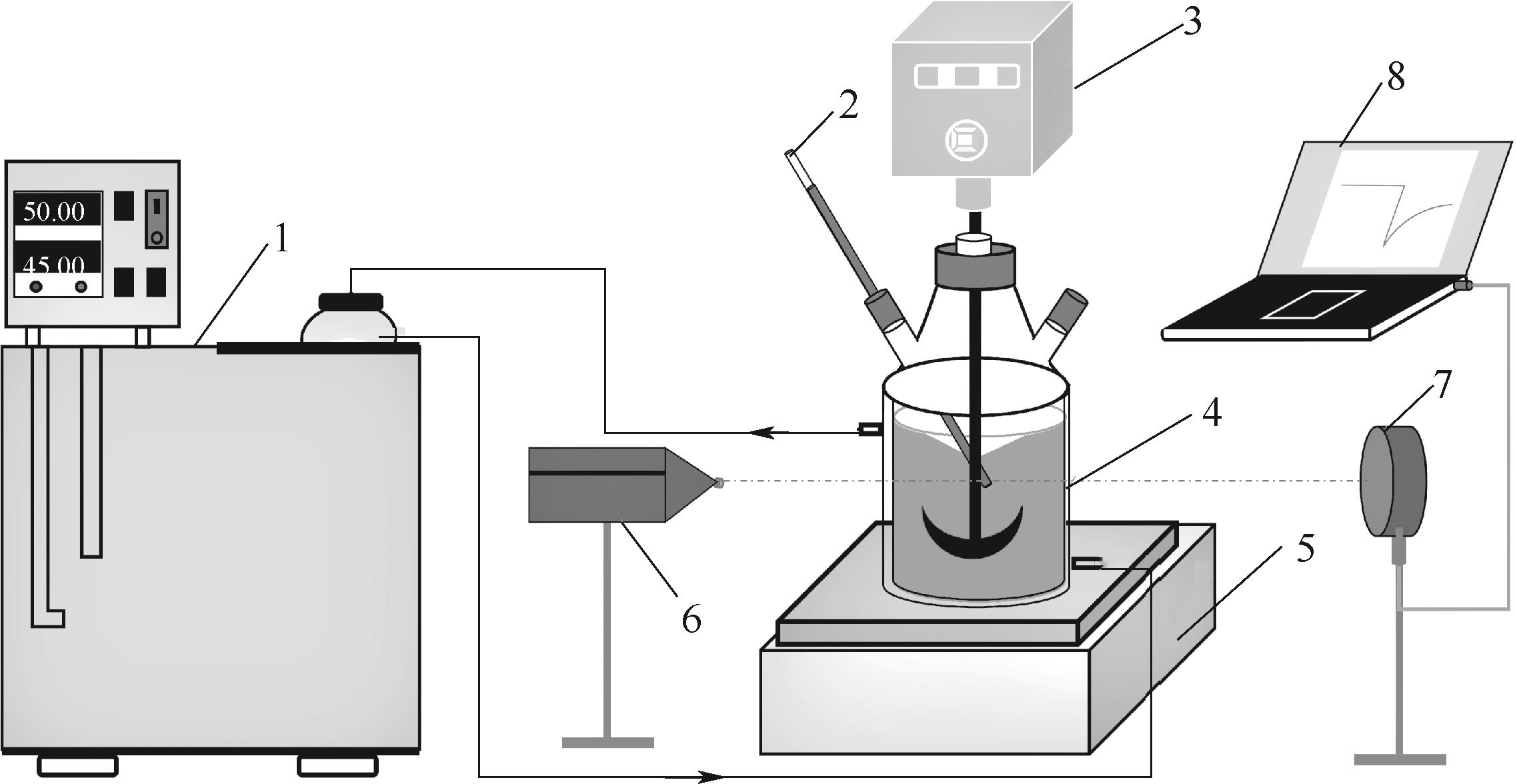
图1 莱鲍迪苷A溶解度与介稳区测定装置图1—水浴恒温槽;2—水银温度计; 3—数显机械搅拌; 4—夹套结晶器; 5—升降台; 6—激光发射器; 7—激光接收器; 8—激光记录仪
Fig.1 Sketch of the apparatus for measurement of solubility and metastable zone of rebaudioside A
| Solvents | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 283.15 K | 288.15 K | 293.15 K | 298.15 K | 303.15 K | 308.15 K | 313.15 K | 318.15 K | 323.15 K | 328.15 K | |
| methanol-water | ||||||||||
| xa= 0.4① | 0.312 | 0.450 | 0.597 | 0.861 | 1.151 | 1.483 | 1.938 | 2.653 | 3.731 | 4.793 |
| xa = 0.5 | 0.279 | 0.392 | 0.496 | 0.655 | 0.886 | 1.196 | 1.555 | 2.175 | 2.915 | 3.805 |
| xa = 0.6 | 0.229 | 0.306 | 0.417 | 0.561 | 0.756 | 0.993 | 1.272 | 1.632 | 2.270 | 2.772 |
| xa = 0.7 | 0.203 | 0.263 | 0.344 | 0.446 | 0.580 | 0.723 | 0.934 | 1.189 | 1.461 | 1.810 |
| ethanol-water | ||||||||||
| xb = 0.5 | 1.868 | 2.239 | 2.640 | 2.962 | 3.556 | 4.117 | 4.772 | 5.474 | 6.271 | 7.154 |
| xb = 0.6 | 1.338 | 1.573 | 1.822 | 2.020 | 2.378 | 2.711 | 3.094 | 3.498 | 3.950 | 4.444 |
| xb = 0.7 | 0.638 | 0.751 | 0.891 | 1.051 | 1.253 | 1.463 | 1.661 | 1.955 | 2.230 | 2.555 |
| n-propanol-water | ||||||||||
| xc = 0.4 | 1.931 | 2.296 | 2.756 | 3.287 | 3.867 | 4.574 | 5.425 | 6.392 | 7.330 | 8.475 |
| xc = 0.5 | 0.945 | 1.147 | 1.407 | 1.713 | 2.149 | 2.472 | 2.974 | 3.452 | 4.194 | 5.090 |
| xc = 0.6 | 0.471 | 0.555 | 0.650 | 0.804 | 1.002 | 1.237 | 1.430 | 1.773 | 2.091 | 2.591 |
| acetone-water | ||||||||||
| xd = 0.3 | 0.344 | 0.463 | 0.619 | 0.820 | 1.073 | 1.340 | 1.819 | 2.294 | 2.909 | 3.663 |
| xd = 0.4 | 0.240 | 0.319 | 0.420 | 0.583 | 0.671 | 0.875 | 1.119 | 1.406 | 1.792 | 2.182 |
| xd = 0.6 | 0.036 | 0.053 | 0.078 | 0.123 | 0.157 | 0.222 | 0.299 | 0.416 | 0.618 | 0.766 |
表3 莱鲍迪苷A在不同二元混合溶剂中的摩尔溶解度
Table 3 Experimental solubility x1exp of rebaudioside A in different binary mixed solvents at different temperatures (P = 0.1 MPa)
| Solvents | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 283.15 K | 288.15 K | 293.15 K | 298.15 K | 303.15 K | 308.15 K | 313.15 K | 318.15 K | 323.15 K | 328.15 K | |
| methanol-water | ||||||||||
