化工学报 ›› 2022, Vol. 73 ›› Issue (6): 2289-2305.DOI: 10.11949/0438-1157.20220062
收稿日期:2022-01-12
修回日期:2022-03-13
出版日期:2022-06-05
发布日期:2022-06-30
通讯作者:
李静,魏子栋
作者简介:张文静(1996—),女,博士研究生,基金资助:
Wenjing ZHANG( ),Jing LI(
),Jing LI( ),Zidong WEI(
),Zidong WEI( )
)
Received:2022-01-12
Revised:2022-03-13
Online:2022-06-05
Published:2022-06-30
Contact:
Jing LI,Zidong WEI
摘要:
电催化过程是时空多尺度的复杂系统,探究认识多孔电极电催化体系中不同层次和尺度下的介尺度行为,对进一步强化电催化反应以及物质扩散传递,提高效率减低能耗具有重要的意义。围绕多孔电极、电催化剂以及隔膜,对多级孔电极结构的构筑、电催化剂表界面活性位调控、介尺度结构可控制备策略和隔膜结构调控中存在的介尺度现象以及介尺度效应进行了详细的综述,发现电催化体系中各层次之间的介尺度行为可以用于优化、调控、指导多孔电极、电催化剂以及隔膜的设计与制备,为丰富和完善电化学催化体系提供了新思路和新角度。
中图分类号:
张文静, 李静, 魏子栋. 介尺度视角下的电催化:从界面、隔膜到多孔电极[J]. 化工学报, 2022, 73(6): 2289-2305.
Wenjing ZHANG, Jing LI, Zidong WEI. Electrocatalysis from a mesoscale perspective: interface, membrane and porous electrode[J]. CIESC Journal, 2022, 73(6): 2289-2305.
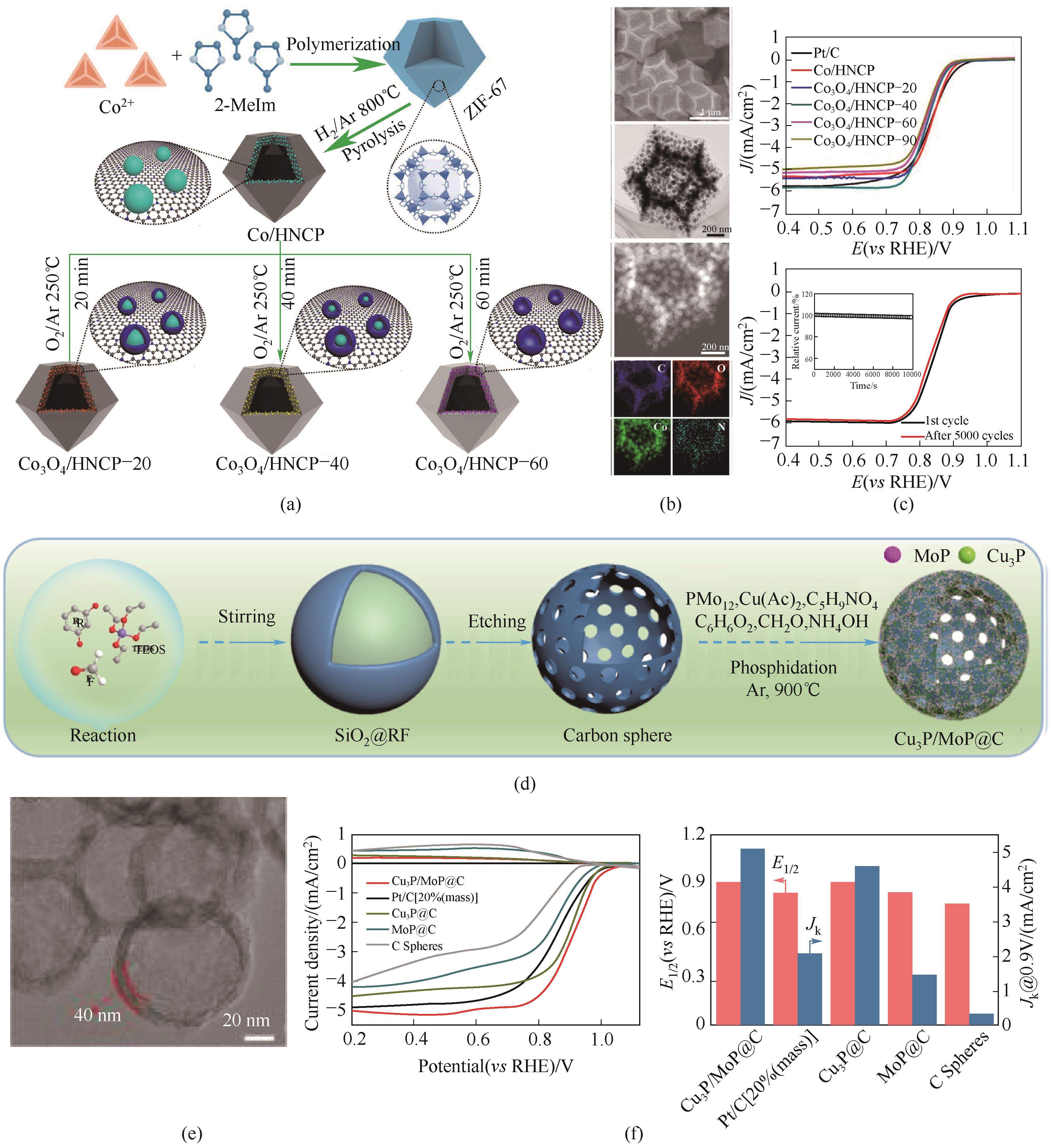
图3 (a)基于Co - Co3O4嵌入空心氮掺杂碳催化剂的示意图;(b)Co3O4/HNCP-40催化剂的SEM, TEM, STEM和EDS mapping图像;(c)ORR极化曲线和5000圈老化前后对比[15];(d)Cu3P/MoP@C催化剂的合成示意图;(e)HR-TEM图像;(f)ORR极化曲线和半波电位以及在0.9 V下的动力学电流密度对比曲线[16]
Fig.3 (a) Illustration of Co-Co3O4-based nanoarchitectures embedded in hollow nitrogen-doped carbon polyhedron; (b) SEM, TEM, STEM, and EDS mappings of Co3O4/HNCP-40; (c) ORR polarization curves and before and after 5000 cycles for the ORR[15]; (d) Schematic illustration of preparation Cu3P/MoP@C; (e) HR-TEM image; (f) ORR polarization and the corresponding half-wave potential (E1/2) and kinetic current density (Jk) at 0.9 V[16]
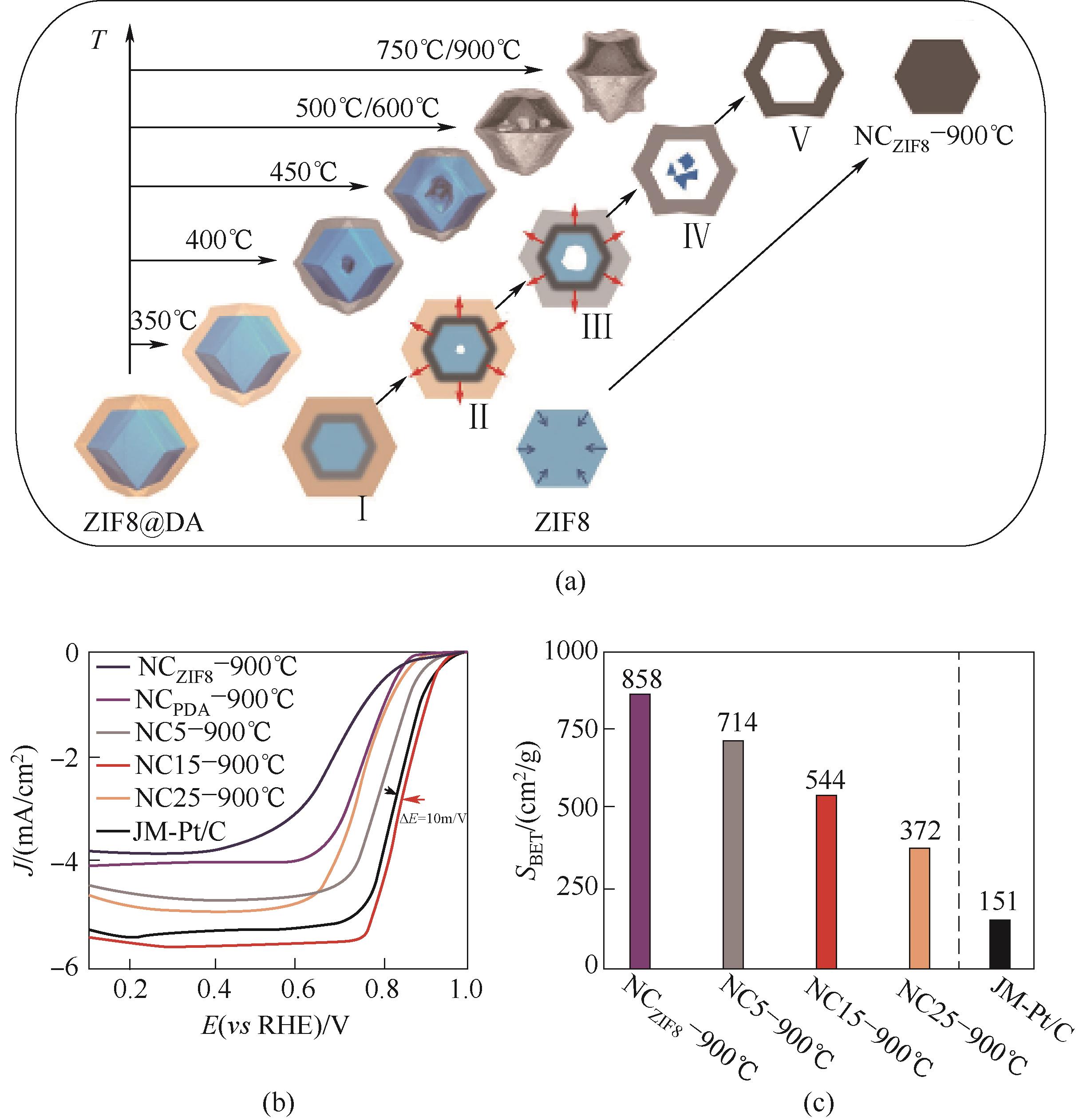
图4 (a)构造空心结构的应力诱导定向收缩机制示意图;(b)在O2饱和的0.1 mol/L KOH 溶液中的ORR极化曲线;(c)催化剂的BET比表面积对比[17]
Fig.4 (a) Schematic illustration of the stresses induced orientation contraction mechanism for constructing the hollow structures; (b) ORR polarization curves measured in O2-saturated 0.1 mol/L KOH solution; (c) The comparison of BET specific surfaces areas[17]
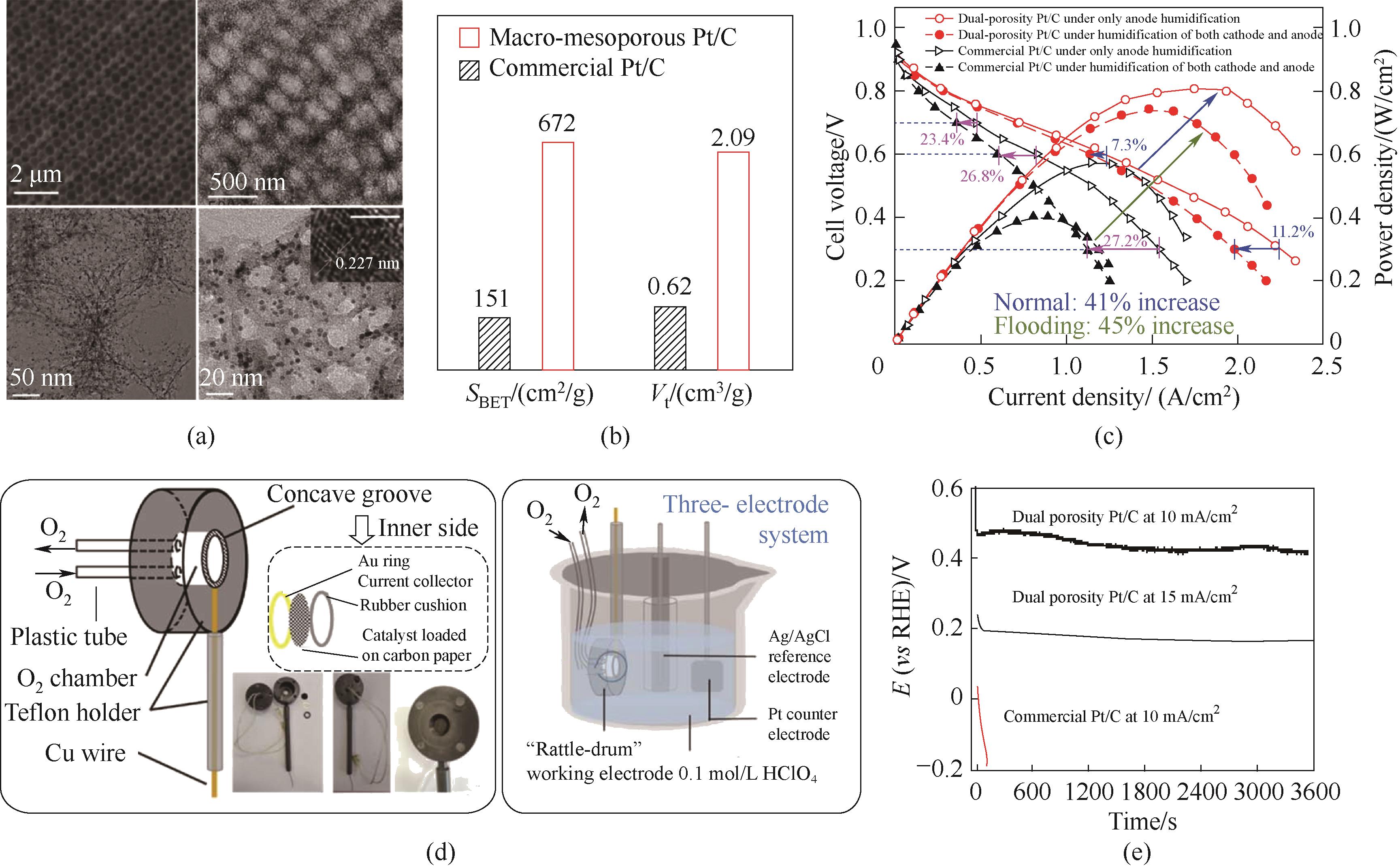
图5 (a)催化剂的SEM和TEM图像;(b)催化剂的比表面积和总孔体积比较;(c)MEA性能测试图;(d)自制“拨浪鼓”光学图片和工作示意图;(e)催化剂以10/15 mA/cm2电流密度恒电流放电时的计时电位曲线[22]
Fig.5 (a) SEM and TEM images of the catalysts; (b) Comparison of the specific surface areas and total pore volumes for the two catalysts; (c) Polarization and power densities curves of MEA; (d) The schematic and optical pictures of the self-made rattle-drum -like working electrode; (e) Chronopotentiometry curves recorded at 10/15 mA/cm2, the catalyst was loaded on the “rattle-drum” working electrode [22]
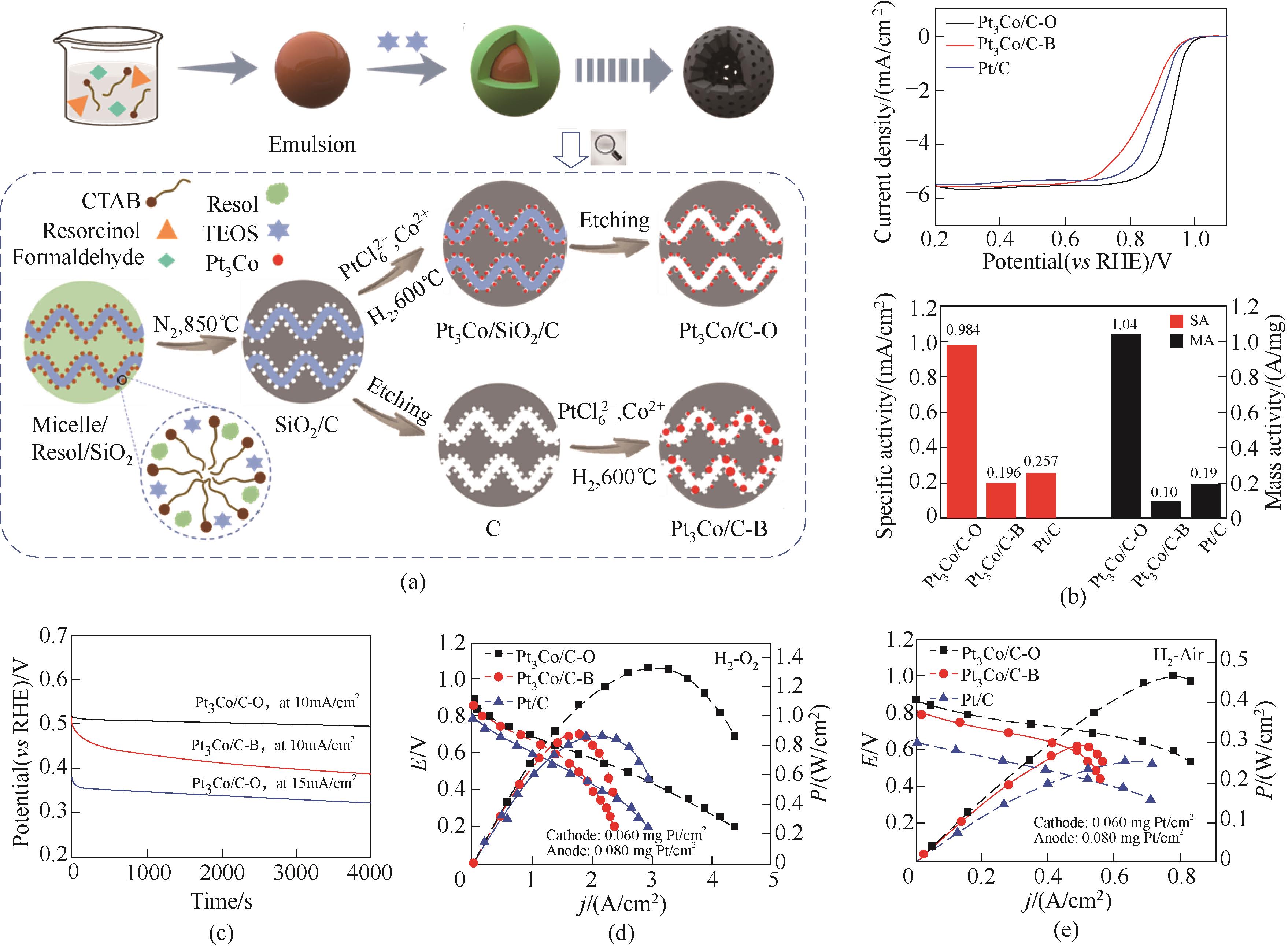
图6 (a) Pt3Co/C-O 和Pt3Co/C-B催化剂的合成示意图; (b) ORR极化曲线以及质量活性(MA)和比活性(SA)比较;(c)催化剂以10/15 mA/cm2电流密度恒电流放电时的计时电位曲线;(d) 催化剂在H2-O2和(e)H2-Air燃料电池中的极化曲线和功率密度曲线[26]
Fig.6 (a) Schematic illustration of the preparation of Pt3Co/C-O and Pt3Co/C-B catalysts; (b) ORR polarization curves and specific activity and mass activity; (c) Chronopotentiometry curves recorded at 10/15 mA/cm2, the catalyst was loaded on the “rattle-drum” working electrode; Polarization curves and power density of H2-O2 (d) and H2-Air (e) fuel cells[26]
