化工学报 ›› 2021, Vol. 72 ›› Issue (10): 5016-5027.DOI: 10.11949/0438-1157.20210617
党永强1( ),李博妮1,李可可1,张建兰1,冯香钰1,张亚婷1,2(
),李博妮1,李可可1,张建兰1,冯香钰1,张亚婷1,2( )
)
收稿日期:2021-05-06
修回日期:2021-08-10
出版日期:2021-10-05
发布日期:2021-10-05
通讯作者:
张亚婷
作者简介:党永强(1986—),男,博士,讲师,基金资助:
Yongqiang DANG1( ),Boni LI1,Keke LI1,Jianlan ZHANG1,Xiangyu FENG1,Yating ZHANG1,2(
),Boni LI1,Keke LI1,Jianlan ZHANG1,Xiangyu FENG1,Yating ZHANG1,2( )
)
Received:2021-05-06
Revised:2021-08-10
Online:2021-10-05
Published:2021-10-05
Contact:
Yating ZHANG
摘要:
利用可再生清洁能源——太阳能,将CO2转化为一氧化碳、甲烷、甲醇等,因同时具有提供可持续燃料和解决全球变暖问题的潜力而受到越来越多的关注。铁基材料因具有金属/半导体的特性和独特的电子结构,在光催化还原CO2领域具有广阔的应用潜力。基于此,各种具有高催化活性的铁基催化剂已经被设计来提高光催化还原CO2的效率。概述了近年来铁基催化剂在光催化还原二氧化碳中的研究进展,对它们的结构特征和催化活性进行了阐述和比较,最后总结了铁基催化剂在光催化还原CO2领域中待解决的问题,并展望了未来发展的方向。
中图分类号:
党永强,李博妮,李可可,张建兰,冯香钰,张亚婷. 铁基催化剂光催化还原CO2研究进展[J]. 化工学报, 2021, 72(10): 5016-5027.
Yongqiang DANG,Boni LI,Keke LI,Jianlan ZHANG,Xiangyu FENG,Yating ZHANG. Research progress in photocatalytic reduction of CO2 with iron-based catalysts[J]. CIESC Journal, 2021, 72(10): 5016-5027.

图1 半导体光催化剂上光催化CO2转化的可能机理 [19]
Fig.1 Schematic illustration of probable mechanism of photocatalytic CO2 conversion over a semiconducting photocatalyst[19]
| Reaction | Eo (vs NHE)/V |
|---|---|
CO2 + 2H+ + 2e- HCOOH HCOOH | -0.61 |
CO2 + 2H+ + 2e- CO + H2O CO + H2O | -0.53 |
CO2 + 4H+ + 4e- ΗCHO + H2O ΗCHO + H2O | -0.48 |
CO2 + 6H+ + 6e- CH3OH + H2O CH3OH + H2O | -0.38 |
CO2 + 8H+ + 8e- CH4 + 2H2O CH4 + 2H2O | -0.24 |
2CO2 + 12H+ + 12e- C2H4 + 4H2O C2H4 + 4H2O | -0.34 |
2CO2 + 12H+ + 12e- C2H5OH + 3H2O C2H5OH + 3H2O | -0.33 |
2CO2 + 14H+ + 14e- C2H6 + 4H2O C2H6 + 4H2O | -0.27 |
表1 在25℃、101.325 kPa、pH 7的水溶液中CO2还原的标准电势
Table 1 Standard potentials of CO2 reduction to various products in aqueous solutions at 25 ℃, 101.325 kPa and pH 7
| Reaction | Eo (vs NHE)/V |
|---|---|
CO2 + 2H+ + 2e- HCOOH HCOOH | -0.61 |
CO2 + 2H+ + 2e- CO + H2O CO + H2O | -0.53 |
CO2 + 4H+ + 4e- ΗCHO + H2O ΗCHO + H2O | -0.48 |
CO2 + 6H+ + 6e- CH3OH + H2O CH3OH + H2O | -0.38 |
CO2 + 8H+ + 8e- CH4 + 2H2O CH4 + 2H2O | -0.24 |
2CO2 + 12H+ + 12e- C2H4 + 4H2O C2H4 + 4H2O | -0.34 |
2CO2 + 12H+ + 12e- C2H5OH + 3H2O C2H5OH + 3H2O | -0.33 |
2CO2 + 14H+ + 14e- C2H6 + 4H2O C2H6 + 4H2O | -0.27 |

图4 空白反应、rGO、InVO4/Fe2O3、InVO4、rGO/InVO4/Fe2O3上CO2转化成甲醇的产率[37]
Fig.4 Conversion of CO2 to methanol over time using blank reaction, rGO, InVO4/Fe2O3, InVO4 and rGO/InVO4/Fe2O3 [37]
| 光催化剂 | 光敏剂 | 还原剂 | 溶剂① | 产物 | TON | 选择性 | 文献 |
|---|---|---|---|---|---|---|---|
| FeTPP | FeTPP | TEA | DMF | CO | 70 | — | [ |
| Fe-p-TMA | Ir(ppy)3 | TEA | ACN∶H2O(3∶8) | CH4 | 81 | 81% | [ |
| FeTMA | CuInS2/ZnS QD | — | H2O | CO | 450 | 99% | [ |
| FeTPP-p-TMA | 无 | BIH | CAN | CO | 63 | — | [ |
| FeTPP-p-TMA | 无 | TEA | CAN | CO | 33 | 100% | [ |
| FeTPP-p-TMA | Ir(ppy)3 | TEA | CAN | CH4 | 89 | 82% | [ |
| FeTPP-o-OH | Ir(ppy)3 | TEA | CAN | CO | 140 | 93% | [ |
| Fe3(CO)12 | [Ru(bpy)3]Cl2 | TEOA | NMP∶TEOA(5∶1) | CO | 36 | — | [ |
| Fe(CO)3bpy | [Ru(bpy)3]Cl2 | TEOA | NMP∶TEOA(5∶1) | CO | 42 | — | [ |
| (环戊二烯酮)铁-三羰基配合物 | Ir PS | TEOA | NMP | CO | 596 | — | [ |
| 环戊二烯酮铁配合物 | Cu PS | BNAH | NMP∶TEOA(5∶1) | CO | 487 | 99% | [ |
| 四联吡啶铁配合物 | BIH | MeCN∶TEOA(4∶1) | CO | 384 | 85% | [ | |
| 四联吡啶铁配合物 | Purpurin | BIH | DMF | CO | 1365 | 92% | [ |
| 四联吡啶铁配合物 | mpg-C3N4 | TEOA | ACN∶TEOA (4∶1) | CO | 155 | 97% | [ |
表2 近几年铁配合物催化剂在光催化还原CO2方面的应用