| xa= 0.4① | 0.312 | 0.450 | 0.597 | 0.861 | 1.151 | 1.483 | 1.938 | 2.653 | 3.731 | 4.793 |
| xa = 0.5 | 0.279 | 0.392 | 0.496 | 0.655 | 0.886 | 1.196 | 1.555 | 2.175 | 2.915 | 3.805 |
| xa = 0.6 | 0.229 | 0.306 | 0.417 | 0.561 | 0.756 | 0.993 | 1.272 | 1.632 | 2.270 | 2.772 |
| xa = 0.7 | 0.203 | 0.263 | 0.344 | 0.446 | 0.580 | 0.723 | 0.934 | 1.189 | 1.461 | 1.810 |
| ethanol-water | ||||||||||
| xb = 0.5 | 1.868 | 2.239 | 2.640 | 2.962 | 3.556 | 4.117 | 4.772 | 5.474 | 6.271 | 7.154 |
| xb = 0.6 | 1.338 | 1.573 | 1.822 | 2.020 | 2.378 | 2.711 | 3.094 | 3.498 | 3.950 | 4.444 |
| xb = 0.7 | 0.638 | 0.751 | 0.891 | 1.051 | 1.253 | 1.463 | 1.661 | 1.955 | 2.230 | 2.555 |
| n-propanol-water | ||||||||||
| xc = 0.4 | 1.931 | 2.296 | 2.756 | 3.287 | 3.867 | 4.574 | 5.425 | 6.392 | 7.330 | 8.475 |
| xc = 0.5 | 0.945 | 1.147 | 1.407 | 1.713 | 2.149 | 2.472 | 2.974 | 3.452 | 4.194 | 5.090 |
| xc = 0.6 | 0.471 | 0.555 | 0.650 | 0.804 | 1.002 | 1.237 | 1.430 | 1.773 | 2.091 | 2.591 |
| acetone-water | ||||||||||
| xd = 0.3 | 0.344 | 0.463 | 0.619 | 0.820 | 1.073 | 1.340 | 1.819 | 2.294 | 2.909 | 3.663 |
| xd = 0.4 | 0.240 | 0.319 | 0.420 | 0.583 | 0.671 | 0.875 | 1.119 | 1.406 | 1.792 | 2.182 |
| xd = 0.6 | 0.036 | 0.053 | 0.078 | 0.123 | 0.157 | 0.222 | 0.299 | 0.416 | 0.618 | 0.766 |
| x | V2/(cm3·mol-1) | Δg12/ (J·mol-1) | Δg21/ (J·mol-1) | RMSD | R2 |
|---|---|---|---|---|---|
| xa =0.4 | 27.52 | -7803 | 7105 | 6.74×10-5 | 0.9984 |
| xa =0.5 | 29.79 | -7820 | 7533 | 6.70×10-5 | 0.9966 |
| xa =0.6 | 32.01 | -7833 | 8181 | 3.54×10-5 | 0.9993 |
| xa =0.7 | 34.19 | -8569 | 12530 | 8.05×10-6 | 0.9998 |
| xb =0.5 | 37.88 | -13094 | 89986 | 7.12×10-4 | 0.9992 |
| xb =0.6 | 41.81 | -11714 | 87851 | 6.28×10-4 | 0.9992 |
| xb =0.7 | 45.74 | -9892 | 88789 | 3.16×10-4 | 0.9997 |
| xc =0.4 | 39.83 | -3269 | 91951 | 5.76×10-4 | 0.9997 |
| xc =0.5 | 45.33 | -11369 | 88705 | 2.61×10-4 | 0.9988 |
| xc =0.6 | 50.92 | -9237 | 91732 | 1.47×10-4 | 0.9962 |
| xd =0.3 | 35.83 | -8949 | 8691 | 2.02×10-5 | 0.9997 |
| xd =0.4 | 41.76 | -8562 | 12163 | 1.84×10-5 | 0.9983 |
| xd =0.6 | 53.37 | -423 | 4922 | 1.47×10-5 | 0.9982 |
表4 基于Wilson方程拟合得到的参数结果