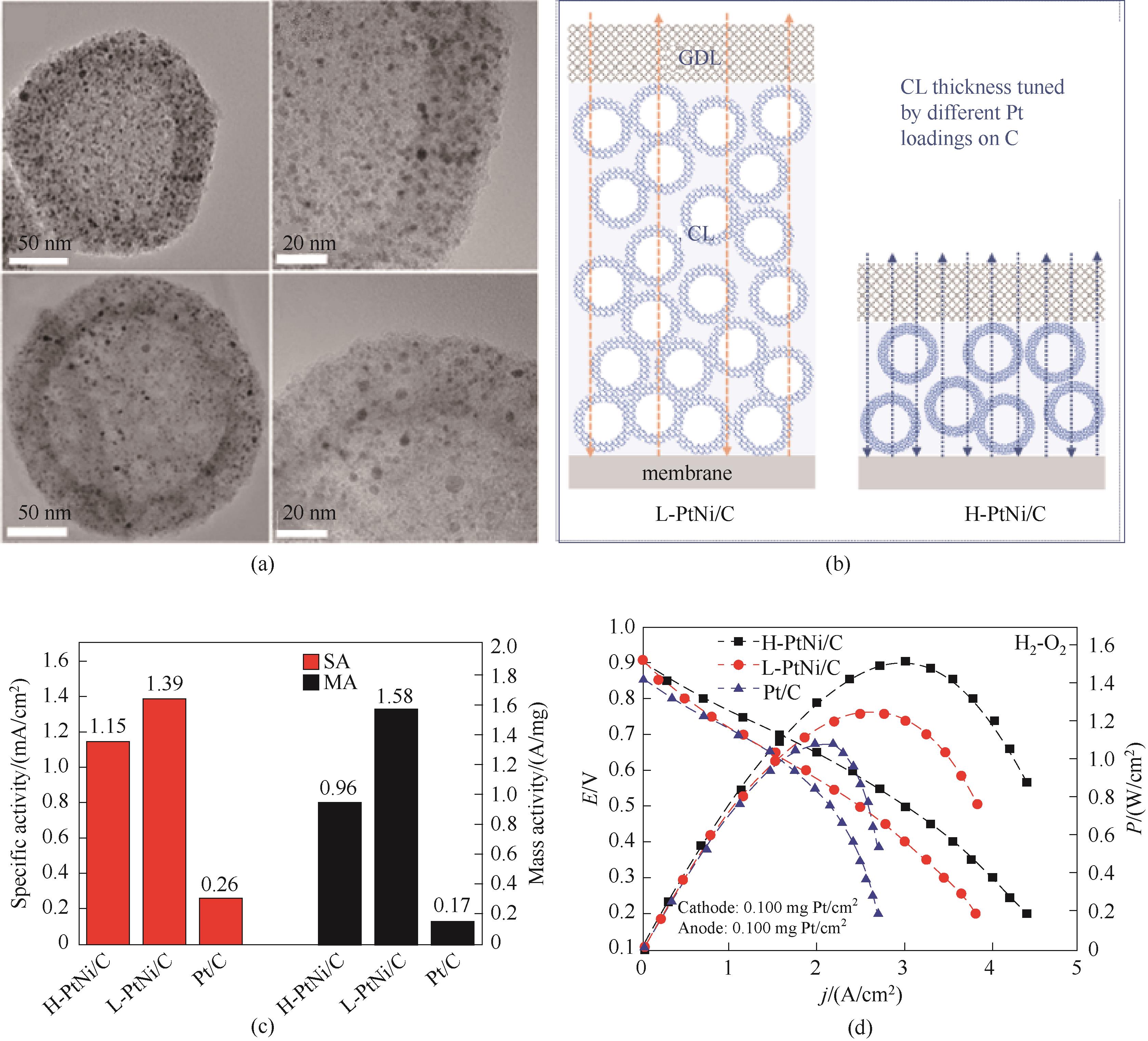
图7 (a) 高Pt (H-PtNi/C)(上图)和低Pt (L-PtNi/C)(下图)催化剂的TEM图像;(b)催化剂负载在碳(GDL为气体扩散层;CL为催化剂层) 催化剂上的膜电极(MEA)示意图;(c)催化剂在0.9 V(vs RHE)下的质量活性和比活性比较;(d)催化剂在H2-O2燃料电池中的极化曲线和功率密度曲线[27]
Fig.7 (a) TEM images of H-PtNi/C (top) and L-PtNi/C (down); (b) Schematic illustration of MEA cathodes constructed by catalysts with a high Pt loading (H-PtNi/C) and a low Pt loading (L-PtNi/C) on carbon (GDL is gas diffusion layer; CL is catalyst layer); (c) Specific activity and mass activity estimated at 0.9 V versus reversible hydrogen electrode (RHE); (d) Polarization curves and power densities of H2-O2 fuel cells[27]

图8 (a) 过电位为0.3 V时的电流密度对比[37];(b)中性pH条件下,MoP700表面HER过程示意图;(c)HER极化曲线及Tafel斜率大小比较[38]
Fig.8 (a) Current densities at η=0.3 V of different metals[37]; (b) Schematic representation of the neutral pH HER on MoP700; (c) HER polarization curves and Tafel plots[38]

图9 (a)IO-Ru-TiO2 /C、Ru-TiO2 /C和Ru/C催化剂的合成示意图[39];(b)Ru@ TiO2的HAADF-STEM图像[40];(c)Pt/O–TiO2 和 Pt/Ti–TiO2上H吸附能和H,OH,H2O的吸附自由能[41]
Fig.9 (a) Schematic diagram of the synthesis of IO-Ru–TiO2/C, Ru–TiO2/C and Ru/C catalysts[39]; (b) HAADF-STEM images of Ru@ TiO2[40]; (c) H adsorption energy (ΔE*H) and free energy (ΔGads) of H, OH and H2O on Pt/O–TiO2 and Pt/Ti–TiO2[41]

图10 Ir/Mo-MoO2,Ir/O-MoO2和Ir/MoO2在H2饱和的0.1 mol/L KOH溶液中的(a)差分电荷密度;(b) pCOHP和PDOS曲线;(c)Ir 4f XPS高分辨光谱;(d)HOR极化曲线[42]
Fig.10 (a) Charge density difference; (b) Projected crystal orbital Hamilton overlap population (pCOHP) curves and partial density of states (PDOS) of interfacial bonds;(c) Ir 4f XPS spectrum; (d) Polarization curves of investigated catalysts for HOR in H2-saturated 0.1 mol/LKOH solutions of Ir/Mo-MoO2, Ir/O-MoO2, and Ir/MoO2[42]

图11 (a)NiO-Ni3S2异质结及其表面催化电解水过程示意图[48];(b)Fe3O4/FeS2-x合成示意图;(c)OER极化曲线;(d)Fe3O4/FeS2-1和Fe3O4/FeS2-2.5的HRTEM图像[49]
Fig.11 (a) The schematic diagram of the monometallic NiO-Ni3S2 heteronanosheets and the water electrolysis process occurred on its surface[48]; (b) Illustration of the preparation of Fe3O4/FeS2-x samples; (c) OER polarization curves; (d) HRTEM images for the samples of Fe3O4/FeS2-1 and Fe3O4/FeS2-2.5[49]
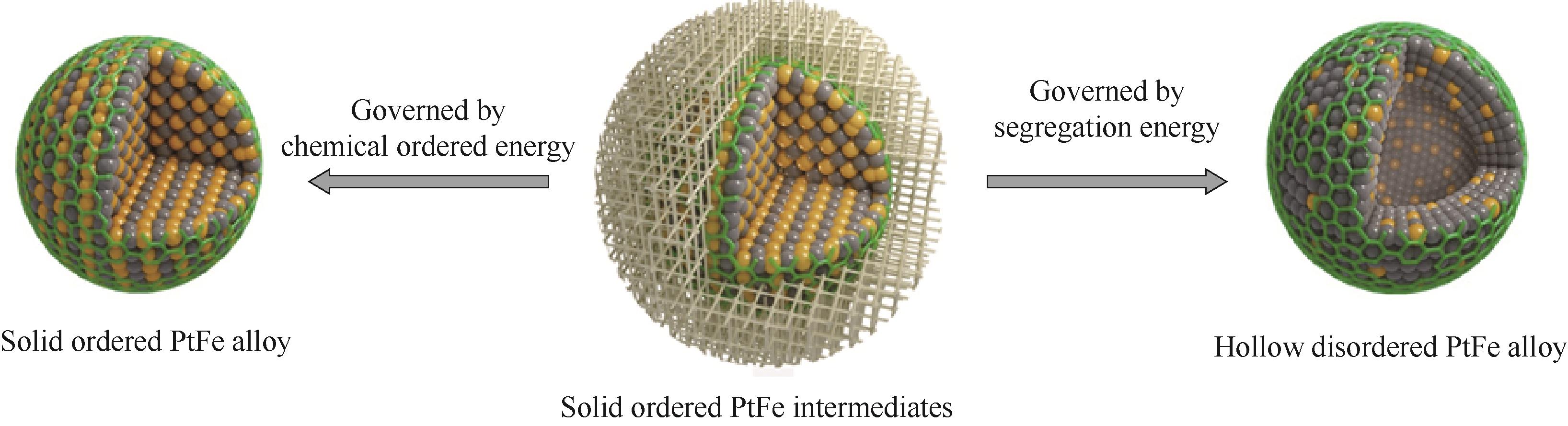
图13 基于化学有序能和表面偏析能主导的催化剂结构演变机理[55]
Fig.13 Mechanism of catalyst structure evolution based on chemical ordered energy and surface segregation energy [55]
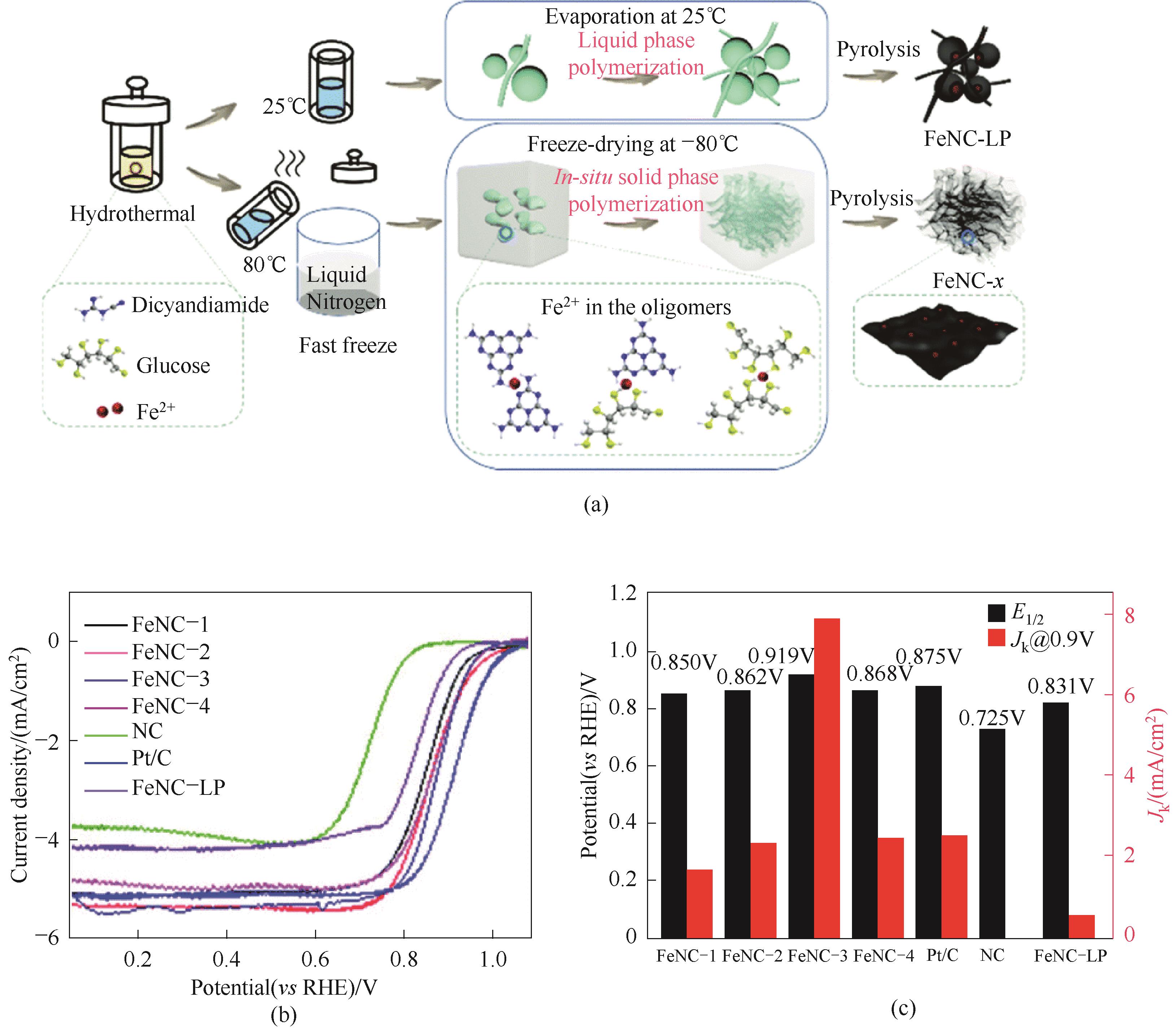
图14 (a)气凝胶结构FeNC材料制备示意图;(b)ORR极化曲线;(c)半波电位和动力学电流密度比较[59]
Fig.14 (a) Schematic illustration of the preparation of the aerogel structured FeNC materials; (b) ORR polarization curves;(c) Comparison of half wave potential and dynamic current density [59]
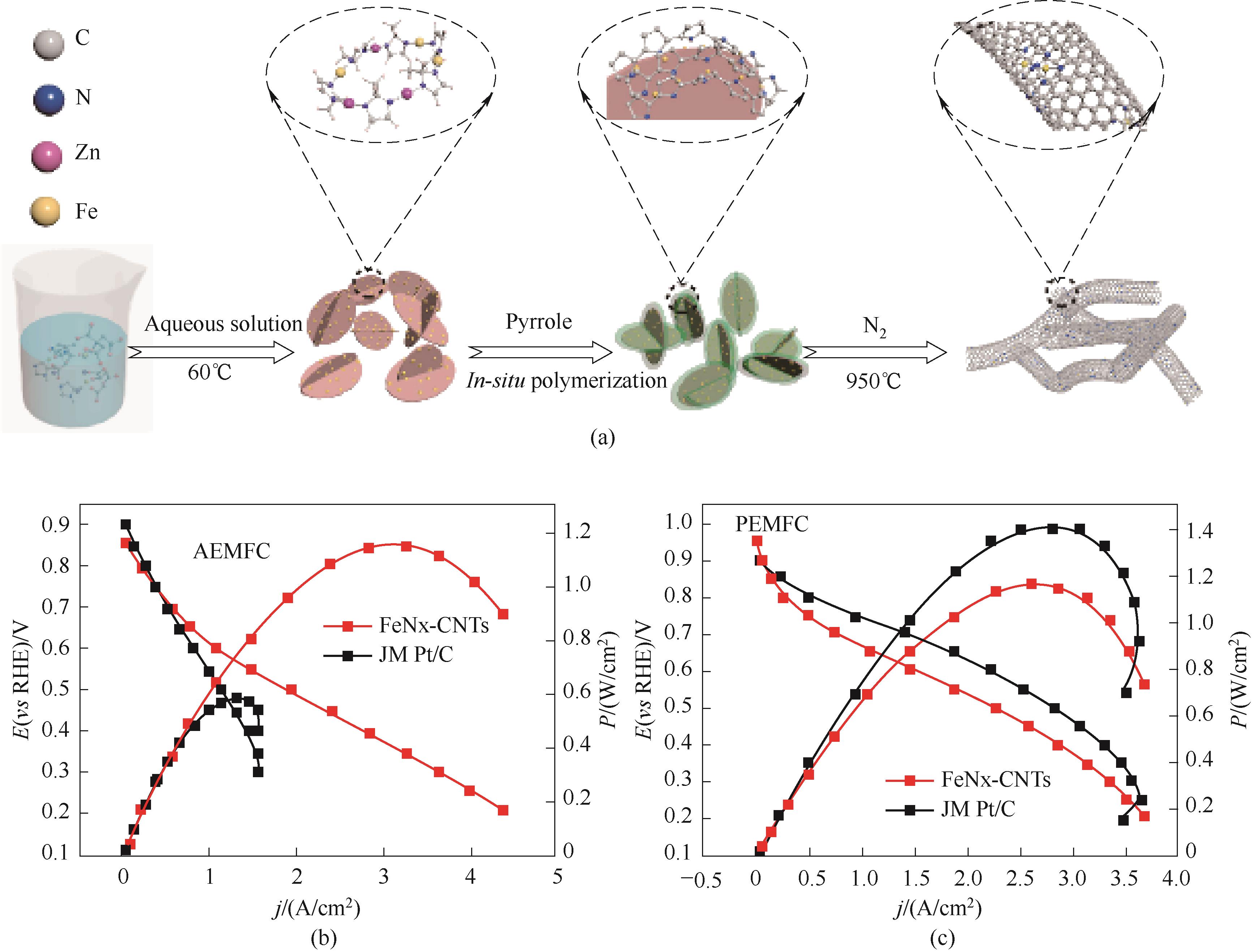
图15 (a) FeNx-CNTs催化剂的制备示意图;(b)阴离子交换膜(AEMFC)和(c)质子膜(PEMFC)H2-O2燃料电池的极化曲线和功率密度曲线[64]