Table 2 Application of iron complex catalyst in photocatalytic reduction of CO2 in recent years
| 光催化剂 | 光敏剂 | 还原剂 | 溶剂① | 产物 | TON | 选择性 | 文献 |
|---|---|---|---|---|---|---|---|
| FeTPP | FeTPP | TEA | DMF | CO | 70 | — | [ |
| Fe-p-TMA | Ir(ppy)3 | TEA | ACN∶H2O(3∶8) | CH4 | 81 | 81% | [ |
| FeTMA | CuInS2/ZnS QD | — | H2O | CO | 450 | 99% | [ |
| FeTPP-p-TMA | 无 | BIH | CAN | CO | 63 | — | [ |
| FeTPP-p-TMA | 无 | TEA | CAN | CO | 33 | 100% | [ |
| FeTPP-p-TMA | Ir(ppy)3 | TEA | CAN | CH4 | 89 | 82% | [ |
| FeTPP-o-OH | Ir(ppy)3 | TEA | CAN | CO | 140 | 93% | [ |
| Fe3(CO)12 | [Ru(bpy)3]Cl2 | TEOA | NMP∶TEOA(5∶1) | CO | 36 | — | [ |
| Fe(CO)3bpy | [Ru(bpy)3]Cl2 | TEOA | NMP∶TEOA(5∶1) | CO | 42 | — | [ |
| (环戊二烯酮)铁-三羰基配合物 | Ir PS | TEOA | NMP | CO | 596 | — | [ |
| 环戊二烯酮铁配合物 | Cu PS | BNAH | NMP∶TEOA(5∶1) | CO | 487 | 99% | [ |
| 四联吡啶铁配合物 | BIH | MeCN∶TEOA(4∶1) | CO | 384 | 85% | [ | |
| 四联吡啶铁配合物 | Purpurin | BIH | DMF | CO | 1365 | 92% | [ |
| 四联吡啶铁配合物 | mpg-C3N4 | TEOA | ACN∶TEOA (4∶1) | CO | 155 | 97% | [ |
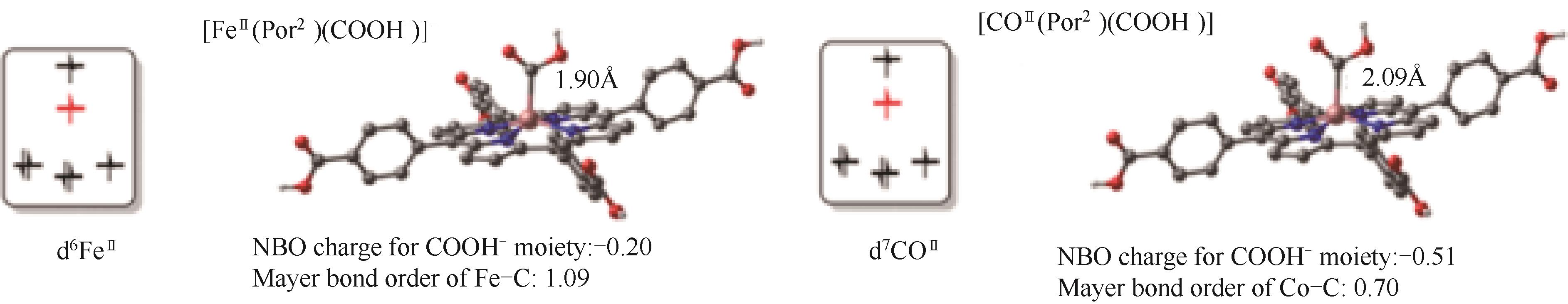
图8 [FeⅡ(Por2-)(COOH-)]-和[CoⅡ(Por2-)(COOH-)]-中金属中心的电子构型对金属-碳相互作用的影响[72]
Fig.8 Influence of the electron configurations of the metal centers on the metal-carbon interactions in [FeⅡ(Por2-)(COOH-)]- and[CoⅡ(Por2-)(COOH-)]-[72]
| 光催化剂 | 合成方法 | 产物 | 产率 | 文献 |
|---|---|---|---|---|
| g-C3N4/α-Fe2O3 | 超声波辅助法 | CO/CH4 | 15.8/3.1 μmol/(g·h) | [ |
| CN-Al-F | 湿化学法 | CO | 24 μmol/(g·h) | [ |
| g-C3N4/α-Fe2O3 | 水热法 | CH3OH | 5.63 μmol/(g·h) | [ |
| rGO/InVO4/Fe2O3 | 沉积-沉淀法 | CH3OH | 16.9 mmol/g(24 h) | [ |
| FeOx/ZSM-5 | 功能离子预吸附法 | CO/CH3CHO | 10.01/3.8 μmol/(g·h) | [ |
| ZnFe2O4 | 溶剂热法 | CH3CHO/CH3CH2OH | 57.8/13.7 μmol/(g·h) | [ |
| ZnFe2O4/Ag/TiO2 | 水热法 | CO/CH4/CH3OH | 606.25/132/31 μmol/(g·h) | [ |
| Au/CuFe2O4 | 溶剂热法 | CO | 537.6 μl/(g·h) | [ |
| NH2-MIL-101(Fe) | 水热法 | HCOO- | 178 μmol(4 h) | [ |
| NH2-MIL-101(Fe) | 水热法 | CO | 17.52 μmol/(g·h) | [ |
| MAPbI3@PCN-221(Fex) | 顺序沉积法 | CO/CH4 | 6.625/12.85 μmol/(g·h) | [ |
| NH2-MIL-101(Fe)/g-C3N4 | 水热法 | CO | 132.8 μmol/g(6 h) | [ |
| In-FenTCPP-MOF | 超声波辅助法 | CO | 3469 μmol/g(24 h) | [ |
| BiFeO3/SWCNTs | 溶胶-凝胶法 | CH3OH | 1000 μmol/g(4~6 h) | [ |
| BiFeO3-ZnO | 水热法 | — | CO2转化率:21% | [ |
| Ag2CrO4/Ag/BiFeO3@RGO | — | CH4 | 260 μmol/g(8 h) | [ |
| TiO2/碳纳米球/N-LaFeO3 | 水热法,热解法 | CO/CH4 | 150/110 μmol/g(8 h) | [ |
| Fe–TiO2 | 水热法 | CH4 | 7.73 μmol/g(12 h) | [ |
| Fe–TiO2 | 溶胶-凝胶法 | CH3OH | 约2125 μmol/g(12 h) | [ |
| Fe-N-TiO2 | 溶胶-凝胶法 | CH4/CH3OH | 38.72/1.73 μmol/(g·h) | [ |
| Fe–CeO2 | 纳米铸造法 | CO/CH4 | 12.38/2.88 μmol/(g·h) | [ |
表3 近几年铁基催化剂在光催化还原CO2方面的应用
Table 3 Application of iron-based catalysts in photocatalytic reduction of CO2 in recent years
| 光催化剂 | 合成方法 | 产物 | 产率 | 文献 |
|---|---|---|---|---|
| g-C3N4/α-Fe2O3 | 超声波辅助法 | CO/CH4 | 15.8/3.1 μmol/(g·h) | [ |
| CN-Al-F | 湿化学法 | CO | 24 μmol/(g·h) | [ |
| g-C3N4/α-Fe2O3 | 水热法 | CH3OH | 5.63 μmol/(g·h) | [ |
| rGO/InVO4/Fe2O3 | 沉积-沉淀法 | CH3OH | 16.9 mmol/g(24 h) | [ |
| FeOx/ZSM-5 | 功能离子预吸附法 | CO/CH3CHO | 10.01/3.8 μmol/(g·h) | [ |
| ZnFe2O4 | 溶剂热法 | CH3CHO/CH3CH2OH | 57.8/13.7 μmol/(g·h) | [ |
| ZnFe2O4/Ag/TiO2 | 水热法 | CO/CH4/CH3OH | 606.25/132/31 μmol/(g·h) | [ |
| Au/CuFe2O4 | 溶剂热法 | CO | 537.6 μl/(g·h) | [ |
| NH2-MIL-101(Fe) | 水热法 | HCOO- | 178 μmol(4 h) | [ |
| NH2-MIL-101(Fe) | 水热法 | CO | 17.52 μmol/(g·h) | [ |
