化工学报 ›› 2022, Vol. 73 ›› Issue (6): 2318-2333.DOI: 10.11949/0438-1157.20220159
汪帆1( ),刘岩博1,李康丽2,童丽2,金美堂2,汤伟伟1,陈明洋1,2(
),刘岩博1,李康丽2,童丽2,金美堂2,汤伟伟1,陈明洋1,2( ),龚俊波1,2,3(
),龚俊波1,2,3( )
)
收稿日期:2022-02-07
修回日期:2022-03-09
出版日期:2022-06-05
发布日期:2022-06-30
通讯作者:
陈明洋,龚俊波
作者简介:汪帆(1999—),男,硕士研究生, 基金资助:
Fan WANG1( ),Yanbo LIU1,Kangli LI2,Li TONG2,Meitang JIN2,Weiwei TANG1,Mingyang CHEN1,2(
),Yanbo LIU1,Kangli LI2,Li TONG2,Meitang JIN2,Weiwei TANG1,Mingyang CHEN1,2( ),Junbo GONG1,2,3(
),Junbo GONG1,2,3( )
)
Received:2022-02-07
Revised:2022-03-09
Online:2022-06-05
Published:2022-06-30
Contact:
Mingyang CHEN,Junbo GONG
摘要:
成核作为溶液结晶的第一步,是决定晶体产品质量的关键因素。目前,成核理论主要包括经典成核理论和非经典成核理论。相比于仅以原子、离子或分子等均匀稳定结构为单元的经典成核理论,非经典成核理论以纳米级前聚体为单元,这类单元涵盖了聚集体、纳米粒子等介尺度非均匀动态结构,导致形成的非经典成核过程更为复杂,需在传统的化学、化学工程和过程系统工程研究方法的基础上,充分利用介尺度科学研究方法完成其核心规律的探究。为此,总结了二步成核理论、预成核团簇理论、粒子附着晶化理论以及其他新提出的非经典成核理论,分析了其中的介尺度结构及其时空动态行为,并探讨了利用介尺度数学模型对现有成核数学模型的修正和优化的思路,最后对溶液结晶中晶体成核的介尺度研究范式及理论发展进行了展望。
中图分类号:
汪帆, 刘岩博, 李康丽, 童丽, 金美堂, 汤伟伟, 陈明洋, 龚俊波. 溶液结晶中的介尺度成核过程研究进展[J]. 化工学报, 2022, 73(6): 2318-2333.
Fan WANG, Yanbo LIU, Kangli LI, Li TONG, Meitang JIN, Weiwei TANG, Mingyang CHEN, Junbo GONG. Research progress on mesoscale nucleation process in solution crystallization[J]. CIESC Journal, 2022, 73(6): 2318-2333.
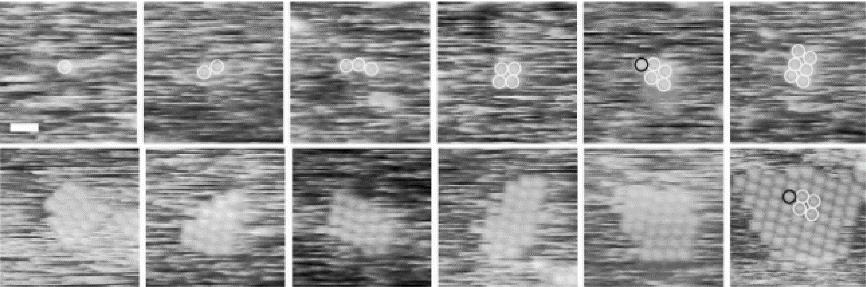
图3 单个线体团簇(大小从1到大于50个分子)的快照 (比例尺,20 nm)[38]
Fig.3 Snapshots of individual clusters ranging in size from 1 to more than 50 molecules (scale bar, 20 nm ) [38]
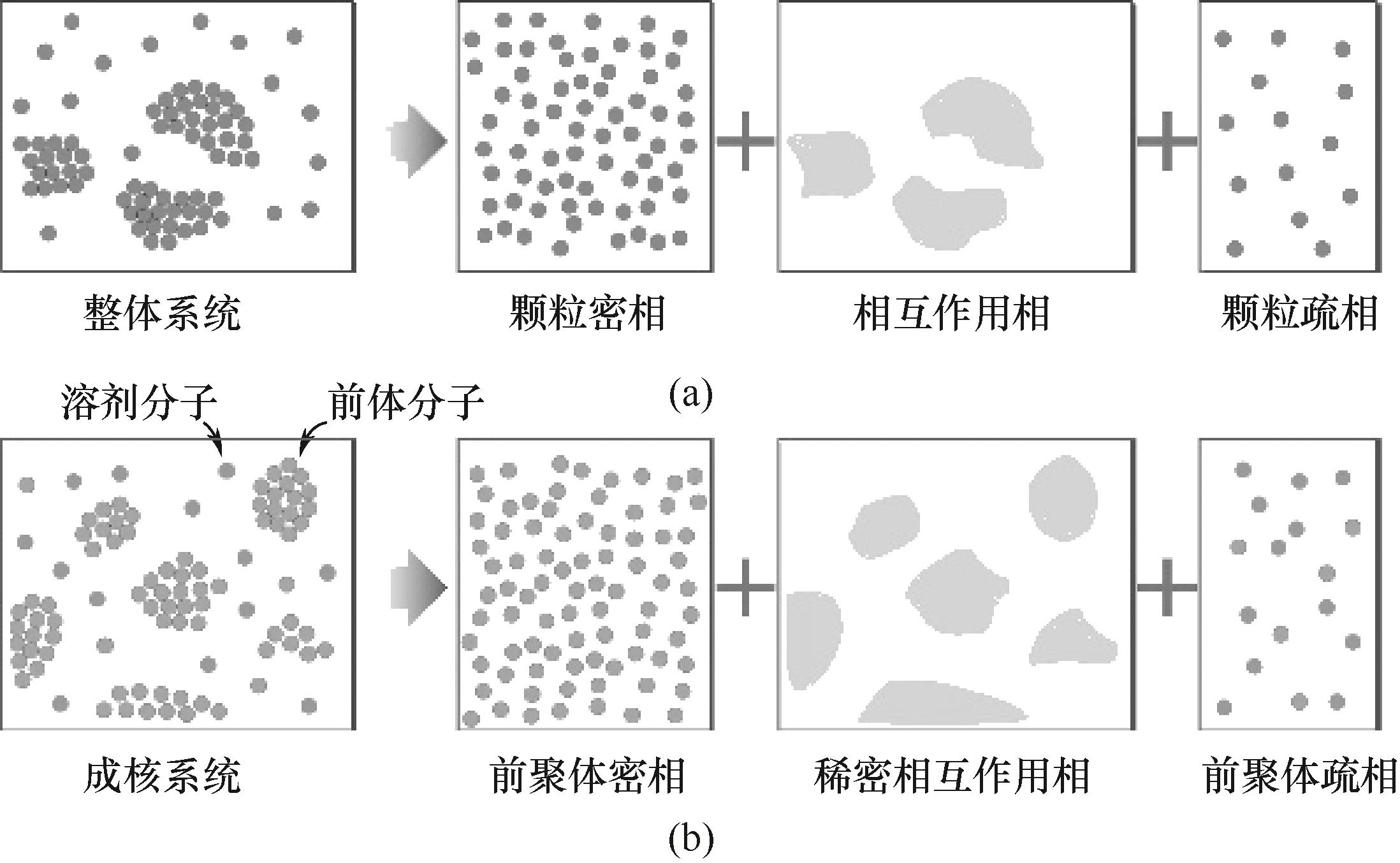
图13 局部非均匀气固流场的分解(a);成核过程非均匀固液流场的分解(b)
Fig.13 Decomposition of local inhomogeneous gas-solid flow field (a); Decomposition of local inhomogeneous solid-liquid flow field during nucleation (b)
| 1 | Liu X W, Chee S W, Raj S, et al. Three-step nucleation of metal-organic framework nanocrystals[J]. Proceedings of the National Academy of Sciences of the United States of America, 2021, 118(10): e2008880118. |
| 2 | Myerson A S, Trout B L. Nucleation from solution[J]. Science, 2013, 341(6148): 855-856. |
| 3 | Tsarfati Y, Biran I, Wiedenbeck E, et al. Continuum crystallization model derived from pharmaceutical crystallization mechanisms[J]. ACS Central Science, 2021, 7(5): 900-908. |
| 4 | Olafson K N, Nguyen T Q, Rimer J D, et al. Antimalarials inhibit hematin crystallization by unique drug-surface site interactions[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(29): 7531-7536. |
| 5 | Davey R J, Schroeder S L M, ter Horst J H. Nucleation of organic crystals—a molecular perspective[J]. Angewandte Chemie International Edition, 2013, 52(8): 2166-2179. |
| 6 | Fang C, Tang W W, Wu S G, et al. Ultrasound-assisted intensified crystallization of L-glutamic acid: crystal nucleation and polymorph transformation[J]. Ultrasonics Sonochemistry, 2020, 68: 105227. |
| 7 | Shi P, Xu S J, Ma Y M, et al. Probing the structural pathway of conformational polymorph nucleation by comparing a series of α, ω-alkanedicarboxylic acids[J]. IUCrJ, 2020, 7(3): 422-433. |
