化工学报 ›› 2022, Vol. 73 ›› Issue (9): 4079-4086.DOI: 10.11949/0438-1157.20220470
收稿日期:2022-04-01
修回日期:2022-06-30
出版日期:2022-09-05
发布日期:2022-10-09
通讯作者:
朱腾龙
作者简介:张婉晨(2000—),女,硕士研究生,1531025188@qq.com
基金资助:
Wanchen ZHANG( ), Xiaoyang CHEN, Qiuqiu LYU, Qin ZHONG, Tenglong ZHU(
), Xiaoyang CHEN, Qiuqiu LYU, Qin ZHONG, Tenglong ZHU( )
)
Received:2022-04-01
Revised:2022-06-30
Online:2022-09-05
Published:2022-10-09
Contact:
Tenglong ZHU
摘要:
化工副产气是具有一定热值的可燃性复杂气体,但一直以来提纯再利用经济效益低,大多采用直接燃烧的方法处理。针对当前化学工业开展绿色低碳转型的需求,研究了固体氧化物燃料电池(SOFC)采用化工副产气发电的性能。以SrTi0.3Fe0.7O3-δ (STF)钙钛矿氧化物为基础,采用高温固相合成法制备了B位Co掺杂的Sr0.95Ti0.33Fe0.6Co0.07O3-δ (STFC)阳极,以不同种类和组成的模拟化工副产气作为燃料,系统研究了单电池的电化学性能和稳定性。结果表明,STFC钙钛矿氧化物在加湿氢气中还原后原位析出Co0.28Fe0.72纳米合金颗粒;以其为阳极的SOFC单电池在多种模拟化工副产气燃料下实现了高性能运行,表现出较小的极化阻抗和良好的电化学性能输出;在含有多种碳氢组分的复杂燃料下表现出卓越的长期运行稳定性。
中图分类号:
张婉晨, 陈晓阳, 吕秋秋, 钟秦, 朱腾龙. Co掺杂SrTi0.3Fe0.7O3-δ 阳极SOFC在化工副产气燃料下的性能及稳定性[J]. 化工学报, 2022, 73(9): 4079-4086.
Wanchen ZHANG, Xiaoyang CHEN, Qiuqiu LYU, Qin ZHONG, Tenglong ZHU. Performance and durability of cobalt doped SrTi0.3Fe0.7O3-δ anode SOFC fueled with by-product gas from chemical industry[J]. CIESC Journal, 2022, 73(9): 4079-4086.
| 化工工艺源 | 副产气组成/%(体积) | 热值(LHV)/(MJ/m3) |
|---|---|---|
| 炼油 | N2 13.1、CO2 0.7、CO 1.3、H2 30.6、CH4 35.1、C2H6 10.9、C3H8 8.3 | 30~32 |
| 丙烷脱氢 | H2、CO2、CO、CH4、C2H4、C2H6、C3H6、C3H8 | 25~27 |
| 炼焦工业 | H2 54~60、CH4 19~24、CO、CO2 | 17~19 |
| 合成氨 | NH3 17.9、H2 46.5、N2 15.1、CH4 46.5、Ar | 13~15 |
| 碳化硅合成 | CO 70~90、CO2 2~3、H2 1~5、N2 1~3、CH4 2~4 | 10~14 |
表1 典型化工副产气的组成及热值
Table 1 Composition and heat value of typical by-product gases
| 化工工艺源 | 副产气组成/%(体积) | 热值(LHV)/(MJ/m3) |
|---|---|---|
| 炼油 | N2 13.1、CO2 0.7、CO 1.3、H2 30.6、CH4 35.1、C2H6 10.9、C3H8 8.3 | 30~32 |
| 丙烷脱氢 | H2、CO2、CO、CH4、C2H4、C2H6、C3H6、C3H8 | 25~27 |