Table 4 The parameter results based on the fitting of Wilson equation
| x | V2/(cm3·mol-1) | Δg12/ (J·mol-1) | Δg21/ (J·mol-1) | RMSD | R2 |
|---|---|---|---|---|---|
| xa =0.4 | 27.52 | -7803 | 7105 | 6.74×10-5 | 0.9984 |
| xa =0.5 | 29.79 | -7820 | 7533 | 6.70×10-5 | 0.9966 |
| xa =0.6 | 32.01 | -7833 | 8181 | 3.54×10-5 | 0.9993 |
| xa =0.7 | 34.19 | -8569 | 12530 | 8.05×10-6 | 0.9998 |
| xb =0.5 | 37.88 | -13094 | 89986 | 7.12×10-4 | 0.9992 |
| xb =0.6 | 41.81 | -11714 | 87851 | 6.28×10-4 | 0.9992 |
| xb =0.7 | 45.74 | -9892 | 88789 | 3.16×10-4 | 0.9997 |
| xc =0.4 | 39.83 | -3269 | 91951 | 5.76×10-4 | 0.9997 |
| xc =0.5 | 45.33 | -11369 | 88705 | 2.61×10-4 | 0.9988 |
| xc =0.6 | 50.92 | -9237 | 91732 | 1.47×10-4 | 0.9962 |
| xd =0.3 | 35.83 | -8949 | 8691 | 2.02×10-5 | 0.9997 |
| xd =0.4 | 41.76 | -8562 | 12163 | 1.84×10-5 | 0.9983 |
| xd =0.6 | 53.37 | -423 | 4922 | 1.47×10-5 | 0.9982 |
| T0/K | Slope | Intercept | m | K | R2 |
|---|---|---|---|---|---|
| 313.15 | 0.354 | -3.880 | 2.83 | 9.46×1026 | 0.9997 |
| 318.15 | 0.301 | -4.164 | 3.32 | 4.17×1027 | 0.9982 |
| 323.15 | 0.277 | -4.366 | 3.61 | 1.32×1028 | 0.9974 |
| 328.15 | 0.255 | -4.472 | 3.93 | 3.38×1028 | 0.9976 |
表5 基于自洽Nyvlt介稳区方程拟合的相关系数及动力学参数
Table 5 Regression cofficient and kinetics parameters calculated by self-consistent Nyvlt-like equation
| T0/K | Slope | Intercept | m | K | R2 |
|---|---|---|---|---|---|
| 313.15 | 0.354 | -3.880 | 2.83 | 9.46×1026 | 0.9997 |
| 318.15 | 0.301 | -4.164 | 3.32 | 4.17×1027 | 0.9982 |
| 323.15 | 0.277 | -4.366 | 3.61 | 1.32×1028 | 0.9974 |
| 328.15 | 0.255 | -4.472 | 3.93 | 3.38×1028 | 0.9976 |
R/ (K·h-1) | Modified Sangwal’s model | ||||
|---|---|---|---|---|---|
| Slope | Intercept | γ/(mJ·m-2) | A | R2 | |
| 2.5 | -102.2 | -1168 | 0.315 | 3.39×1023 | 0.9972 |
| 5 | -70.26 | -802.6 | 0.357 | 6.81×1023 | 0.9899 |
| 10 | -49.38 | -563.9 | 0.402 | 1.37×1024 | 0.9843 |
| 20 | -32.54 | -371.2 | 0.462 | 2.76×1024 | 0.9736 |
表6 采用修正后的Sangwal模型计算得到的动力学参数
Table 6 Regression cofficient and kinetics parameters calculated by modified Sangwal's model
R/ (K·h-1) | Modified Sangwal’s model | ||||
|---|---|---|---|---|---|