| 8 | Davey R J, Allen K, Blagden N, et al. Crystal engineering—nucleation, the key step[J]. CrystEngComm, 2002, 4(47): 257-264. |
| 9 | Coquerel G. Crystallization of molecular systems from solution: phase diagrams, supersaturation and other basic concepts[J]. Chemical Society Reviews, 2014, 43(7): 2286-2300. |
| 10 | Martin S T. Phase transitions of aqueous atmospheric particles[J]. Chemical Reviews, 2000, 100(9): 3403-3454. |
| 11 | Karthika S, Radhakrishnan T K, Kalaichelvi P. A review of classical and nonclassical nucleation theories[J]. Crystal Growth & Design, 2016, 16(11): 6663-6681. |
| 12 | Li X, Yin Q X, Zhang M J, et al. Antisolvent crystallization of erythromycin ethylsuccinate in the presence of liquid-liquid phase separation[J]. Industrial & Engineering Chemistry Research, 2016, 55(3): 766-776. |
| 13 | Cui P L, Zhang X W, Yin Q X, et al. Evidence of hydrogen-bond formation during crystallization of cefodizime sodium from induction-time measurements and in situ Raman spectroscopy[J]. Industrial & Engineering Chemistry Research, 2012, 51(42): 13663-13669. |
| 14 | Gower L B, Odom D J. Deposition of calcium carbonate films by a polymer-induced liquid-precursor (PILP) process[J]. Journal of Crystal Growth, 2000, 210(4): 719-734. |
| 15 | Gebauer D, Cölfen H. Prenucleation clusters and non-classical nucleation[J]. Nano Today, 2011, 6(6): 564-584. |
| 16 | Thanh N T K, MacLean N, Mahiddine S. Mechanisms of nucleation and growth of nanoparticles in solution[J]. Chemical Reviews, 2014, 114(15): 7610-7630. |
| 17 | Klaerner G, Padmanabhan R. Multi-step/step-wise polymerization of well-defined oligomers[M]//Reference Module in Materials Science and Materials Engineering. Amsterdam: Elsevier, 2016. |
| 18 | Sun M M, Tang W W, Du S C, et al. Understanding the roles of oiling-out on crystallization of β-alanine: unusual behavior in metastable zone width and surface nucleation during growth stage[J]. Crystal Growth & Design, 2018, 18(11): 6885-6890. |
| 19 | Vekilov P G. Dense liquid precursor for the nucleation of ordered solid phases from solution[J]. Crystal Growth & Design, 2004, 4(4): 671-685. |
| 20 | Chen J J, Zhu E B, Liu J, et al. Building two-dimensional materials one row at a time: avoiding the nucleation barrier[J]. Science, 2018, 362(6419): 1135-1139. |
| 21 | Li J H, Huang W L, Chen J H. Possible roadmap to advancing the knowledge system and tackling challenges from complexity[J]. Chemical Engineering Science, 2021, 237:116548. |
| 22 | Ge W, Chen F G, Gao J, et al. Analytical multi-scale method for multi-phase complex systems in process engineering—bridging reductionism and holism[J]. Chemical Engineering Science, 2007, 62(13): 3346-3377. |
| 23 | Ge W, Wang W, Yang N, et al. Meso-scale oriented simulation towards virtual process engineering (VPE)—the EMMS paradigm[J]. Chemical Engineering Science, 2011, 66(19): 4426-4458. |
| 24 | Smeets P J M, Cho K R, Kempen R G E, et al. Calcium carbonate nucleation driven by ion binding in a biomimetic matrix revealed by in situ electron microscopy[J]. Nature Materials, 2015, 14(4): 394-399. |