| MAPbI3@PCN-221(Fex) | 顺序沉积法 | CO/CH4 | 6.625/12.85 μmol/(g·h) | [ |
| NH2-MIL-101(Fe)/g-C3N4 | 水热法 | CO | 132.8 μmol/g(6 h) | [ |
| In-FenTCPP-MOF | 超声波辅助法 | CO | 3469 μmol/g(24 h) | [ |
| BiFeO3/SWCNTs | 溶胶-凝胶法 | CH3OH | 1000 μmol/g(4~6 h) | [ |
| BiFeO3-ZnO | 水热法 | — | CO2转化率:21% | [ |
| Ag2CrO4/Ag/BiFeO3@RGO | — | CH4 | 260 μmol/g(8 h) | [ |
| TiO2/碳纳米球/N-LaFeO3 | 水热法,热解法 | CO/CH4 | 150/110 μmol/g(8 h) | [ |
| Fe–TiO2 | 水热法 | CH4 | 7.73 μmol/g(12 h) | [ |
| Fe–TiO2 | 溶胶-凝胶法 | CH3OH | 约2125 μmol/g(12 h) | [ |
| Fe-N-TiO2 | 溶胶-凝胶法 | CH4/CH3OH | 38.72/1.73 μmol/(g·h) | [ |
| Fe–CeO2 | 纳米铸造法 | CO/CH4 | 12.38/2.88 μmol/(g·h) | [ |
| 5 | 张睿哲, 李可可, 张凯博, 等. 煤基碳量子点/氮化碳复合材料制备及其光催化还原CO2性能[J]. 化工学报, 2020, 71(6): 2788-2794. |
| Zhang R Z, Li K K, Zhang K B, et al. Coal-based carbon quantum dots/carbon nitride composites for photocatalytic CO2 reduction[J]. CIESC Journal, 2020, 71(6): 2788-2794. | |
| 6 | Ismael M. A review on graphitic carbon nitride (g-C3N4) based nanocomposites: synthesis, categories, and their application in photocatalysis[J]. Journal of Alloys and Compounds, 2020, 846: 156446. |
| 7 | Wang Y O, Liu X, Han X Y, et al. Unique hole-accepting carbon-dots promoting selective carbon dioxide reduction nearly 100% to methanol by pure water[J]. Nature Communications, 2020, 11: 2531. |
| 8 | 张轩, 黄耀桢, 邵秀丽, 等. 结构化铜基催化剂电化学还原CO2为多碳产物研究进展[J]. 化工进展, 2021, 40(7): 3736-3746. |
| Zhang X, Huang Y Z, Shao X L, et al. Recent progress in structured Cu-based catalysts for electrochemical CO2 reduction to C2+ products[J]. Chemical Industry and Engineering Progress, 2021, 40(7): 3736-3746. | |
| 9 | Li B, Sun L Q, Bian J, et al. Controlled synthesis of novel Z-scheme iron phthalocyanine/porous WO3 nanocomposites as efficient photocatalysts for CO2 reduction[J]. Applied Catalysis B: Environmental, 2020, 270: 118849. |
| 10 | Zhang X H, Zhang L, Deng B W, et al. Visible light-responding perovskite oxide catalysts for photo-thermochemical CO2 reduction[J]. Catalysis Communications, 2020, 138: 105955. |
| 11 | Lehn J M, Ziessel R. Photochemical generation of carbon monoxide and hydrogen by reduction of carbon dioxide and water under visible light irradiation[J]. Proceedings of the National Academy of Sciences of the United States of America, 1982, 79(2): 701-704. |
| 12 | Lingampalli S R, Ayyub M M, Rao C N R. Recent progress in the photocatalyticr reduction of carbon dioxide[J]. ACS Omega, 2017, 2(6): 2740-2748. |
| 13 | Ren X H, Philo D, Li Y X, et al. Recent advances of low-dimensional phosphorus-based nanomaterials for solar-driven photocatalytic reactions[J]. Coordination Chemistry Reviews, 2020, 424: 213516. |
| 14 | Tjandra A D, Huang J. Photocatalytic carbon dioxide reduction by photocatalyst innovation[J]. Chinese Chemical Letters, 2018, 29(6): 734-746. |
| 15 | Chan S L F, Lam T L, Yang C, et al. A robust and efficient cobalt molecular catalyst for CO2 reduction[J]. Chemical Communications (Cambridge, England), 2015, 51(37): 7799-7801. |
| 1 | Cao S W, Li Y, Zhu B C, et al. Facet effect of Pd cocatalyst on photocatalytic CO2 reduction over g-C3N4[J]. Journal of Catalysis, 2017, 349: 208-217. |
| 2 | Liu H M, Song H, Zhou W, et al. A promising application of optical hexagonal TaN in photocatalytic reactions[J]. Angewandte Chemie International Editon, 2018, 57(51): 16781-16784. |