| Slope | Intercept | γ/(mJ·m-2) | A | R2 | |
| 2.5 | -102.2 | -1168 | 0.315 | 3.39×1023 | 0.9972 |
| 5 | -70.26 | -802.6 | 0.357 | 6.81×1023 | 0.9899 |
| 10 | -49.38 | -563.9 | 0.402 | 1.37×1024 | 0.9843 |
| 20 | -32.54 | -371.2 | 0.462 | 2.76×1024 | 0.9736 |
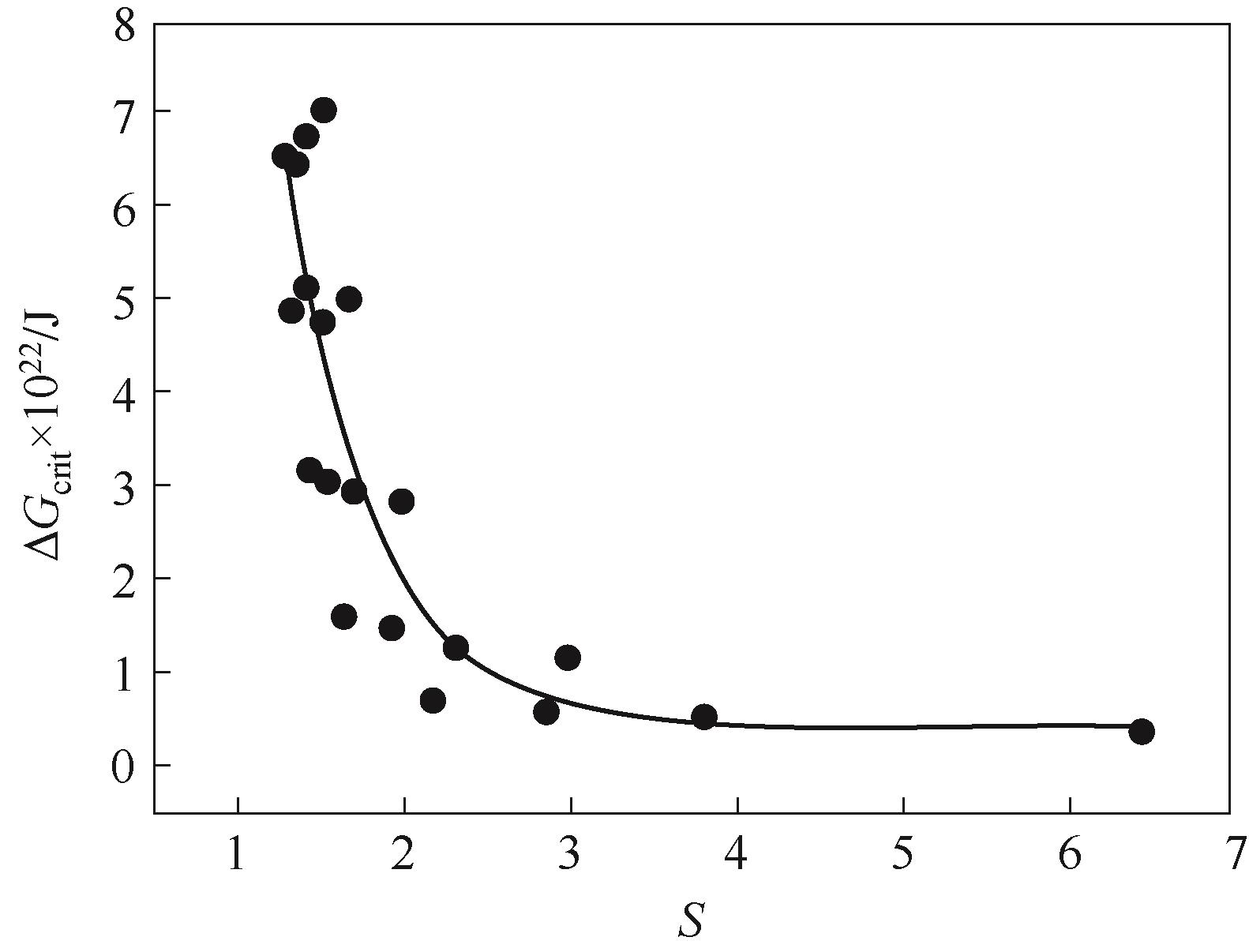
图9 由式(25)得到的过饱和度比S与临界Gibbs自由能ΔGcrit关系
Fig.9 Relationship between critical Gibbs free energy (ΔGcrit) and supersaturation ratio (S) according to Eq.(25)
搅拌转速/ (r·min-1) | 平均粒径/μm | 粒径标准偏差/μm | 粒径横纵比 | 变异系数(CV) |
|---|---|---|---|---|
| 100 | 154.9 | 65.7 | 0.217 | 42.4 |
| 200 | 171.9 | 72.0 | 0.231 | 41.8 |
| 300 | 201.3 | 83.1 | 0.242 | 41.2 |
| 400 | 149.7 | 65.9 | 0.267 | 43.9 |
| 500 | 117.3 | 50.8 | 0.292 | 43.3 |
表7 搅拌速率对莱鲍迪苷A结晶产品尺寸的影响
Table 7 Effect of different stirring rate on particle size of rebaudioside A
搅拌转速/ (r·min-1) | 平均粒径/μm | 粒径标准偏差/μm | 粒径横纵比 | 变异系数(CV) |
|---|---|---|---|---|
| 100 | 154.9 | 65.7 | 0.217 | 42.4 |
| 200 | 171.9 | 72.0 | 0.231 | 41.8 |
| 300 | 201.3 | 83.1 | 0.242 | 41.2 |
| 400 | 149.7 | 65.9 | 0.267 | 43.9 |
| 500 | 117.3 | 50.8 | 0.292 | 43.3 |
| 冷却速率 | 平均粒径/μm | 粒径标准偏差/μm | 粒径横纵比 | 变异系数值(CV) |
|---|---|---|---|---|
| 2.5 K·h-1 (线性) | 118.3 | 41.1 | 0.258 | 34.75 |
| 5 K·h-1 (线性) | 91.9 | 32.5 | 0.230 | 35.37 |
| 程序降温 | 134.0 | 46.3 | 0.236 | 34.52 |
表8 冷却速率对莱鲍迪苷A结晶产品尺寸的影响
Table 8 Effect of different cooling rate on particle size of rebaudioside A
| 冷却速率 | 平均粒径/μm | 粒径标准偏差/μm | 粒径横纵比 | 变异系数值(CV) |
|---|---|---|---|---|
| 2.5 K·h-1 (线性) | 118.3 | 41.1 | 0.258 | 34.75 |
| 5 K·h-1 (线性) | 91.9 | 32.5 | 0.230 | 35.37 |
| 程序降温 | 134.0 | 46.3 | 0.236 | 34.52 |
| 1 | Gao Z G, Rohani S, Gong J B, et al. Recent developments in the crystallization process: toward the pharmaceutical industry[J]. Engineering, 2017, 3(3): 343-353. |
| 2 | 赵绍磊, 王耀国, 张腾, 等. 制药结晶中的先进过程控制[J]. 化工学报, 2020, 71(2): 459-474. |
| Zhao S L, Wang Y G, Zhang T, et al. Advanced process control of pharmaceutical crystallization[J]. CIESC Journal, 2020, 71(2): 459-474. | |
| 3 | 赵绍磊, 王灵宇, 吴送姑. 药物多晶型的研究进展[J]. 化学工业与工程, 2018, 35(3): 12-21. |
| Zhao S L, Wang L Y, Wu S G. Progress in the research of pharmaceutical polymorph[J]. Chemical Industry and Engineering, 2018, 35(3): 12-21. | |