Fig.15 (a) Schematic illustration of the preparation process for the FeNx-CNTs catalysts; (b),(c) Polarization curves and power densities of H2-O2 AEMFC and PEMFC [64]
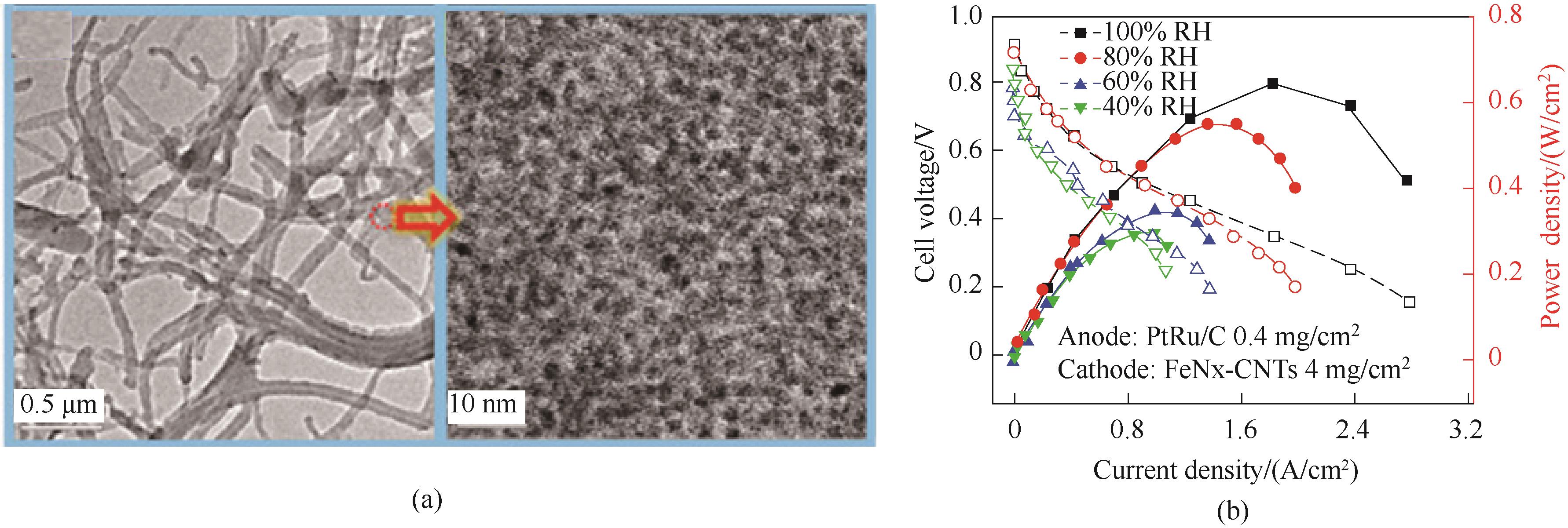
图16 (a)低倍和高倍阴离子交换膜图像(IPN AEM);(b)碱性膜(AEMFC)H2-空燃料电池的极化曲线和功率密度曲线[74]
Fig.16 (a) Low and high magnification TEM images of IPN AEM; (b) Polarization curves and power densities of H2-air AEMFC [74]
| 1 | 李宇明, 刘梓烨, 张启扬, 等. 氮掺杂碳材料的制备及其在催化领域中的应用[J]. 化工学报, 2021, 72(8): 3919-3932. |
| Li Y M, Liu Z Y, Zhang Q Y, et al. Preparation of nitrogen-doped carbon materials and their applications in catalysis[J]. CIESC Journal, 2021, 72(8): 3919-3932. | |
| 2 | Rabiee H, Ge L, Zhang X Q, et al. Gas diffusion electrodes (GDEs) for electrochemical reduction of carbon dioxide, carbon monoxide, and dinitrogen to value-added products: a review[J]. Energy & Environmental Science, 2021, 14(4): 1959-2008. |
| 3 | Wang M, Zhang L, He Y J, et al. Recent advances in transition-metal-sulfide-based bifunctional electrocatalysts for overall water splitting[J]. Journal of Materials Chemistry A, 2021, 9(9): 5320-5363. |
| 4 | Wu Z Z, Gao F Y, Gao M R. Regulating the oxidation state of nanomaterials for electrocatalytic CO2 reduction[J]. Energy & Environmental Science, 2021, 14(3): 1121-1139. |
| 5 | Wang Y, Huang X, Wei Z D. Recent developments in the use of single-atom catalysts for water splitting[J]. Chinese Journal of Catalysis, 2021, 42(8): 1269-1286. |
| 6 | 葛蔚, 刘新华, 任瑛, 等. 从多尺度到介尺度: 复杂化工过程模拟的新挑战[J]. 化工学报, 2010, 61(7): 1613-1620. |
| Ge W, Liu X H, Ren Y, et al. From multi-scale to meso-scale: new challenges for simulation of complex processes in chemical engineering[J]. CIESC Journal, 2010, 61(7): 1613-1620. | |
| 7 | 朱育丹, 陆小华, 郭晓静, 等. 材料化学工程科学内涵及方法初探: 从介观尺度界面流体行为出发认知材料[J]. 化工学报, 2013, 64(1): 148-154. |
| Zhu Y D, Lu X H, Guo X J, et al. Preliminary discussion on scientific connotation and research method of aterial-oriented chemical engineering: understanding materials based on confined interfacial fluid behavior on mesoscale[J]. CIESC Journal, 2013, 64(1): 148-154. | |
| 8 | 李静海, 胡英, 袁权. 探索介尺度科学: 从新角度审视老问题[J]. 中国科学: 化学, 2014, 44(3): 277-281. |
| Li J H, Hu Y, Yuan Q. Mesoscience: exploring old problems from a new angle[J]. Scientia Sinica Chimica, 2014, 44(3): 277-281. | |
| 9 | 叶艳玲, 李静海. 介科学: 探索介尺度共性原理[J]. 中国科技术语, 2017, 19(5): 69. |
| Ye Y L, Li J H. Mesoscience: exploring the common principle at mesoscales[J]. China Terminology, 2017, 19(5): 69. | |
| 10 | Peng L S, Wei Z D. Recent progress of mesoscience in design of electrocatalytic materials for hydrogen energy conversion[J]. Particuology, 2020, 48: 19-33. |
| 11 | 张文静, 李静, 魏子栋. 燃料电池空气电极的孔道结构调控[J]. 化工学报, 2020, 71(10): 4553-4574. |
| Zhang W J, Li J, Wei Z D. Strategies for tuning porous structures of air electrode in fuel cells[J]. CIESC Journal, 2020, 71(10): 4553-4574. | |