| 25 | Yang J, Koo J, Kim S, et al. Amorphous-phase-mediated crystallization of Ni nanocrystals revealed by high-resolution liquid-phase electron microscopy[J]. Journal of the American Chemical Society, 2019, 141(2): 763-768. |
| 26 | Baumgartner J, Dey A, Bomans P H H, et al. Nucleation and growth of magnetite from solution[J]. Nature Materials, 2013, 12(4): 310-314. |
| 27 | Xu S J, Chen Y F, Gong J B, et al. Interplay between kinetics and thermodynamics on the probability nucleation rate of a urea-water crystallization system[J]. Crystal Growth & Design, 2018, 18(4): 2305-2315. |
| 28 | Yang J, Xu S J, Wang J K, et al. Nucleation behavior of ethyl vanillin: balance between chemical potential difference and saturation temperature[J]. Journal of Molecular Liquids, 2020, 303: 112609. |
| 29 | Xu S J, Wang J K, Zhang K K, et al. Nucleation behavior of eszopiclone-butyl acetate solutions from metastable zone widths[J]. Chemical Engineering Science, 2016, 155: 248-257. |
| 30 | Volmer M, Weber. Keimbildung in übersättigten gebilden[J]. Zeitschrift Für Physikalische Chemie, 1926, 119U(1): 277-301. |
| 31 | Farkas L. Keimbildungsgeschwindigkeit in übersättigten dämpfen[J]. Zeitschrift Für Physikalische Chemie, 1927, 125U(1): 236-242. |
| 32 | Becker R, Döring W. Kinetische behandlung der keimbildung in übersättigten dämpfen[J]. Annalen Der Physik, 1935, 416(8): 719-752. |
| 33 | Bai G, Gao D, Liu Z, et al. Probing the critical nucleus size for ice formation with graphene oxide nanosheets[J]. Nature, 2019, 576(7787): 437-441. |
| 34 | Garten V A, Head R B. Homogeneous nucleation and the phenomenon of crystalloluminescence[J]. Philosophical Magazine, 1966, 14(132): 1243-1253. |
| 35 | Gilra N K. Precrystallization theory applied to ultrasonic velocity in supercooled water[J]. Journal of the Physical Society of Japan, 1967, 23(6): 1431. |
| 36 | Adamski T. Commination of crystal nucleation by a precipitation method[J]. Nature, 1963, 197(4870): 894. |
| 37 | Yau S T, Vekilov P G. Direct observation of nucleus structure and nucleation pathways in apoferritin crystallization[J]. Journal of the American Chemical Society, 2001, 123(6): 1080-1089. |
| 38 | Sleutel M, Lutsko J, van Driessche A E S, et al. Observing classical nucleation theory at work by monitoring phase transitions with molecular precision[J]. Nature Communications, 2014, 5: 5598. |
| 39 | Tidhar Y, Weissman H, Tworowski D, et al. Mechanism of crystalline self-assembly in aqueous medium: a combined cryo-TEM/kinetic study[J]. Chemistry - A European Journal, 2014, 20(33): 10332-10342. |
| 40 | Wallace A F, Hedges L O, Fernandez-Martinez A, et al. Microscopic evidence for liquid-liquid separation in supersaturated CaCO3 solutions[J]. Science, 2013, 341(6148): 885-889. |
| 41 | Galkin O, Pan W C, Filobelo L, et al. Two-step mechanism of homogeneous nucleation of sickle cell hemoglobin polymers[J]. Biophysical Journal, 2007, 93(3): 902-913. |