| 4 | Xu S J, Wang J K, Zhang K K, et al. Nucleation behavior of eszopiclone-butyl acetate solutions from metastable zone widths[J]. Chemical Engineering Science, 2016, 155: 248-257. |
| 5 | 张春桃, 王海蓉, 王永莉. 头孢曲松钠结晶诱导期的测定及其晶体生长机理的辨识[J]. 中国抗生素杂志, 2011, 36(2): 125-128. |
| Zhang C T, Wang H R, Wang Y L. Determination of induction period and crystal growth mechanism of ceftriaxone sodium in acetone-water system[J]. Chinese Journal of Antibiotics, 2011, 36(2): 125-128. | |
| 6 | Hanson J R, de Oliveira B H. Stevioside and related sweet diterpenoid glycosides[J]. Natural Product Reports, 1993, 10(3): 301-309. |
| 7 | Ghanta S, Banerjee A, Poddar A, et al. Oxidative DNA damage preventive activity and antioxidant potential of Stevia rebaudiana (Bertoni) Bertoni, a natural sweetener[J]. Journal of Agricultural and Food Chemistry, 2007, 55(26): 10962-10967. |
| 8 | Ruiz-Ruiz J C, Moguel-Ordoñez Y B, Segura-Campos M R. Biological activity of Stevia rebaudiana Bertoni and their relationship to health[J]. Critical Reviews in Food Science and Nutrition, 2017, 57(12): 2680-2690. |
| 9 | Yadav S K, Guleria P. Steviol glycosides from stevia: biosynthesis pathway review and their application in foods and medicine[J]. Critical Reviews in Food Science and Nutrition, 2012, 52(11): 988-998. |
| 10 | 娄力行. 甜菊糖及其衍生物的研究进展[J]. 中国糖料, 2008, 30(2): 70-72. |
| Lou L X. Research progress of stevioside and steviol[J]. Sugar Crops of China, 2008, 30(2): 70-72. | |
| 11 | 魏婷婷. 低莱鲍迪苷A(RA)甜菊糖母液的提纯工艺研究[D]. 天津: 天津大学, 2015. |
| Wei T T. Study on purification technology of low-rebodioside A(RA) stevia mother liquor[D]. Tianjin: Tianjin University, 2015. | |
| 12 | 沈建, 范刚, 杨健. 高纯度莱鲍迪甙A的结晶制备工艺[J]. 轻工机械, 2015, 33(5): 1-5. |
| Shen J, Fan G, Yang J. Crystallization preparation technology on high purity rebaudioside A from steviol glycosides[J]. Light Industry Machinery, 2015, 33(5): 1-5. | |
| 13 | 毛娅. 多溶剂溶析冷却结晶法提纯莱鲍迪甙A的工艺研究[J]. 化工设计通讯, 2018, 44(11): 145-146. |
| Mao Y. Purification of rebaudioside A by mixed solventing-out cooling crystallization[J]. Chemical Engineering Design Communications, 2018, 44(11): 145-146. | |