| 炼焦工业 | H2 54~60、CH4 19~24、CO、CO2 | 17~19 |
| 合成氨 | NH3 17.9、H2 46.5、N2 15.1、CH4 46.5、Ar | 13~15 |
| 碳化硅合成 | CO 70~90、CO2 2~3、H2 1~5、N2 1~3、CH4 2~4 | 10~14 |
| 编号 | 气体组成 | 比例 |
|---|---|---|
| 1 | H2、CO2、CO | x(CO2)=10%,y(H2)=6%~45 %,z(CO)=100%-x-y |
| 2 | H2、CO2、CH4 | x(CO2)=10%,y(CH4)=3%~12 %,z(H2)=100%-x-y |
| 3 | 某化工企业炼油工段副产气 | N2 (13.1%)、CO2 (0.7%)、CO (1.3%)、H2 (30.6%)、CH4 (35.1%)、C2H6 (10.9%)、C3H8 (8.3%) |
表2 模拟化工副产气及炼油某工段副产气组成
Table 2 The compositions of simulated by-product gas and by-product gas in refinery section
| 编号 | 气体组成 | 比例 |
|---|---|---|
| 1 | H2、CO2、CO | x(CO2)=10%,y(H2)=6%~45 %,z(CO)=100%-x-y |
| 2 | H2、CO2、CH4 | x(CO2)=10%,y(CH4)=3%~12 %,z(H2)=100%-x-y |
| 3 | 某化工企业炼油工段副产气 | N2 (13.1%)、CO2 (0.7%)、CO (1.3%)、H2 (30.6%)、CH4 (35.1%)、C2H6 (10.9%)、C3H8 (8.3%) |
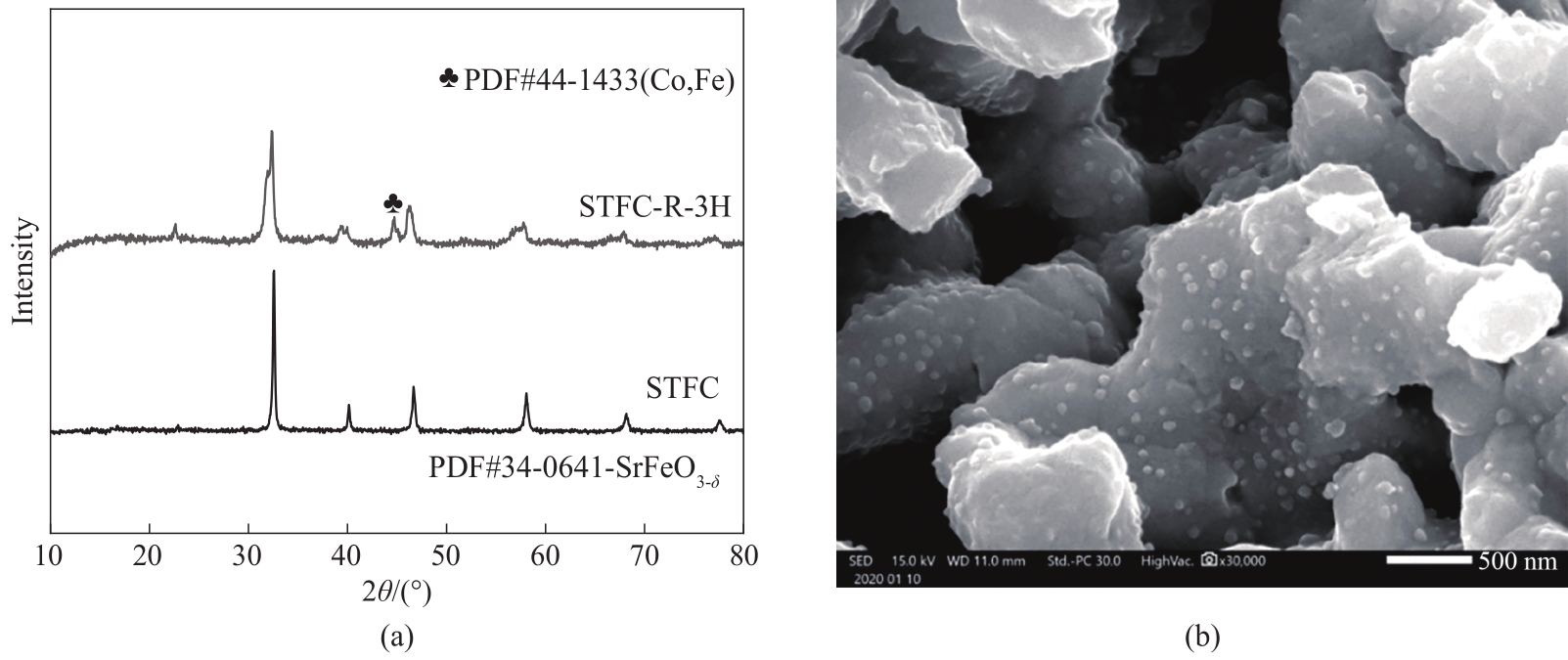
图1 STFC粉体在1100℃空气煅烧和在800℃氢气(约3% H2O)还原后XRD谱图(a); STFC粉体在800℃氢气(约3% H2O)还原后SEM图(b)
Fig.1 XRD pattern of STFC calcined in air at 1100℃ and reduced in wet hydrogen at 800℃ (a); SEM image of STFC reduced in wet hydrogen at 800℃ (b)
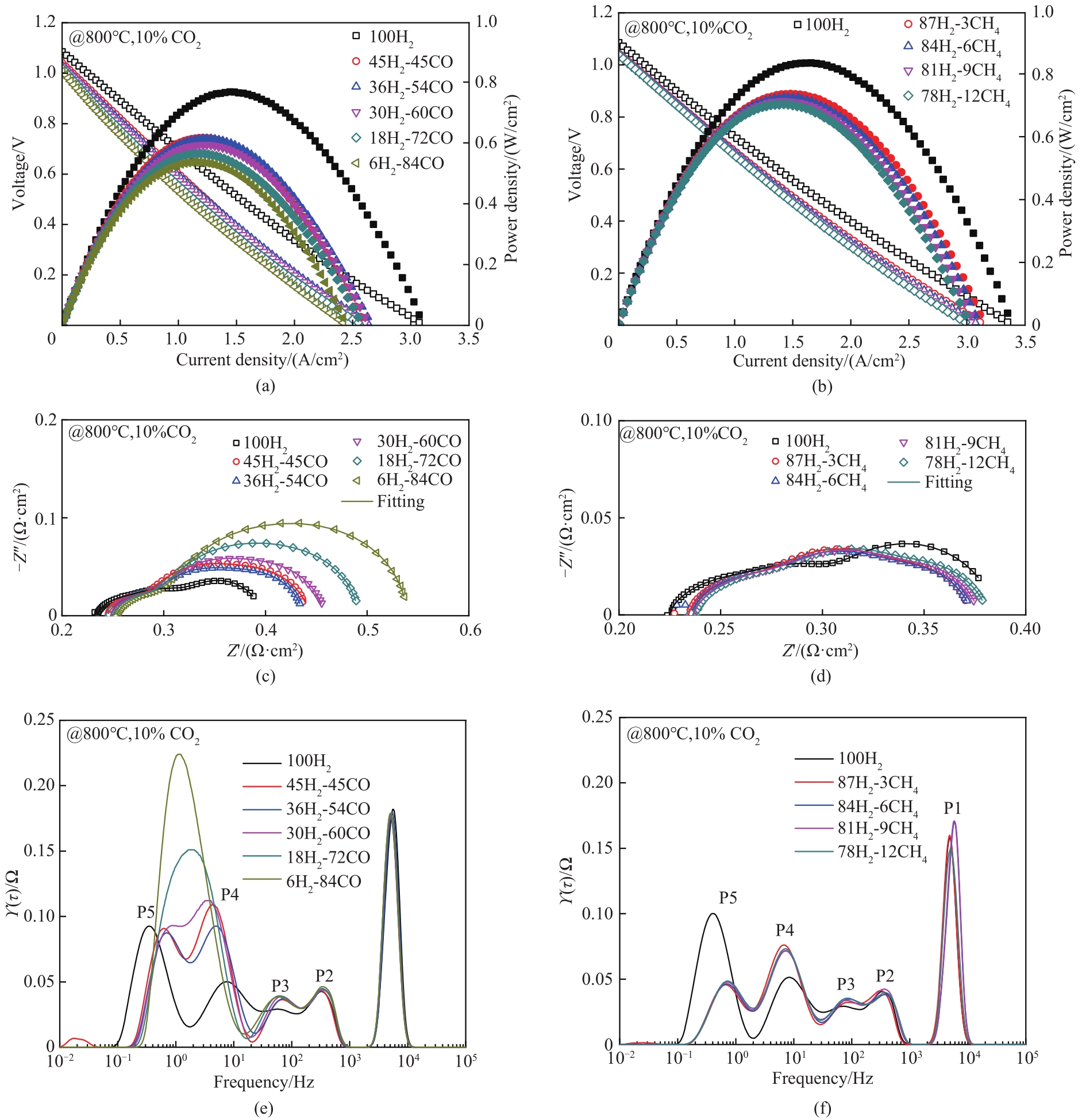
图2 800℃时STFC阳极在不同组成模拟副产气中的电化学性能:(a), (b) CO2-H2-CO及CO2-H2-CH4燃料体系下单电池的电流密度-电压-功率密度(J-V-P)曲线;(c), (e), (d), (f) CO2-H2-CO及CO2-H2-CH4燃料体系下单电池的电化学阻抗谱(EIS)与相应的DRT分析
Fig.2 Electrochemical performance of STFC cell under simulated by-product gases at 800℃.(a), (b) Current density-voltage-power density (J-V-P) curves of STFC cell under CO2-H2-CO and CO2-H2-CH4 fuels; (c), (e), (d), (f) Electrochemical impedance spectroscopy (EIS) and the corresponding DRT analysis of STFC cell under CO2-H2-CO and CO2-H2-CH4 fuels
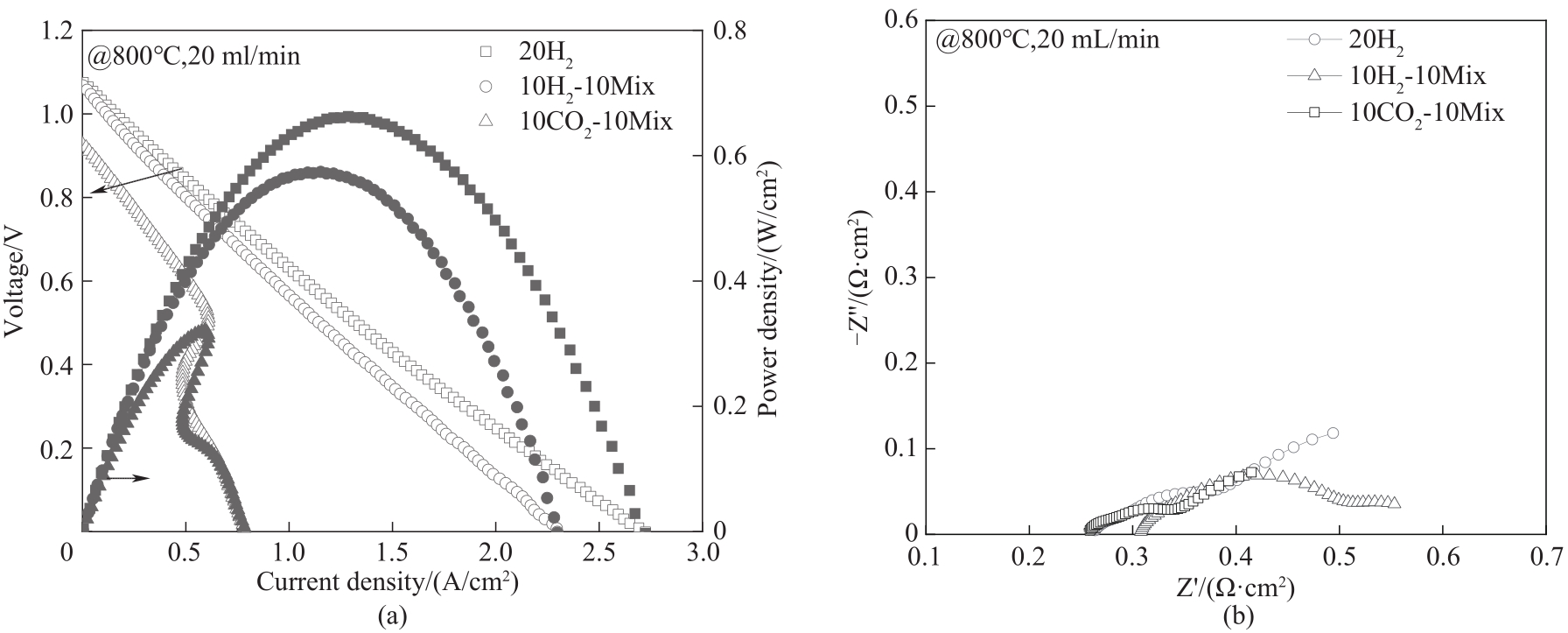
图4 800 ℃时STFC单电池在副产气燃料下的电流密度-电压-功率密度曲线及(a); 800℃时STFC单电池在副产气燃料下的电化学阻抗谱图(b)