| 42 | ten Wolde P R, Frenkel D. Enhancement of protein crystal nucleation by critical density fluctuations[J]. Science, 1997, 277(5334): 1975-1978. |
| 43 | Talanquer V, Oxtoby D W. Crystal nucleation in the presence of a metastable critical point[J]. The Journal of Chemical Physics, 1998, 109(1): 223-227. |
| 44 | Sleutel M, van Driessche A E S. Role of clusters in nonclassical nucleation and growth of protein crystals[J]. PNAS, 2014, 111(5): 201309320. |
| 45 | Lutsko J F. How crystals form: a theory of nucleation pathways[J]. Science Advances, 2019, 5(4): eaav7399. |
| 46 | Garcia N A, Malini R I, Freeman C L, et al. Simulation of calcium phosphate prenucleation clusters in aqueous solution: association beyond ion pairing[J]. Crystal Growth & Design, 2019, 19(11): 6422-6430. |
| 47 | Gebauer D, Völkel A, Cölfen H. Stable prenucleation calcium carbonate clusters[J]. Science, 2008, 322(5909): 1819-1822. |
| 48 | Scheck J, Wu B H, Drechsler M, et al. The molecular mechanism of iron(Ⅲ) oxide nucleation[J]. The Journal of Physical Chemistry Letters, 2016, 7(16): 3123-3130. |
| 49 | Gebauer D, Kellermeier M, Gale J D, et al. Pre-nucleation clusters as solute precursors in crystallisation[J]. Chemical Society Reviews, 2014, 43(7): 2348-2371. |
| 50 | van Vleet M J, Weng T T, Li X Y, et al. In situ, time-resolved, and mechanistic studies of metal-organic framework nucleation and growth[J]. Chemical Reviews, 2018, 118(7): 3681-3721. |
| 51 | Kellermeier M, Rosenberg R, Moise A, et al. Amino acids form prenucleation clusters: ESI-MS as a fast detection method in comparison to analytical ultracentrifugation[J]. Faraday Discussions, 2012, 159: 23. |
| 52 | Nielsen M H, Aloni S, de Yoreo J J. In situ TEM imaging of CaCO₃ nucleation reveals coexistence of direct and indirect pathways[J]. Science, 2014, 345(6201): 1158-1162. |
| 53 | Shi P, Xu S J, Yang H Y, et al. Use of additives to regulate solute aggregation and direct conformational polymorph nucleation of pimelic acid[J]. IUCrJ, 2021, 8(Pt 2): 161-167. |
| 54 | Li S, Tang W W, Shi P, et al. A new perspective of gallic acid on calcium oxalate nucleation[J]. Crystal Growth & Design, 2020, 20(5): 3173-3181. |
| 55 | Zhao S L, Gao J, Ma S Y, et al. Mechanism and modelling of reactive crystallization process of lithium carbonate[J]. Processes, 2019, 7(5): 248. |
| 56 | 龚俊波, 李斯, 陈明洋, 等. 氯吡格雷硫酸氢盐的溶析结晶过程研究[J]. 中国医药工业杂志, 2018, 49(5): 677-683. |
| Gong J B, Li S, Chen M Y, et al. Study on the process of antisolvent crystallization of clopidogrel hydrogen sulfate[J]. Chinese Journal of Pharmaceuticals, 2018, 49(5): 677-683. | |
| 57 | Chen M Y, Du S C, Zhang T, et al. Spherical crystallization and the mechanism of clopidogrel hydrogen sulfate[J]. Chemical Engineering & Technology, 2018, 41(6): 1259-1265. |
| 58 | Pouget E M, Bomans P H H, Goos J A C M, et al. The initial stages of template-controlled CaCO3 formation revealed by cryo-TEM[J]. Science, 2009, 323(5920): 1455-1458. |