| 14 | 赵昊, 彭奇均. 溶析结晶法分离莱鲍迪甙A的工艺研究[J]. 应用化工, 2011, 40(8): 1310-1313. |
| Zhao H, Peng Q J. Study on the separation of rebaudioside A by solventing-out crystallization[J]. Applied Chemical Industry, 2011, 40(8): 1310-1313. | |
| 15 | 马庆, 许晓东, 陆文通, 等. 一种常温下从甜菊糖中提取高纯度莱鲍迪苷A的方法: 102485736A[P]. 2012-06-06. |
| Ma Q, Xu X D, Lu W T, et al. Method of extracting high purity rebaudiodside A from stevioside at normal temperature: 102485736A[P]. 2012-06-06. | |
| 16 | 赵宏宇. 莱鲍迪苷A的结晶工艺及其结晶热力学研究[D]. 无锡: 江南大学, 2012. |
| Zhao H Y. Studies on the crystallization process and crystallization thermodynamics of rebaudioside A[D]. Wuxi: Jiangnan University, 2012. | |
| 17 | 郭亚军, 刘文举, 余林达, 等. 酒石酸钠二水合物的溶解度测定及关联[J]. 化学工程, 2017, 45(12): 48-52. |
| Guo Y J, Liu W J, Yu L D, et al. Measurement and correlation of solubility of disodium tartrate dehydrate[J]. Chemical Engineering (China), 2017, 45(12): 48-52. | |
| 18 | 刘欣玉, 孙杰, 罗义芬, 等. ADN的溶解度、结晶介稳区及诱导期的测定[J]. 含能材料, 2019, 27(9): 766-772. |
| Liu X Y, Sun J, Luo Y F, et al. Measurement of solubility, metastable zone and induction period of ADN[J]. Chinese Journal of Energetic Materials, 2019, 27(9): 766-772. | |
| 19 | Cao Y, Du S C, Ke X, et al. Interplay between thermodynamics and kinetics on polymorphic behavior of vortioxetine hydrobromide in reactive crystallization[J]. Organic Process Research & Development, 2020, 24(7): 1233-1243. |
| 20 | Gu C H, Li H, Gandhi R B, et al. Grouping solvents by statistical analysis of solvent property parameters: implication to polymorph screening[J]. International Journal of Pharmaceutics, 2004, 283(1/2): 117-125. |
| 21 | Wilson G M. Vapor-liquid equilibrium(Ⅺ): A new expression for the excess free energy of mixing[J]. Journal of the American Chemical Society, 1964, 86(2): 127-130. |
| 22 | Ouyang J B, Chen J, Huang H Q, et al. Solid-liquid equilibrium and dissolution thermodynamics of 4-methylumbelliferon in different solvents[J]. Journal of Molecular Liquids, 2020, 306: 112797. |