Fig.4 J-V-P curves of single cell under by-product gas at 800℃ (a); EIS results of single cell under by-product gas at 800℃ (b)
| 1 | 韩敏芳, 彭苏萍. 碳基燃料固体氧化物燃料电池发展前景[J]. 中国工程科学, 2013, 15(2): 4-6, 26. |
| Han M F, Peng S P. Prospect of carbon-based solid oxide fuel cells[J]. Engineering Sciences, 2013, 15(2): 4-6, 26. | |
| 2 | Lai K Y, Manthiram A. Self-regenerating Co-Fe nanoparticles on perovskite oxides as a hydrocarbon fuel oxidation catalyst in solid oxide fuel cells[J]. Chemistry of Materials, 2018, 30(8): 2515-2525. |
| 3 | 杨晓勤, 肖聪, 杨晓华, 等. 合成氨尾气综合回收副产LNG的研究与实践[J]. 中氮肥, 2015(1): 11-13. |
| Yang X Q, Xiao C, Yang X H, et al. Research and practice on comprehensive recovery by-product LNG by synthesis ammonia tail gas[J]. M-Sized Nitrogenous Fertilizer Progress, 2015(1): 11-13. | |
| 4 | 齐景丽, 孔繁荣. 我国焦炉气化工利用现状及前景展望[J]. 天然气化工(C1化学与化工), 2013, 38(1): 60-64. |
| Qi J L, Kong F R. Status and prospect for chemical utilization of coke oven gas in China[J]. Natural Gas Chemical Industry, 2013, 38(1): 60-64. | |
| 5 | 付鹏兵, 蔡俊艳. 煤制油项目炼油装置副产气回收利用探讨[J]. 山西化工, 2018, 38(3): 85-87. |
| Fu P B, Cai J Y. Discussion on recovery and utilization of by-product gas from refining unit of coal-to-oil project [J]. Shanxi Chemical Industry, 2018, 38(3): 85-87. | |
| 6 | 张炜. 炼厂制氢技术路线选择和成本分析[J]. 化学工程, 2010, 38(10): 141-145. |
| Zhang W. Selection of route for hydrogen production in refinery and its cost analysis[J]. Chemical Engineering(China), 2010, 38(10): 141-145. | |
| 7 | Zhu B, Bai X Y, Chen G X, et al. Fundamental study on biomass-fuelled ceramic fuel cell[J]. International Journal of Energy Research, 2002, 26(1): 57-66. |
| 8 | Guerra C, Lanzini A, Leone P, et al. Optimization of dry reforming of methane over Ni/YSZ anodes for solid oxide fuel cells[J]. Journal of Power Sources, 2014, 245: 154-163. |