| 59 | Xing J, Schweighauser L, Okada S, et al. Atomistic structures and dynamics of prenucleation clusters in MOF-2 and MOF-5 syntheses[J]. Nature Communications, 2019, 10: 3608. |
| 60 | Bewernitz M A, Gebauer D, Long J, et al. A metastable liquid precursor phase of calcium carbonate and its interactions with polyaspartate[J]. Faraday Discussions, 2012, 159: 291. |
| 61 | de Yoreo J J, Gilbert P U P A, Sommerdijk N A J M, et al. Crystallization by particle attachment in synthetic, biogenic, and geologic environments[J]. Science, 2015, 349(6247): aaa6760. |
| 62 | Olszta M J, Cheng X G, Jee S S, et al. Bone structure and formation: a new perspective[J]. Materials Science and Engineering: R: Reports, 2007, 58(3/4/5): 77-116. |
| 63 | Ivanov V K, Fedorov P P, Ye Baranchikov A, et al. Oriented attachment of particles: 100 years of investigations of non-classical crystal growth[J]. Russian Chemical Reviews, 2014, 83(12): 1204-1222. |
| 64 | Li D S, Nielsen M H, Lee J R I, et al. Direction-specific interactions control crystal growth by oriented attachment[J]. Science, 2012, 336(6084): 1014-1018. |
| 65 | Rodríguez-Navarro C, Ruiz-Agudo E, Harris J, et al. Nonclassical crystallization in vivo et in vitro (Ⅱ): Nanogranular features in biomimetic minerals disclose a general colloid-mediated crystal growth mechanism[J]. Journal of Structural Biology, 2016, 196(2): 260-287. |
| 66 | Li M Y, Li S, Tang W W, et al. Understanding the crystallization pathway of monosodium urate monohydrate in a biomimetic matrix[J]. Crystal Growth & Design, 2020, 20(2): 804-812. |
| 67 | Mirabello G, Ianiro A, Bomans P H H, et al. Crystallization by particle attachment is a colloidal assembly process[J]. Nature Materials, 2020, 19(4): 391-396. |
| 68 | Michaels T C T, Lazell H W, Arosio P, et al. Dynamics of protein aggregation and oligomer formation governed by secondary nucleation[J]. The Journal of Chemical Physics, 2015, 143(5): 054901. |
| 69 | Li H Y, Chavez A D, Li H F, et al. Nucleation and growth of covalent organic frameworks from solution: the example of COF-5[J]. Journal of the American Chemical Society, 2017, 139(45): 16310-16318. |
| 70 | Chen H, Li M, Lu Z, et al. Multistep nucleation and growth mechanisms of organic crystals from amorphous solid states[J]. Nature Communications, 2019, 10: 3872. |
| 71 | Kashchiev D, van Rosmalen G M. Review: nucleation in solutions revisited[J]. Crystal Research and Technology, 2003, 38(78): 555-574. |
| 72 | Tang W W, Sima A D, Gong J B, et al. Kinetic difference between concomitant polymorphism and solvent-mediated phase transformation: a case of tolfenamic acid[J]. Crystal Growth & Design, 2020, 20(3): 1779-1788. |
| 73 | Du W, Yin Q X, Bao Y, et al. Concomitant polymorphism of prasugrel hydrochloride in reactive crystallization[J]. Industrial & Engineering Chemistry Research, 2013, 52(46): 16182-16189. |
| 74 | Zhang K K, Xu S J, Liu S Y, et al. Novel strategy to control polymorph nucleation of gamma pyrazinamide by preferred intermolecular interactions during heterogeneous nucleation[J]. Crystal Growth & Design, 2018, 18(9): 4874-4879. |