| 16 | Khalil M, Gunlazuardi J, Ivandini T A, et al. Photocatalytic conversion of CO2 using earth-abundant catalysts: a review on mechanism and catalytic performance[J]. Renewable and Sustainable Energy Reviews, 2019, 113: 109246. |
| 17 | Wang Y G, Wang F, Chen Y T, et al. Enhanced photocatalytic performance of ordered mesoporous Fe-doped CeO2 catalysts for the reduction of CO2 with H2O under simulated solar irradiation[J]. Applied Catalysis B: Environmental, 2014, 147: 602-609. |
| 18 | Sun L T, Tang Y M, Zuo W. Coronavirus pushes education online[J]. Nature Materials, 2020, 19(6): 687. |
| 19 | Li K, Peng B S, Peng T Y. Recent advances in heterogeneous photocatalytic CO2 conversion to solar fuels[J]. ACS Catalysis, 2016, 6(11): 7485-7527. |
| 20 | Karamian E, Sharifnia S. On the general mechanism of photocatalytic reduction of CO2[J]. Journal of CO2 Utilization, 2016, 16: 194-203. |
| 21 | Kang Y F, Li Y H, Fang Y W, et al. Carbon quantum dots for zebrafish fluorescence imaging[J]. Scientific Reports, 2015, 5: 11835. |
| 22 | Li K, An X Q, Park K H, et al. A critical review of CO2 photoconversion: catalysts and reactors[J]. Catalysis Today, 2014, 224: 3-12. |
| 23 | Ma Y J, Wang Z M, Xu X F, et al. Review on porous nanomaterials for adsorption and photocatalytic conversion of CO2[J]. Chinese Journal of Catalysis, 2017, 38(12): 1956-1969. |
| 24 | Iqbal F, Mumtaz A, Shahabuddin S, et al. Photocatalytic reduction of CO2 to methanol over ZnFe2O4/TiO2 (p-n) heterojunctions under visible light irradiation[J]. Journal of Chemical Technology & Biotechnology, 2020, 95(8): 2208-2221. |
| 25 | Sekizawa K, Sato S, Arai T, et al. Solar-driven photocatalytic CO2 reduction in water utilizing a ruthenium complex catalyst on p-type Fe2O3 with a multiheterojunction[J]. ACS Catalysis, 2018, 8(2): 1405-1416. |
| 26 | Sahara G, Ishitani O. Efficient photocatalysts for CO2 reduction[J]. Inorganic Chemistry, 2015, 54(11): 5096-5104. |
| 27 | Kulandaivalu T, Mohamed A R, Ali K A, et al. Photocatalytic carbon dioxide reforming of methane as an alternative approach for solar fuel production—a review[J]. Renewable and Sustainable Energy Reviews, 2020, 134: 110363. |
| 28 | Vu N N, Kaliaguine S, Do T O. Critical aspects and recent advances in structural engineering of photocatalysts for sunlight-driven photocatalytic reduction of CO2 into fuels[J]. Advanced Functional Materials, 2019, 29(31): 1901825. |
| 29 | Yamazaki Y, Takeda H, Ishitani O. Photocatalytic reduction of CO2 using metal complexes[J]. Journal of Photochemistry and Photobiology C: Photochemistry Reviews, 2015, 25: 106-137. |
| 30 | Sokol K, Robinson W E, Oliveira A R, et al. Photoreduction of CO2 with a formate dehydrogenase driven by photosystem II using a semi-artificial Z-scheme architecture[J]. Journal of the American Chemical Society, 2018, 140(48): 16418-16422. |
| 31 | He Y, Zhang L, Teng B, et al. New application of Z-scheme Ag3PO4/g-C3N4 composite in converting CO2 to fuel[J]. Environmental Science & Technology, 2015, 49(1): 649-656. |
| 32 | Yu W L, Xu D F, Peng T Y. Enhanced photocatalytic activity of g-C3N4 for selective CO2 reduction to CH3OH via facile coupling of ZnO: a direct Z-scheme mechanism[J]. Journal of Materials Chemistry A, 2015, 3(39): 19936-19947. |