| 23 | Xie Y, Shi H W, Du C B, et al. Solubility determination and modeling for 4, 4'-dihydroxydiphenyl sulfone in mixed solvents of (acetone, ethyl acetate, or acetonitrile) + methanol and acetone + ethanol from (278.15 to 313.15) K[J]. Journal of Chemical & Engineering Data, 2016, 61(10): 3519-3526. |
| 24 | Wang L P, Feng H T, Peng J Y, et al. Solubility, metastable zone width, and nucleation kinetics of sodium dichromate dihydrate[J]. Journal of Chemical & Engineering Data, 2015, 60(1): 185-191. |
| 25 | Kubota N. A new interpretation of metastable zone widths measured for unseeded solutions[J]. Journal of Crystal Growth, 2008, 310(3): 629-634. |
| 26 | 龚俊波, 李康, 何兵兵, 等. 果糖在高黏度水溶液中的生长模型及机理[J]. 化工进展, 2020, 39(5): 1714-1721. |
| Gong J B, Li K, He B B, et al. Model and mechanism of fructose crystal growth in aqueous solution with high viscosity[J]. Chemical Industry and Engineering Progress, 2020, 39(5): 1714-1721. | |
| 27 | Jacobsen C, Garside J, Hoare M. Nucleation and growth of microbial lipase crystals from clarified concentrated fermentation broths[J]. Biotechnology and Bioengineering, 1998, 57(6): 666-675. |
| 28 | Kobari M, Kubota N, Hirasawa I. Deducing primary nucleation parameters from metastable zone width and induction time data determined with simulation[J]. CrystEngComm, 2013, 15(6): 1199-1209. |
| 29 | Sangwal K. A novel self-consistent Nývlt-like equation for metastable zone width determined by the polythermal method[J]. Crystal Research and Technology, 2009, 44(3): 231-247. |
| 30 | Sangwal K. Novel approach to analyze metastable zone width determined by the polythermal method: physical interpretation of various parameters[J]. Crystal Growth & Design, 2009, 9(2): 942-950. |
| 31 | Kadam S S, Kramer H J M, ter Horst J H. Combination of a single primary nucleation event and secondary nucleation in crystallization processes[J]. Crystal Growth & Design, 2011, 11(4): 1271-1277. |
| 32 | Kashchiev D, Borissova A, Hammond R B, et al. Effect of cooling rate on the critical undercooling for crystallization[J]. Journal of Crystal Growth, 2010, 312(5): 698-704. |
| 33 | Upreti M, Smit J P, Hagen E J, et al. Single crystal growth and structure determination of the natural “high potency” sweetener rebaudioside A[J]. Crystal Growth & Design, 2012, 12(2): 990-993. |