| 75 | 林家伟, 石鹏, 龚俊波, 等. 表面诱导药物多晶型成核的研究进展[J]. 化工学报, 2021, 72(2): 814-827. |
| Lin J W, Shi P, Gong J B, et al. Progress on surface-induced nucleation of drug for controlling polymorphism[J]. CIESC Journal, 2021, 72(2): 814-827. | |
| 76 | 赵绍磊, 王灵宇, 吴送姑. 药物多晶型的研究进展[J]. 化学工业与工程, 2018, 35(3): 12-21. |
| Zhao S L, Wang L Y, Wu S G. Progress in the research of pharmaceutical polymorph[J]. Chemical Industry and Engineering, 2018, 35(3): 12-21. | |
| 77 | 汤伟伟, 李斯, 龚俊波. 有机晶体成核分子机理研究进展[J]. 化学工业与工程, 2018, 35(3): 2-11. |
| Tang W W, Li S, Gong J B. Research progress on molecular mechanism of nucleation of organic crystals[J]. Chemical Industry and Engineering, 2018, 35(3): 2-11. | |
| 78 | Erdemir D, Lee A Y, Myerson A S. Nucleation of crystals from solution: classical and two-step models[J]. Accounts of Chemical Research, 2009, 42(5): 621-629. |
| 79 | Lee J, Yang J, Kwon S G, et al. Nonclassical nucleation and growth of inorganic nanoparticles[J]. Nature Reviews Materials, 2016, 1: 16034. |
| 80 | Gebauer D, Wolf S E. Designing solid materials from their solute state: a shift in paradigms toward a holistic approach in functional materials chemistry[J]. Journal of the American Chemical Society, 2019, 141(11): 4490-4504. |
| 81 | Gilbert P U P A, Porter S M, Sun C Y, et al. Biomineralization by particle attachment in early animals[J]. PNAS, 2019, 116(36): 17659-17665. |
| 82 | Yin Y, Alivisatos A P. Colloidal nanocrystal synthesis and the organic-inorganic interface[J]. Nature, 2005, 437(7059): 664-670. |
| 83 | Wu Z H, Yang S L, Wu W. Shape control of inorganic nanoparticles from solution[J]. Nanoscale, 2016, 8(3): 1237-1259. |
| 84 | Zhang Q, Liu S J, Yu S H. Recent advances in oriented attachment growth and synthesis of functional materials: concept, evidence, mechanism, and future[J]. J. Mater. Chem., 2009, 19(2): 191-207. |
| 85 | Guo L, Wu J, Li J H. Complexity at mesoscales: a common challenge in developing artificial intelligence[J]. Engineering, 2019,5(5):924-929. |
| 86 | Li J H. Approaching virtual process engineering with exploring mesoscience[J]. Chemical Engineering Journal, 2015, 278: 541-555. |
| 87 | Li J H, Huang W L. Towards Mesoscience: the Principle of Compromise in Competition[M]. Berlin, Heidelberg: Springer, 2014. |
| 88 | Li J H, Tung Y, Kwauk M. Method of energy minimization in multi-scale modeling of particle-fluid two-phase flow[M]//Circulating Fluidized Bed Technology. Amsterdam: Elsevier, 1988: 89-103. |
| 89 | Yang N, Wu Z Y, Chen J H, et al. Multi-scale analysis of gas-liquid interaction and CFD simulation of gas-liquid flow in bubble columns[J]. Chemical Engineering Science, 2011, 66(14): 3212-3222. |
| 90 | Wang L M, Qiu X P, Zhang L, et al. Turbulence originating from the compromise-in-competition between viscosity and inertia[J]. Chemical Engineering Journal, 2016, 300: 83-97. |
| 91 | Li J H, Zhang Z D, Ge W, et al. A simple variational criterion for turbulent flow in pipe[J]. Chemical Engineering Science, 1999, 54(8): 1151-1154. |