| 9 | Sameshima S, Furukawa N, Hirata Y, et al. Cell performance of SOFC using CH4-CO2 mixed gases[J]. Ceramics International, 2014, 40(4): 6279-6284. |
| 10 | Shabri H A, Othman M H D, Mohamed M A, et al. Recent progress in metal-ceramic anode of solid oxide fuel cell for direct hydrocarbon fuel utilization: a review[J]. Fuel Processing Technology, 2021, 212: 106626. |
| 11 | Singhal S C, Kendall K. High-temperature solid oxide fuel cells: fundamentals, design and applications[J]. Materials Today, 2002, 5(12): 55. |
| 12 | Maček J, Novosel B, Marinšek M. Ni-YSZ SOFC anodes-Minimization of carbon deposition[J]. Journal of the European Ceramic Society, 2007, 27(2/3): 487-491. |
| 13 | Ishihara T. Perovskite Oxide for Solid Oxide Fuel Cells[M]. New York: Springer Science+Business Media, LLC, 2009. |
| 14 | Fowler D E, Messner A C, Miller E C, et al. Decreasing the polarization resistance of (La,Sr)CrO3- δ solid oxide fuel cell anodes by combined Fe and Ru substitution[J]. Chemistry of Materials, 2015, 27(10): 3683-3693. |
| 15 | 杜志鸿. 钙钛矿基固体氧化物燃料电池电极材料结构及性能的研究[D]. 北京: 北京科技大学, 2016. |
| Du Z H. Struture and properties of perovskite-based electrode materials for solid oxide fuel cell[D]. Beijing: University of Science and Technology Beijing, 2016. | |
| 16 | Song J N, Zhu T L, Chen X Y, et al. Cobalt and titanium substituted SrFeO3 based perovskite as efficient symmetrical electrode for solid oxide fuel cell[J]. Journal of Materiomics, 2020, 6(2): 377-384. |
| 17 | Neagu D, Tsekouras G, Miller D N, et al. In situ growth of nanoparticles through control of non-stoichiometry[J]. Nature Chemistry, 2013, 5(11): 916-923. |
| 18 | Yang C H, Yang Z B, Jin C, et al. Sulfur-tolerant redox-reversible anode material for direct hydrocarbon solid oxide fuel cells[J]. Advanced Materials, 2012, 24(11): 1439-1443. |