| 34 | 丁绪淮, 谈遒. 工业结晶[M]. 北京: 化学工业出版社, 1985: 79. |
| Ding X H, Tan Q. Industrial Crystallization [M]. Beijing: Chemical Industry Press, 1985: 79. | |
| 35 | Kashchiev D, van Rosmalen G M. Review: nucleation in solutions revisited[J]. Crystal Research and Technology, 2003, 38(7/8): 555-574. |
| 36 | Kashchiev D. Nucleation: Basic Theory with Applications [M]. London: Butterworth-Heinemann, 2000: 156-163. |
| 37 | Mullin J W. Crystallization [M]. 4th ed. London: Butterworths, 2001: 181-215 |
| 38 | Granberg R A, Ducreux C, Gracin S, et al. Primary nucleation of paracetamol in acetone-water mixtures[J]. Chemical Engineering Science, 2001, 56(7): 2305-2313. |
| [1] | 胡超, 董玉明, 张伟, 张红玲, 周鹏, 徐红彬. 浓硫酸活化五氧化二钒制备高浓度全钒液流电池正极电解液[J]. 化工学报, 2023, 74(S1): 338-345. |
| [2] | 王皓, 唐思扬, 钟山, 梁斌. MEA吸收CO2富液解吸过程中固体颗粒表面的强化作用分析[J]. 化工学报, 2023, 74(4): 1539-1548. |
| [3] | 王瑞恒, 何品晶, 吕凡, 章骅. 垃圾焚烧飞灰水洗后三种固液分离方法参数比较及优化[J]. 化工学报, 2023, 74(4): 1712-1723. |
| [4] | 张炜, 李昊阳, 徐纯刚, 李小森. 气体水合物生成微观机理及分析方法研究进展[J]. 化工学报, 2022, 73(9): 3815-3827. |
| [5] | 鲁统鹏, 潘晓林, 吴鸿飞, 李煜, 于海燕. 有机絮凝剂对铁矿相沉降性能影响及其吸附机理[J]. 化工学报, 2022, 73(9): 4122-4132. |
| [6] | 顾仁杰, 张加威, 靳雪阳, 文利雄. 微撞击流反应器制备镍钴复合氢氧化物超级电容器材料及其性能研究[J]. 化工学报, 2022, 73(8): 3749-3757. |
| [7] | 乃学瑛, 吴鹏, 程远, 肖剑飞, 刘鑫, 董亚萍. 水热生长碱式硫酸镁纳米线结晶动力学研究[J]. 化工学报, 2022, 73(7): 3038-3044. |
| [8] | 李雯, 兰忠, 强伟丽, 任文芝, 杜宾港, 马学虎. 蒸汽冷凝近壁过渡区团簇演化特性[J]. 化工学报, 2022, 73(7): 2865-2873. |
| [9] | 赵庆杰, 胡晓红, 张超, 凡凤仙. 蒸汽在含有不可溶核和可溶无机盐的细颗粒物表面的核化特性[J]. 化工学报, 2022, 73(7): 3251-3261. |
| [10] | 汪帆, 刘岩博, 李康丽, 童丽, 金美堂, 汤伟伟, 陈明洋, 龚俊波. 溶液结晶中的介尺度成核过程研究进展[J]. 化工学报, 2022, 73(6): 2318-2333. |
| [11] | 白浩隆, 付亮亮, 许光文, 白丁荣. 流化床煤燃烧过程不同气氛下的气态氮释放特征[J]. 化工学报, 2022, 73(2): 876-886. |
| [12] | 戴军涛, 刘莉, 刘帅, 顾汉洋, 王科. 基于丝网探针的螺旋管内气液两相流气泡行为研究[J]. 化工学报, 2022, 73(10): 4377-4388. |
| [13] | 丰闪闪, 刘晓斌, 郭石麟, 何兵兵, 高振国, 陈明洋, 龚俊波. 锂枝晶的成核、生长与抑制[J]. 化工学报, 2022, 73(1): 97-109. |
| [14] | 周闻, 鄂承林, 李永祺, 郭玉娇, 李子轩, 卢春喜. 新型多旋臂气液分离器入口旋流头的预分离特性研究[J]. 化工学报, 2021, 72(9): 4775-4785. |
| [15] | 张学平, 崔瑞芝, 桑世华. NaBr-CaBr2-H2O和KBr-CaBr2-H2O三元体系273.15 K相平衡实验及计算[J]. 化工学报, 2021, 72(9): 4479-4486. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号