| 92 | Huang W L, Li J H. Mesoscale model for heterogeneous catalysis based on the principle of compromise in competition[J]. Chemical Engineering Science, 2016, 147: 83-90. |
| 93 | 王维, 洪坤, 鲁波娜, 等. 流态化模拟: 基于介尺度结构的多尺度CFD[J]. 化工学报, 2013, 64(1): 95-106. |
| Wang W, Hong K, Lu B N, et al. Fluidized bed simulation: structure-dependent multiscale CFD[J]. CIESC Journal, 2013, 64(1): 95-106. | |
| 94 | Li J H, Kwauk M. Multiscale nature of complex fluid-particle systems[J]. Industrial & Engineering Chemistry Research, 2001, 40(20): 4227-4237. |
| 95 | 李静海. 两相流多尺度作用模型和能量最小方法[D]. 北京: 中国科学院, 1987. |
| Li J H. Multiscale action model and energy minimization method for two-phase flow[D]. Beijing: Chinese Academy of Sciences, 1987. | |
| 96 | 李飞, 陈程, 王锦生, 等. 稠密气固两相QL-EMMS曳力模型及改进[J]. 工程热物理学报, 2011, 32(1): 75-79. |
| Li F, Chen C, Wang J S, et al. QL-EMMS drag model & its revision for fluidized dense gas-solid two-phase flow[J]. Journal of Engineering Thermophysics, 2011, 32(1): 75-79. | |
| 97 | 佟颖, Nouman Ahmad, 鲁波娜, 等. 基于EMMS介尺度模型的双分散鼓泡流化床的模拟[J]. 化工学报, 2019, 70(5): 1682-1692. |
| Tong Y, Nouman A, Lu B N, et al. Numerical investigation of bubbling fluidized bed with binary particle mixture using EMMS mesoscale drag model[J]. CIESC Journal, 2019, 70(5): 1682-1692. | |
| 98 | Horio M, Kuroki H. Three-dimensional flow visualization of dilutely dispersed solids in bubbling and circulating fluidized beds[J]. Chemical Engineering Science, 1994, 49(15): 2413-2421. |
| 99 | Bi H T, Grace J R. Effect of measurement method on the velocities used to demarcate the onset of turbulent fluidization[J]. The Chemical Engineering Journal and the Biochemical Engineering Journal, 1995, 57(3): 261-271. |
| 100 | Zhu H Y, Zhu J. Characterization of fluidization behavior in the bottom region of CFB risers[J]. Chemical Engineering Journal, 2008, 141(1/2/3): 169-179. |
| 101 | Li J H, Zhang J Y, Ge W, et al. Multi-scale methodology for complex systems[J]. Chemical Engineering Science, 2004, 59(8/9): 1687-1700. |
| 102 | Zhang J Y, Ge W, Li J H. Simulation of heterogeneous structures and analysis of energy consumption in particle-fluid systems with pseudo-particle modeling[J]. Chemical Engineering Science, 2005, 60(11): 3091-3099. |
| 103 | Li J H, Cheng C L, Zhang Z D, et al. The EMMS model—its application, development and updated concepts[J]. Chemical Engineering Science, 1999, 54(22): 5409-5425. |
| 104 | Li J H, Ge W, Wang W, et al. Focusing on mesoscales: from the energy-minimization multiscale model to mesoscience[J]. Current Opinion in Chemical Engineering, 2016, 13: 10-23. |
| 105 | Li J H, Ge W, Wang W, et al. From Multiscale Modeling to Meso-science[M]. Berlin, Heidelberg: Springer, 2013. |
| 106 | Ren Y, Gao J, Xu J, et al. Key factors in chaperonin-assisted protein folding[J]. Particuology, 2012, 10(1): 105-116. |
| 107 | Li J H, Huang W L, Chen J H, et al. Mesoscience based on the EMMS principle of compromise in competition[J]. Chemical Engineering Journal, 2018, 333: 327-335. |