| 19 | Kobsiriphat W, Madsen B D, Wang Y, et al. Nickel- and ruthenium-doped lanthanum chromite anodes: effects of nanoscale metal precipitation on solid oxide fuel cell performance[J]. Journal of the Electrochemical Society, 2010, 157(2): B279. |
| 20 | Bierschenk D M, Potter-Nelson E, Hoel C, et al. Pd-substituted (La,Sr)CrO3– δ -Ce0.9Gd0.1O2- δ solid oxide fuel cell anodes exhibiting regenerative behavior[J]. Journal of Power Sources, 2011, 196(6): 3089-3094. |
| 21 | Zhu T L, Troiani H, Mogni L V, et al. Exsolution and electrochemistry in perovskite solid oxide fuel cell anodes: role of stoichiometry in Sr(Ti,Fe,Ni)O3 [J]. Journal of Power Sources, 2019, 439: 227077. |
| 22 | Sun Y F, Li J H, Zeng Y M, et al. A-site deficient perovskite: the parent for in situ exsolution of highly active, regenerable nano-particles as SOFC anodes[J]. Journal of Materials Chemistry A, 2015, 3(20): 11048-11056. |
| 23 | Zhang S L, Wang H Q, Lu M Y, et al. Cobalt-substituted SrTi0.3Fe0.7O3- δ : a stable high-performance oxygen electrode material for intermediate-temperature solid oxide electrochemical cells[J]. Energy & Environmental Science, 2018, 11(7): 1870-1879. |
| 24 | Zhu T L, Troiani H E, Mogni L V, et al. Ni-substituted Sr(Ti,Fe)O3 SOFC anodes: achieving high performance via metal alloy nanoparticle exsolution[J]. Joule, 2018, 2(3): 478-496. |
| 25 | 朱腾龙. SrFeO3基钙钛矿阳极结构演化、机理及应用研究[D]. 北京: 中国矿业大学(北京), 2016. |
| Zhu T L. Structure evolution, mechanism and application of SrFeO3 based perovskite anode[D]. Beijing: China University of Mining & Technology, Beijing, 2016. | |
| 26 | Nenning A, Volgger L, Miller E, et al. The electrochemical properties of Sr(Ti,Fe)O3- δ for anodes in solid oxide fuel cells[J]. Journal of the Electrochemical Society, 2017, 164(4): F364-F371. |