| 12 | Yang Z C, Zhang Y, Kong J H, et al. Hollow carbon nanoparticles of tunable size and wall thickness by hydrothermal treatment of α-cyclodextrin templated by F127 block copolymers[J]. Chemistry of Materials, 2013, 25(5): 704-710. |
| 13 | Hwang J, Kim S, Wiesner U, et al. Generalized access to mesoporous inorganic particles and hollow spheres from multicomponent polymer blends[J]. Advanced Materials, 2018, 30(27): 1801127. |
| 14 | Li B Q, Zhang S Y, Kong L, et al. Porphyrin organic framework hollow spheres and their applications in lithium-sulfur batteries[J]. Advanced Materials, 2018, 30(23): e1707483. |
| 15 | Ding D N, Shen K, Chen X D, et al. Multi-level architecture optimization of MOF-templated co-based nanoparticles embedded in hollow N-doped carbon polyhedra for efficient OER and ORR[J]. ACS Catalysis, 2018, 8(9): 7879-7888. |
| 16 | Guo M, Xu M J, Qu Y, et al. Electronic/mass transport increased hollow porous Cu3P/MoP nanospheres with strong electronic interaction for promoting oxygen reduction in Zn-air batteries[J]. Applied Catalysis B: Environmental, 2021, 297: 120415. |
| 17 | Wang M J, Mao Z X, Liu L, et al. Preparation of hollow nitrogen doped carbon via stresses induced orientation contraction[J]. Small, 2018, 14(52): e1804183. |
| 18 | Borup R, Meyers J, Pivovar B, et al. Scientific aspects of polymer electrolyte fuel cell durability and degradation[J]. Chemical Reviews, 2007, 107(10): 3904-3951. |
| 19 | Choi J Y, Yang L J, Kishimoto T, et al. Is the rapid initial performance loss of Fe/N/C non precious metal catalysts due to micropore flooding? [J]. Energy & Environmental Science, 2017, 10(1): 296-305. |
| 20 | Guo L, Jiang W J, Zhang Y, et al. Embedding Pt nanocrystals in N-doped porous carbon/carbon nanotubes toward highly stable electrocatalysts for the oxygen reduction reaction[J]. ACS Catalysis, 2015, 5(5): 2903-2909. |
| 21 | Takenaka S, Miyamoto H, Utsunomiya Y, et al. Catalytic activity of highly durable Pt/CNT catalysts covered with hydrophobic silica layers for the oxygen reduction reaction in PEFCs[J]. The Journal of Physical Chemistry C, 2014, 118(2): 774-783. |
| 22 | Wang M J, Zhao T, Luo W, et al. Quantified mass transfer and superior antiflooding performance of ordered macro-mesoporous electrocatalysts[J]. AIChE Journal, 2018, 64(7): 2881-2889. |
| 23 | Ao X, Zhang W, Zhao B T, et al. Atomically dispersed Fe–N–C decorated with Pt-alloy core–shell nanoparticles for improved activity and durability towards oxygen reduction[J]. Energy & Environmental Science, 2020, 13(9): 3032-3040. |
| 24 | Zhao W Y, Ye Y K, Jiang W J, et al. Mesoporous carbon confined intermetallic nanoparticles as highly durable electrocatalysts for the oxygen reduction reaction[J]. Journal of Materials Chemistry A, 2020, 8(31): 15822-15828. |