| 33 | Yamamoto M, Yoshida T, Yamamoto N, et al. Photocatalytic reduction of CO2 with water promoted by Ag clusters in Ag/Ga2O3 photocatalysts[J]. Journal of Materials Chemistry A, 2015, 3(32): 16810-16816. |
| 34 | Wang Z Y, Luan D Y, Madhavi S, et al. Assembling carbon-coated α-Fe2O3 hollow nanohorns on the CNT backbone for superior lithium storage capability[J]. Energy Environ Sci, 2012, 5(1): 5252-5256. |
| 35 | Wang J S, Qin C L, Wang H J, et al. Exceptional photocatalytic activities for CO2 conversion on ALO bridged g-C3N4/α- Fe2O3 z-scheme nanocomposites and mechanism insight with isotopesZ[J]. Applied Catalysis B: Environmental, 2018, 221: 459-466. |
| 36 | Guo H W, Chen M Q, Zhong Q, et al. Synthesis of Z-scheme α-Fe2O3/g-C3N4 composite with enhanced visible-light photocatalytic reduction of CO2 to CH3OH[J]. Journal of CO2 Utilization, 2019, 33: 233-241. |
| 37 | Kumar A, Prajapati P K, Pal U, et al. Ternary rGO/InVO4/ Fe2O3Z-scheme heterostructured photocatalyst for CO2 reduction under visible light irradiation[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(7): 8201-8211. |
| 38 | Zhao X J, Chen W, Li G H, et al. Gas-phase CO2 photoreduction via iron/ZSM-5 composites[J]. Applied Catalysis A: General, 2020, 595: 117503. |
| 39 | 何小刚, 齐高璨. 铁酸盐的形貌调控及其催化还原CO2的性能研究[J]. 天津理工大学学报, 2020, 36(2): 59-64. |
| He X G, Qi G C. Morphology control of ferrites and its catalytic reduction of CO2[J]. Journal of Tianjin University of Technology, 2020, 36(2): 59-64. | |
| 40 | Gao H Y, Wang J Y, Jia M Y, et al. Construction of TiO2 nanosheets/tetra (4-carboxyphenyl) porphyrin hybrids for efficient visible-light photoreduction of CO2[J]. Chemical Engineering Journal, 2019, 374: 684-693. |
| 41 | Yadav N G, Chaudhary L S, Sakhare P A, et al. Impact of collected sunlight on Zn2FeO4 nanoparticles for photocatalytic application[J]. Journal of Colloid and Interface Science, 2018, 527: 289-297. |
| 42 | Xiao J, Yang W Y, Gao S, et al. Fabrication of ultrafine Zn2FeO4 nanoparticles for efficient photocatalytic reduction CO2 under visible light illumination[J]. Journal of Materials Science & Technology, 2018, 34(12): 2331-2336. |
| 43 | Tahir M. Well-designed Zn2FeO4/Ag/ TiO2 nanorods heterojunction with Ag as electron mediator for photocatalytic CO2 reduction to fuels under UV/visible light[J]. Journal of CO2 Utilization, 2020, 37: 134-146. |
| 44 | Chen L, Guo Z, Wei X G, et al. Molecular catalysis of the electrochemical and photochemical reduction of CO2 with earth-abundant metal complexes, selective production of CO vs HCOOH by switching of the metal center[J]. Journal of the American Chemical Society, 2015, 137(34): 10918-10921. |
| 45 | Grodkowski J, Dhanasekaran T, Neta P, et al. Reduction of cobalt and iron phthalocyanines and the role of the reduced species in catalyzed photoreduction of CO2[J]. The Journal of Physical Chemistry A, 2000, 104(48): 11332-11339. |
| 46 | Sorokin A B. Phthalocyanine metal complexes in catalysis[J]. Chemical Reviews, 2013, 113(10): 8152-8191. |
| 47 | Lin L, Hou C C, Zhang X H, et al. Highly efficient visible-light driven photocatalytic reduction of CO2 over g-C3N4 nanosheets/tetra(4-carboxyphenyl)porphyrin iron(Ⅲ) chloride heterogeneous catalysts[J]. Applied Catalysis B: Environmental, 2018, 221: 312-319. |
| 48 | Grodkowski J, Neta P. Ferrous ions as catclysts for photochemical reduction of CO2 in homogeneous solutions[J]. The Journal of Physical Chemistry A, 2000, 104(19): 4475-4479. |