| 27 | 倪维婕, 朱腾龙, 陈晓阳, 等. Co/Ni掺杂SrTi0.3Fe0.7O3- δ 钙钛矿电极材料制备及性能[J]. 硅酸盐学报, 2019, 47(3): 313-319. |
| Ni W J, Zhu T L, Chen X Y, et al. Fabrication and characterization of Co/Ni substituted SrTi0.3Fe0.7O3- δ perovskite electrode[J]. Journal of the Chinese Ceramic Society, 2019, 47(3): 313-319. | |
| 28 | Ohnuma I, Enoki H, Ikeda O, et al. Phase equilibria in the Fe-Co binary system[J]. Acta Materialia, 2002, 50(2): 379-393. |
| 29 | Chen X Y, Ni W J, Wang J, et al. Exploration of Co-Fe alloy precipitation and electrochemical behavior hysteresis using lanthanum and cobalt co-substituted SrFeO3- δ SOFC anode[J]. Electrochimica Acta, 2018, 277: 226-234. |
| 30 | Wang X X, Wei K W, Yan S L, et al. Efficient and stable conversion of oxygen-bearing low-concentration coal mine methane by the electrochemical catalysis of SOFC anode: from pollutant to clean energy[J]. Applied Catalysis B: Environmental, 2020, 268: 118413. |
| 31 | Xu C M, Sun W, Ren R Z, et al. A highly active and carbon-tolerant anode decorated with in situ grown cobalt nano-catalyst for intermediate-temperature solid oxide fuel cells[J]. Applied Catalysis B: Environmental, 2021, 282: 119553. |
| 32 | Hjelm J, Soegaard M, Knibbe R, et al. Electrochemical characterization of planar anode supported SOFC with strontium-doped lanthanum cobalt oxide cathodes[J]. ECS Transactions, 2008, 13(26): 285-299. |
| 33 | Ebbesen S D, Mogensen M. Kinetics of oxidation of H2 and reduction of H2O in Ni-YSZ based solid oxide cells[J]. ECS Transactions, 2013, 50(49): 167-182. |
| 34 | Zhu T L, Fowler D E, Poeppelmeier K R, et al. Hydrogen oxidation mechanisms on perovskite solid oxide fuel cell anodes[J]. Journal of the Electrochemical Society, 2016, 163(8): F952-F961. |