| 25 | Galeano C, Meier J C, Peinecke V, et al. Toward highly stable electrocatalysts via nanoparticle pore confinement[J]. Journal of the American Chemical Society, 2012, 134(50): 20457-20465. |
| 26 | Hong W, Shen X R, Wang F Z, et al. A bimodal-pore strategy for synthesis of Pt3Co/C electrocatalyst toward oxygen reduction reaction[J]. Chemical Communications, 2021, 57(35): 4327-4330. |
| 27 | Hong W, Shen X R, Wang J, et al. High-loading Pt-alloy catalysts for boosted oxygen reduction reaction performance[J]. Chinese Journal of Chemical Engineering, 2021 |
| 28 | Zhang X, Wang Y T, Liu C B, et al. Recent advances in non-noble metal electrocatalysts for nitrate reduction[J]. Chemical Engineering Journal, 2021, 403: 126269. |
| 29 | Lim K R G, Handoko A D, Nemani S K, et al. Rational design of two-dimensional transition metal carbide/nitride (MXene) hybrids and nanocomposites for catalytic energy storage and conversion[J]. ACS Nano, 2020, 14(9): 10834-10864. |
| 30 | Guo Y H, Bae J, Fang Z W, et al. Hydrogels and hydrogel-derived materials for energy and water sustainability[J]. Chemical Reviews, 2020, 120(15): 7642-7707. |
| 31 | Wang Q C, Lei Y P, Wang Y C, et al. Atomic-scale engineering of chemical-vapor-deposition-grown 2D transition metal dichalcogenides for electrocatalysis[J]. Energy & Environmental Science, 2020, 13(6): 1593-1616. |
| 32 | Xu H, Shang H Y, Wang C, et al. Ultrafine Pt-based nanowires for advanced catalysis[J]. Advanced Functional Materials, 2020, 30(28): 2000793. |
| 33 | 郑星群, 李莉, 魏子栋. 介尺度视角下的电催化剂调控策略[J]. 化工学报, 2020, 71(10): 4445-4461. |
| Zheng X Q, Li L, Wei Z D. Constructing and regulating electrocatalysts: from perspective of mesoscale[J]. CIESC Journal, 2020, 71(10): 4445-4461. | |
| 34 | Subbaraman R, Tripkovic D, Strmcnik D, et al. Enhancing hydrogen evolution activity in water splitting by tailoring Li⁺-Ni(OH)₂-Pt interfaces[J]. Science, 2011, 334(6060): 1256-1260. |
| 35 | Xiang R, Peng L S, Wei Z D. Tuning interfacial structures for better catalysis of water electrolysis[J]. Chemistry - A European Journal, 2019, 25(42): 9799-9815. |
| 36 | Yang L, Liu R M, Jiao L F. Electronic redistribution: construction and modulation of interface engineering on CoP for enhancing overall water splitting[J]. Advanced Functional Materials, 2020, 30(14): 1909618. |
| 37 | Danilovic N, Subbaraman R, Strmcnik D, et al. Enhancing the alkaline hydrogen evolution reaction activity through the bifunctionality of Ni(OH)2/metal catalysts[J]. Angewandte Chemie International Edition, 2012, 124(50): 12663-12666. |
| 38 | Xie X H, Song M, Wang L G, et al. Electrocatalytic hydrogen evolution in neutral pH solutions: dual-phase synergy[J]. ACS Catalysis, 2019, 9(9): 8712-8718. |
| 39 | Jiang J X, Tao S C, He Q, et al. Interphase-oxidized ruthenium metal with half-filled d-orbitals for hydrogen oxidation in an alkaline solution[J]. Journal of Materials Chemistry A, 2020, 8(20): 10168-10174. |