| 49 | Takeda H, Ohashi K, Sekine A, et al. Photocatalytic CO2 reduction using Cu(Ⅰ) photosensitizers with a Fe(Ⅱ) catalyst[J]. Journal of the American Chemical Society, 2016, 138(13): 4354-4357. |
| 50 | Grodkowski J, Behar D, Neta P, et al. Iron porphyrin-catalyzed reduction of CO2. photochemical and radiation chemical studies[J]. The Journal of Physical Chemistry A, 1997, 101(3): 248-254. |
| 51 | Rao H, Bonin J, Robert M. Toward visible-light photochemical CO2-to-CH4 conversion in aqueous solutions using sensitized molecular catalysis[J]. The Journal of Physical Chemistry C, 2018, 122(25): 13834-13839. |
| 52 | Rao H, Bonin J, Robert M. Non-sensitized selective photochemical reduction of CO2 to CO under visible light with an iron molecular catalyst[J]. Chemical Communications, 2017, 53(19): 2830-2833. |
| 53 | Rao H, Schmidt L C, Bonin J, et al. Visible-light-driven methane formation from CO2 with a molecular iron catalyst[J]. Nature, 2017, 548(7665): 74-77. |
| 54 | Bonin J, Robert M, Routier M. Selective and efficient photocatalytic CO2 reduction to CO using visible light and an iron-based homogeneous catalyst[J]. Journal of the American Chemical Society, 2014, 136(48): 16768-16771. |
| 55 | Alsabeh P G, Rosas-Hernández A, Barsch E, et al. Iron-catalyzed photoreduction of carbon dioxide to synthesis gas[J]. Catalysis Science & Technology, 2016, 6(10): 3623-3630. |
| 56 | Rosas-Hernández A, Alsabeh P G, Barsch E, et al. Highly active and selective photochemical reduction of CO2 to CO using molecular-defined cyclopentadienone iron complexes[J]. Chemical Communications, 2016, 52(54): 8393-8396. |
| 57 | Rosas-Hernández A, Steinlechner C, Junge H, et al. Earth-abundant photocatalytic systems for the visible-light-driven reduction of CO2 to CO[J]. Green Chemistry, 2017, 19(10): 2356-2360. |
| 58 | Guo Z G, Cheng S W, Cometto C, et al. Highly efficient and selective photocatalytic CO2 reduction by iron and cobalt quaterpyridine complexes[J]. Journal of the American Chemical Society, 2016, 138(30): 9413-9416. |
| 59 | Cometto C, Kuriki R, Chen L J, et al. A carbon nitride/Fe quaterpyridine catalytic system for photostimulated CO2-to-CO conversion with visible light[J]. Journal of the American Chemical Society, 2018, 140(24): 7437-7440. |
| 60 | Gewirth A A, Varnell J A, DiAscro A M. Nonprecious metal catalysts for oxygen reduction in heterogeneous aqueous systems[J]. Chemical Reviews, 2018, 118(5): 2313-2339. |
| 61 | Lian S, Kodaimati M S, Weiss E A. Photocatalytically active superstructures of quantum dots and iron porphyrins for reduction of CO2 to CO in water[J]. ACS Nano, 2018, 12(1): 568-575. |
| 62 | Alkhatib I I, Garlisi C, Pagliaro M, et al. Metal-organic frameworks for photocatalytic CO2 reduction under visible radiation: a review of strategies and applications[J]. Catalysis Today, 2020, 340: 209-224. |
| 63 | Dhakshinamoorthy A, Asiri A M, Garcia H. Metall-organische Gerüstverbindungen: Photokatalysatoren für Redoxreaktion und Die Produktion von Solarbrennstoffen[J]. Angewandte Chemie, 2016, 128(18): 5504-5535. |
| 64 | Luo Y H, Dong L Z, Liu J, et al. From molecular metal complex to metal-organic framework: the CO2 reduction photocatalysts with clear and tunable structure[J]. Coordination Chemistry Reviews, 2019, 390: 86-126. |