| 108 | Li J H, Huang W L. From multiscale to mesoscience: addressing mesoscales in mesoregimes of different levels[J]. Annual Review of Chemical and Biomolecular Engineering, 2018, 9: 41-60. |
| 109 | Liu Z K, Yin Q X, Zhang H, et al. Investigation of the crystallization of disodium 5'-inosinate in a water + ethanol system: solubility, nucleation mechanism, and crystal morphology[J]. Industrial & Engineering Chemistry Research, 2014, 53(21): 8913-8919. |
| 110 | Zhang K K, Xu S J, Gong J B, et al. Revealing the critical role of template functional group ordering in the template-directed crystallization of pyrazinamide[J]. CrystEngComm, 2019, 21(42): 6382-6389. |
| 111 | Zhang J L, Liu A Y, Han Y, et al. Effects of self-assembled monolayers on selective crystallization of tolbutamide[J]. Crystal Growth & Design, 2011, 11(12): 5498-5506. |
| 112 | 李静海, 胡英, 袁权. 探索介尺度科学: 从新角度审视老问题[J]. 中国科学: 化学, 2014, 44(3): 277-281. |
| Li J H, Hu Y, Yuan Q. Mesoscience: exploring old problems from a new angle[J]. Chinese Science: Chemistry, 2014, 44(3): 277-281. | |
| 113 | 杨宁, 李静海. 化学工程中的介尺度科学与虚拟过程工程: 分析与展望[J]. 化工学报, 2014, 65(7): 2403-2409. |
| Yang N, Li J H. Mesoscience in chemical engineering and virtual process engineering: analysis and perspective[J]. CIESC Journal, 2014, 65(7): 2403-2409. | |
| 114 | Shafiq S I, Sanin C, Szczerbicki E, et al. Virtual engineering factory: creating experience base for industry 4.0[J]. Cybernetics and Systems, 2016, 47(1/2): 32-47. |
| 115 | Ge W, Guo L, Liu X H, et al. Mesoscience-based virtual process engineering[J]. Computers & Chemical Engineering, 2019, 126: 68-82. |
| [1] | 肖明堃, 杨光, 黄永华, 吴静怡. 浸没孔液氧气泡动力学数值研究[J]. 化工学报, 2023, 74(S1): 87-95. |
| [2] | 周绍华, 詹飞龙, 丁国良, 张浩, 邵艳坡, 刘艳涛, 郜哲明. 短管节流阀内流动噪声的实验研究及降噪措施[J]. 化工学报, 2023, 74(S1): 113-121. |
| [3] | 江河, 袁俊飞, 王林, 邢谷雨. 均流腔结构对微细通道内相变流动特性影响的实验研究[J]. 化工学报, 2023, 74(S1): 235-244. |
| [4] | 温凯杰, 郭力, 夏诏杰, 陈建华. 一种耦合CFD与深度学习的气固快速模拟方法[J]. 化工学报, 2023, 74(9): 3775-3785. |
| [5] | 王玉兵, 李杰, 詹宏波, 朱光亚, 张大林. R134a在菱形离散肋微小通道内的流动沸腾换热实验研究[J]. 化工学报, 2023, 74(9): 3797-3806. |
| [6] | 袁佳琦, 刘政, 黄锐, 张乐福, 贺登辉. 泡状入流条件下旋流泵能量转换特性研究[J]. 化工学报, 2023, 74(9): 3807-3820. |
| [7] | 何松, 刘乔迈, 谢广烁, 王斯民, 肖娟. 高浓度水煤浆管道气膜减阻两相流模拟及代理辅助优化[J]. 化工学报, 2023, 74(9): 3766-3774. |
| [8] | 邢雷, 苗春雨, 蒋明虎, 赵立新, 李新亚. 井下微型气液旋流分离器优化设计与性能分析[J]. 化工学报, 2023, 74(8): 3394-3406. |
| [9] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [10] | 郭雨莹, 敬加强, 黄婉妮, 张平, 孙杰, 朱宇, 冯君炫, 陆洪江. 稠油管道水润滑减阻及压降预测模型修正[J]. 化工学报, 2023, 74(7): 2898-2907. |
| [11] | 高金明, 郭玉娇, 鄂承林, 卢春喜. 一种封闭罩内顺流多旋臂气液分离器的分离特性研究[J]. 化工学报, 2023, 74(7): 2957-2966. |
| [12] | 何宣志, 何永清, 闻桂叶, 焦凤. 磁液液滴颈部自相似破裂行为[J]. 化工学报, 2023, 74(7): 2889-2897. |
| [13] | 牛超, 沈胜强, 杨艳, 潘泊年, 李熠桥. 甲烷BOG喷射器流动过程计算与性能分析[J]. 化工学报, 2023, 74(7): 2858-2868. |
| [14] | 江锦波, 彭新, 许文烜, 门日秀, 刘畅, 彭旭东. 泵出型螺旋槽油气密封泄漏特性及参数影响研究[J]. 化工学报, 2023, 74(6): 2538-2554. |
| [15] | 刘起超, 周云龙, 陈聪. 起伏振动垂直上升管气液两相流截面含气率分析与计算[J]. 化工学报, 2023, 74(6): 2391-2403. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号