| 40 | Zhou Y, Xie Z, Jiang J, et al. Lattice-confined Ru clusters with high CO tolerance and activity for the hydrogen oxidation reaction[J]. Nature Catalysis, 2020, 3(5) : 454-462. |
| 41 | Zheng X Q, Li L, Deng M M, et al. Understanding the effect of interfacial interaction on metal/metal oxide electrocatalysts for hydrogen evolution and hydrogen oxidation reactions on the basis of first-principles calculations[J]. Catalysis Science & Technology, 2020, 10(14): 4743-4751. |
| 42 | Li M T, Xie Z Y, Zheng X Q, et al. Revealing the regulation mechanism of Ir–MoO2 interfacial chemical bonding for improving hydrogen oxidation reaction[J]. ACS Catalysis, 2021, 11(24): 14932-14940. |
| 43 | Peng L S, Liao M S, Zheng X Q, et al. Accelerated alkaline hydrogen evolution on M(OH) x /M-MoPO x (M = Ni, Co, Fe, Mn) electrocatalysts by coupling water dissociation and hydrogen ad-desorption steps[J]. Chemical Science, 2020, 11(9): 2487-2493. |
| 44 | Whittaker T, Kumar K B S, Peterson C, et al. H2 oxidation over supported Au nanoparticle catalysts: evidence for heterolytic H2 activation at the metal-support interface[J]. Journal of the American Chemical Society, 2018, 140(48): 16469-16487. |
| 45 | Miller H A, Vizza F, Marelli M, et al. Highly active nanostructured palladium-ceria electrocatalysts for the hydrogen oxidation reaction in alkaline medium[J]. Nano Energy, 2017, 33: 293-305. |
| 46 | Feng L L, Yu G T, Wu Y Y, et al. High-index faceted Ni3S2 nanosheet arrays as highly active and ultrastable electrocatalysts for water splitting[J]. Journal of the American Chemical Society, 2015, 137(44): 14023-14026. |
| 47 | Choi J, Kim D, Zheng W R, et al. Interface engineered NiFe2O4-x/NiMoO4 nanowire arrays for electrochemical oxygen evolution[J]. Applied Catalysis B: Environmental, 2021, 286: 119857. |
| 48 | Peng L S, Shen J J, Zheng X Q, et al. Rationally design of monometallic NiO-Ni3S2/NF heteronanosheets as bifunctional electrocatalysts for overall water splitting[J]. Journal of Catalysis, 2019, 369: 345-351. |
| 49 | Wang M J, Zheng X Q, Song L L, et al. Fe3O4/FeS2 heterostructures enable efficient oxygen evolution reaction[J]. Journal of Materials Chemistry A, 2020, 8(28): 14145-14151. |
| 50 | Liu L C, Corma A. Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles[J]. Chemical Reviews, 2018, 118(10): 4981-5079. |
| 51 | Yang F, Deng D H, Pan X L, et al. Understanding nano effects in catalysis[J]. National Science Review, 2015, 2(2): 183-201. |
| 52 | Cheng N C, Sun X L. Single atom catalyst by atomic layer deposition technique[J]. Chinese Journal of Catalysis, 2017, 38(9): 1508-1514. |
| 53 | Qiao B T, Liang J X, Wang A Q, et al. Single atom gold catalysts for low-temperature CO oxidation[J]. Chinese Journal of Catalysis, 2016, 37(10): 1580-1586. |