| 65 | 封啸, 任颜卫, 江焕峰. 金属-有机框架材料在光催化二氧化碳还原中的应用[J]. 化学进展, 2020, 32(11): 1697-1709. |
| Feng X, Ren Y W, Jiang H F. Application of metal-organic framework materials in the photocatalytic carbon dioxide reduction[J]. Progress in Chemistry, 2020, 32(11): 1697-1709. | |
| 66 | Laurier K G M, Vermoortele F, Ameloot R, et al. Iron(Ⅲ)-based metal-organic frameworks as visible light photocatalysts[J]. Journal of the American Chemical Society, 2013, 135(39): 14488-14491. |
| 67 | Wang D K, Huang R K, Liu W J, et al. Fe-based MOFs for photocatalytic CO2 reduction: role of coordination unsaturated sites and dual excitation pathways[J]. ACS Catalysis, 2014, 4(12): 4254-4260. |
| 68 | Dao X Y, Guo J H, Wei Y P, et al. Solvent-free photoreduction of CO2 to CO catalyzed by Fe-MOFs with superior selectivity[J]. Inorganic Chemistry, 2019, 58(13): 8517-8524. |
| 69 | Chen H, Liu Y T, Cai T, et al. Boosting photocatalytic performance in mixed-valence MIL-53(Fe) by changing FeⅡ/FeⅢ ratio[J]. ACS Applied Materials & Interfaces, 2019, 11(32): 28791-28800. |
| 70 | Wu L Y, Mu Y F, Guo X X, et al. Encapsulating perovskite quantum dots in iron-based metal-organic frameworks (MOFs) for efficient photocatalytic CO2 reduction[J]. Angewandte Chemie International Esition, 2019, 58(28): 9491-9495. |
| 71 | Dao X Y, Xie X F, Guo J H, et al. Boosting photocatalytic CO2 reduction efficiency by heterostructures of NH2-MIL-101(Fe)/g-C3N4[J]. ACS Applied Energy Materials, 2020, 3(4): 3946-3954. |
| 72 | Wang S S, Huang H H, Liu M, et al. Encapsulation of single iron sites in a metal-porphyrin framework for high-performance photocatalytic CO2 reduction[J]. Inorganic Chemistry, 2020, 59(9): 6301-6307. |
| 73 | 王丽梅, 刘宇威, 周杰, 等. 钙钛矿材料光催化还原CO2综述[J]. 分子科学学报, 2020, 36(2): 96-101. |
| Wang L M, Liu Y W, Zhou J, et al. Photocatalytic reduction of CO2via perovskite materials: a review[J]. Journal of Molecular Science, 2020, 36(2): 96-101. | |
| 74 | 赵润, 杨浩. 多铁性钙钛矿薄膜的氧空位调控研究进展[J]. 物理学报, 2018, 67(15): 156101. |
| Zhao R, Yang H. Oxygen vacancies induced tuning effect on physical properties of multiferroic perovskite oxide thin films[J]. Acta Physica Sinica, 2018, 67(15): 156101. | |
| 75 | Hu Z J, Chen D, Wang S, et al. Facile synthesis of Sm-doped BiFeO3 nanoparticles for enhanced visible light photocatalytic performance[J]. Materials Science and Engineering: B, 2017, 220: 1-12. |
| 76 | 李鑫, 何世育, 李忠. 碳纳米管改性铁酸铋光催化还原CO2合成甲醇[J]. 硅酸盐学报, 2009, 37(11): 1869-1872. |
| Li X, He S Y, Li Z. Methanol synthesis in the catalytic reduction of CO2 under the visible light by BiFeO3 modified with carbon nanotubes[J]. Journal of the Chinese Ceramic Society, 2009, 37(11): 1869-1872. | |
| 77 | Karamian E, Sharifnia S. Enhanced visible light photocatalytic activity of BiFeO3-ZnO p-n heterojunction for CO2 reduction[J]. Materials Science and Engineering: B, 2018, 238: 142-148. |
| 78 | Kumar A, Sharma G, Naushad M, et al. Highly visible active Ag2CrO4/Ag/BiFeO3@RGO nano-junction for photoreduction of CO2 and photocatalytic removal of ciprofloxacin and bromate ions: the triggering effect of Ag and RGO[J]. Chemical Engineering Journal, 2019, 370: 148-165. |
| 79 | Liu Y, Ma Y J, Liu W W, et al. Facet and morphology dependent photocatalytic hydrogen evolution with CdS nanoflowers using a novel mixed solvothermal strategy[J]. Journal of Colloid and Interface Science, 2018, 513: 222-230. |