| 35 | Cui X T, Kær S K. Thermodynamic analysis of steam reforming and oxidative steam reforming of propane and butane for hydrogen production[J]. International Journal of Hydrogen Energy, 2018, 43(29): 13009-13021. |
| 36 | Shin T H, Ida S, Ishihara T. Doped CeO2-LaFeO3 composite oxide as an active anode for direct hydrocarbon-type solid oxide fuel cells[J]. Journal of the American Chemical Society, 2011, 133(48): 19399-19407. |
| [1] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [2] | 陈哲文, 魏俊杰, 张玉明. 超临界水煤气化耦合SOFC发电系统集成及其能量转化机制[J]. 化工学报, 2023, 74(9): 3888-3902. |
| [3] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [4] | 刘远超, 关斌, 钟建斌, 徐一帆, 蒋旭浩, 李耑. 单层XSe2(X=Zr/Hf)的热电输运特性研究[J]. 化工学报, 2023, 74(9): 3968-3978. |
| [5] | 陈佳起, 赵万玉, 姚睿充, 侯道林, 董社英. 开心果壳基碳点的合成及其对Q235碳钢的缓蚀行为研究[J]. 化工学报, 2023, 74(8): 3446-3456. |
| [6] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| [7] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [8] | 张蒙蒙, 颜冬, 沈永峰, 李文翠. 电解液类型对双离子电池阴阳离子储存行为的影响[J]. 化工学报, 2023, 74(7): 3116-3126. |
| [9] | 邢美波, 张中天, 景栋梁, 张洪发. 磁调控水基碳纳米管协同多孔材料强化相变储/释能特性[J]. 化工学报, 2023, 74(7): 3093-3102. |
| [10] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| [11] | 葛加丽, 管图祥, 邱新民, 吴健, 沈丽明, 暴宁钟. 垂直多孔碳包覆的FeF3正极的构筑及储锂性能研究[J]. 化工学报, 2023, 74(7): 3058-3067. |
| [12] | 屈园浩, 邓文义, 谢晓丹, 苏亚欣. 活性炭/石墨辅助污泥电渗脱水研究[J]. 化工学报, 2023, 74(7): 3038-3050. |
| [13] | 张谭, 刘光, 李晋平, 孙予罕. Ru基氮还原电催化剂性能调控策略[J]. 化工学报, 2023, 74(6): 2264-2280. |
| [14] | 董茂林, 陈李栋, 黄六莲, 吴伟兵, 戴红旗, 卞辉洋. 酸性助水溶剂制备木质纳米纤维素及功能应用研究进展[J]. 化工学报, 2023, 74(6): 2281-2295. |
| [15] | 杨琴, 秦传鉴, 李明梓, 杨文晶, 赵卫杰, 刘虎. 用于柔性传感的双形状记忆MXene基水凝胶的制备及性能研究[J]. 化工学报, 2023, 74(6): 2699-2707. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号