| 54 | Wang Q M, Chen S G, Shi F, et al. Structural evolution of solid Pt nanoparticles to a hollow PtFe alloy with a Pt-skin surface via space-confined pyrolysis and the nanoscale kirkendall effect[J]. Advanced Materials, 2016, 28(48): 10673-10678. |
| 55 | Zou X, Chen S G, Wang Q M, et al. Leaching- and sintering-resistant hollow or structurally ordered intermetallic PtFe alloy catalysts for oxygen reduction reactions[J]. Nanoscale, 2019, 11(42): 20115-20122. |
| 56 | Wang J, Huang Z Q, Liu W, et al. Design of N-coordinated dual-metal sites: a stable and active Pt-free catalyst for acidic oxygen reduction reaction[J]. Journal of the American Chemical Society, 2017, 139(48): 17281-17284. |
| 57 | Zhang H G, Hwang S, Wang M Y, et al. Single atomic iron catalysts for oxygen reduction in acidic media: particle size control and thermal activation[J]. Journal of the American Chemical Society, 2017, 139(40): 14143-14149. |
| 58 | Chen Y J, Ji S F, Wang Y G, et al. Isolated single iron atoms anchored on N-doped porous carbon as an efficient electrocatalyst for the oxygen reduction reaction[J]. Angewandte Chemie International Edition, 2017, 56(24): 6937-6941. |
| 59 | Hong W, Feng X, Tan L Q, et al. Preparation of monodisperse ferrous nanoparticles embedded in carbon aerogels via in situ solid phase polymerization for electrocatalytic oxygen reduction[J]. Nanoscale, 2020, 12(28): 15318-15324. |
| 60 | Jahnke H, Schönborn M, Zimmermann G. Organic dyestuffs as catalysts for fuel cells[J]. Topics in Current Chemistry, 1976, 61:131-181. |
| 61 | Gupta S, Tryk D, Bae I, et al. Heat-treated polyacrylonitrile-based catalysts for oxygen electroreduction[J]. Journal of Applied Electrochemistry, 1989, 19(1): 19-27. |
| 62 | Masa J, Xia W, Muhler M, et al. On the role of metals in nitrogen-doped carbon electrocatalysts for oxygen reduction[J]. Angewandte Chemie International Edition, 2015, 54(35): 10102-10120. |
| 63 | Ren Q, Wang H, Lu X F, et al. Recent progress on MOF-derived heteroatom-doped carbon-based electrocatalysts for oxygen reduction reaction[J]. Advanced Science, 2018, 5(3): 1700515. |
| 64 | He Q, Zeng L P, Wang J, et al. Polymer-coating-induced synthesis of FeN x enriched carbon nanotubes as cathode that exceeds 1.0 W·cm-2 peak power in both proton and anion exchange membrane fuel cells[J]. Journal of Power Sources, 2021, 489: 229499. |
| 65 | 李存璞, 王建川, 魏子栋. 电化学反应器隔膜材料的分子设计与介尺度策略[J]. 化工学报, 2020, 71(10): 4490-4501. |
| Li C P, Wang J C, Wei Z D. Mesoscopic strategies and molecular design of diaphragm for electrochemical reactors[J]. CIESC Journal, 2020, 71(10): 4490-4501. | |
| 66 | Varcoe J R, Atanassov P, Dekel D R, et al. Anion-exchange membranes in electrochemical energy systems[J]. Energy & Environmental Science, 2014, 7(10): 3135-3191. |
| 67 | Wang Y J, Qiao J L, Baker R, et al. Alkaline polymer electrolyte membranes for fuel cell applications[J]. Chemical Society Reviews, 2013, 42(13): 5768-5787. |