| 80 | Rather R A, Khan M, Lo I M C. High charge transfer response of g-C3N4/Ag/AgCl/BiVO4 microstructure for the selective photocatalytic reduction of CO2 to CH4 under alkali activation[J]. Journal of Catalysis, 2018, 366: 28-36. |
| 81 | Humayun M, Qu Y, Raziq F, et al. Exceptional visible-light activities of TiO2-coupled N-doped porous perovskite LaFeO3 for 2, 4-dichlorophenol decomposition and CO2 conversion[J]. Environmental Science & Technology, 2016, 50(24): 13600-13610. |
| 3 | 郭红霞, 崔继方, 刘利. 氧空位增强光催化还原CO2性能方面的研究进展[J]. 应用化学, 2020, 37(3): 256-263. |
| Guo H X, Cui J F, Liu L. Research progress in photocatalytic reduction of CO2 enhanced by oxygen vacancy[J]. Chinese Journal of Applied Chemistry, 2020, 37(3): 256-263. | |
| 4 | Wu Y A, McNulty I, Liu C, et al. Facet-dependent active sites of a single Cu2O particle photocatalyst for CO2 reduction to methanol[J]. Nature Energy, 2019, 4(11): 957-968. |
| 82 | Ileperuma O A, Tennakone K, Dissanayake W. Photocatalytic behavior of metal-doped titanium dioxide: studies on the photochemical synthesis of ammonia on Mg/TiO2 catalyst systems[J]. Applied Catalysis, 1990, 62: 1-5. |
| 83 | Abdullah H, Khan M M R, Ong H R, et al. Modified TiO2 photocatalyst for CO2 photocatalytic reduction: an overview[J]. Journal of CO2 Utilization, 2017, 22: 15-32. |
| 84 | Shehzad N, Tahir M, Johari K, et al. A critical review on TiO2 based photocatalytic CO2 reduction system: strategies to improve efficiency[J]. Journal of CO2 Utilization, 2018, 26: 98-122. |
| 85 | Xu M, Wu H, Tang Y W, et al. One-step in situ synthesis of porous Fe3+-doped TiO2 octahedra toward visible-light photocatalytic conversion of CO2 into solar fuel[J]. Microporous and Mesoporous Materials, 2020, 309: 110539. |
| 86 | Chen X Y, Ye X Z, He J X, et al. Preparation of Fe3+-doped TiO2 aerogels for photocatalytic reduction of CO2 to methanol[J]. Journal of Sol-Gel Science and Technology, 2020, 95(2): 353-359. |
| 87 | Khalilzadeh A, Shariati A. Photoconversion of CO2 over Fe-N-Ti@xSBA nanocomposite to produce hydrocarbon fuels[J]. Journal of CO2 Utilization, 2019, 33: 21-30. |
| [1] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [2] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [3] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [4] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [5] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [6] | 洪瑞, 袁宝强, 杜文静. 垂直上升管内超临界二氧化碳传热恶化机理分析[J]. 化工学报, 2023, 74(8): 3309-3319. |
| [7] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| [8] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [9] | 张谭, 刘光, 李晋平, 孙予罕. Ru基氮还原电催化剂性能调控策略[J]. 化工学报, 2023, 74(6): 2264-2280. |
| [10] | 张艳梅, 袁涛, 李江, 刘亚洁, 孙占学. 高效SRB混合菌群构建及其在酸胁迫条件下的性能研究[J]. 化工学报, 2023, 74(6): 2599-2610. |
| [11] | 胡南, 陶德敏, 杨照岚, 王学兵, 张向旭, 刘玉龙, 丁德馨. 铁炭微电解与硫酸盐还原菌耦合修复铀尾矿库渗滤水的研究[J]. 化工学报, 2023, 74(6): 2655-2667. |
| [12] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [13] | 蔺彩虹, 王丽, 吴瑜, 刘鹏, 杨江峰, 李晋平. 沸石中碱金属阳离子对CO2/N2O吸附分离性能的影响[J]. 化工学报, 2023, 74(5): 2013-2021. |
| [14] | 李晨曦, 刘永峰, 张璐, 刘海峰, 宋金瓯, 何旭. O2/CO2氛围下正庚烷的燃烧机理研究[J]. 化工学报, 2023, 74(5): 2157-2169. |
| [15] | 王皓, 唐思扬, 钟山, 梁斌. MEA吸收CO2富液解吸过程中固体颗粒表面的强化作用分析[J]. 化工学报, 2023, 74(4): 1539-1548. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号