| 68 | Wang L Q, Peng X, Mustain W E, et al. Radiation-grafted anion-exchange membranes: the switch from low- to high-density polyethylene leads to remarkably enhanced fuel cell performance[J]. Energy & Environmental Science, 2019, 12(5): 1575-1579. |
| 69 | Wang J, Zhao Y, Setzler B P, et al. Poly(aryl piperidinium) membranes and ionomers for hydroxide exchange membrane fuel cells [J]. Nature Energy, 2019, 4(5): 392-398. |
| 70 | Hamada T, Hasegawa S, Fukasawa H, et al. Poly(ether ether ketone) (PEEK)-based graft-type polymer electrolyte membranes having high crystallinity for high conducting and mechanical properties under various humidified conditions[J]. Journal of Materials Chemistry A, 2015, 3(42): 20983-20991. |
| 71 | Lee W H, Kim Y S, Bae C. Robust hydroxide ion conducting poly(biphenyl alkylene)s for alkaline fuel cell membranes[J]. ACS Macro Letters, 2015, 4(8): 814-818. |
| 72 | Zhao W F, He C, Nie C X, et al. Synthesis and characterization of ultrahigh ion-exchange capacity polymeric membranes[J]. Industrial & Engineering Chemistry Research, 2016, 55(36): 9667-9675. |
| 73 | Park A M, Turley F E, Wycisk R J, et al. Electrospun and cross-linked nanofiber composite anion exchange membranes[J]. Macromolecules, 2014, 47(1): 227-235. |
| 74 | Zeng L P, Liao Y C, Wang J C, et al. Construction of highly efficient ion channel within anion exchange membrane based on interpenetrating polymer network for H2/Air (CO2-free) alkaline fuel cell[J]. Journal of Power Sources, 2021, 486: 229377. |
| 75 | Zeng L P, He Q, Liao Y C, et al. Anion exchange membrane based on interpenetrating polymer network with ultrahigh ion conductivity and excellent stability for alkaline fuel cell[J]. Research, 2020, 2020: 4794706. |
| [1] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [2] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [3] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [4] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [5] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [6] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [7] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| [8] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| [9] | 涂玉明, 邵高燕, 陈健杰, 刘凤, 田世超, 周智勇, 任钟旗. 钙基催化剂的设计合成及应用研究进展[J]. 化工学报, 2023, 74(7): 2717-2734. |
| [10] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [11] | 张希庆, 王琰婷, 徐彦红, 常淑玲, 孙婷婷, 薛定, 张立红. Mg量影响的纳米片负载Pt-In催化异丁烷脱氢性能[J]. 化工学报, 2023, 74(6): 2427-2435. |
| [12] | 周继鹏, 何文军, 李涛. 异形催化剂上乙烯催化氧化失活动力学反应工程计算[J]. 化工学报, 2023, 74(6): 2416-2426. |
| [13] | 张谭, 刘光, 李晋平, 孙予罕. Ru基氮还原电催化剂性能调控策略[J]. 化工学报, 2023, 74(6): 2264-2280. |
| [14] | 王辰, 史秀锋, 武鲜凤, 魏方佳, 张昊虹, 车寅, 吴旭. 氧化还原法制备Mn3O4催化剂及其甲苯催化氧化性能与机理研究[J]. 化工学报, 2023, 74(6): 2447-2457. |
| [15] | 李勇, 高佳琦, 杜超, 赵亚丽, 李伯琼, 申倩倩, 贾虎生, 薛晋波. Ni@C@TiO2核壳双重异质结的构筑及光热催化分解水产氢[J]. 化工学报, 2023, 74(6): 2458-2467. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号