化工学报 ›› 2020, Vol. 71 ›› Issue (11): 5265-5277.DOI: 10.11949/0438-1157.20200158
收稿日期:2020-02-18
修回日期:2020-06-21
出版日期:2020-11-05
发布日期:2020-11-05
通讯作者:
张磊,曾亮
作者简介:苏迎辉(1992—),男,硕士研究生,基金资助:
Yinghui SU1( ),Hao ZHENG1(
),Hao ZHENG1( ),Lei ZHANG1,2(
),Lei ZHANG1,2( ),Liang ZENG1(
),Liang ZENG1( )
)
Received:2020-02-18
Revised:2020-06-21
Online:2020-11-05
Published:2020-11-05
Contact:
Lei ZHANG,Liang ZENG
摘要:
采用溶胶-凝胶法制备了B位Fe和Co共取代的LaMn1-x-yFexCoyO3-δ钙钛矿型复合氧化物,并用于化学链甲烷部分氧化制合成气。X射线衍射(XRD)结果表明Fe和Co均进入了LaMnO3的晶格形成钙钛矿晶相,活性和稳定性测试表明LaMn1/3Fe1/3Co1/3O3-δ载氧体具有最佳的化学链甲烷部分氧化性能。CH4程序升温还原(CH4-TPR)表征发现LaMn1/3Fe1/3Co1/3O3-δ具有比LaBO3(B=Co, Mn, Fe)更高的甲烷活化能力和晶格氧迁移性能。甲烷恒温脉冲反应(CH4-pulse reaction)进一步证实了B位离子的协同作用可以提高LaBO3(B=Co, Mn, Fe)的表面反应速率。程序升温氢气还原(H2-TPR)表明,LaMn1/3Fe1/3Co1/3O3-δ中晶格氧具有适中的氧化还原能力,适合用于化学链甲烷部分氧化。
中图分类号:
苏迎辉,郑浩,张磊,曾亮. LaMn1-x-yFexCoyO3-δ钙钛矿载氧体用于化学链部分氧化[J]. 化工学报, 2020, 71(11): 5265-5277.
Yinghui SU,Hao ZHENG,Lei ZHANG,Liang ZENG. LaMn1-x-yFexCoyO3-δ perovskite based oxygen carriers for chemical looping partial oxidation[J]. CIESC Journal, 2020, 71(11): 5265-5277.
| 样品 | 晶胞参数 a0 /? | (110) 晶面 2θ/(°) | 晶粒尺寸 /nm |
|---|---|---|---|
| LaMnO3 | 3.911 | 32.368 | 47.1 |
| LaMn1/3Fe1/3Co1/3O3-δ | 3.888 | 32.628 | 24.4 |
| LaMn1/3Fe1/6Co1/2O3-δ | 3.897 | 32.572 | 31.4 |
| LaMn1/6Fe1/3Co1/2O3-δ | 3.901 | 32.369 | 25.1 |
| LaMn7/15Fe1/3Co1/5O3-δ | 3.891 | 32.716 | 30.9 |
| LaMn1/3Fe1/2Co1/6O3-δ | 3.899 | 32.639 | 28.1 |
表1 新鲜LaMn1-x-yFexCoyO3-δ载氧体晶胞结构参数
Table 1 Structure parameters of the fresh LaMn1-x-yFexCoyO3-δ oxygen carriers
| 样品 | 晶胞参数 a0 /? | (110) 晶面 2θ/(°) | 晶粒尺寸 /nm |
|---|---|---|---|
| LaMnO3 | 3.911 | 32.368 | 47.1 |
| LaMn1/3Fe1/3Co1/3O3-δ | 3.888 | 32.628 | 24.4 |
| LaMn1/3Fe1/6Co1/2O3-δ | 3.897 | 32.572 | 31.4 |
| LaMn1/6Fe1/3Co1/2O3-δ | 3.901 | 32.369 | 25.1 |
| LaMn7/15Fe1/3Co1/5O3-δ | 3.891 | 32.716 | 30.9 |
| LaMn1/3Fe1/2Co1/6O3-δ | 3.899 | 32.639 | 28.1 |
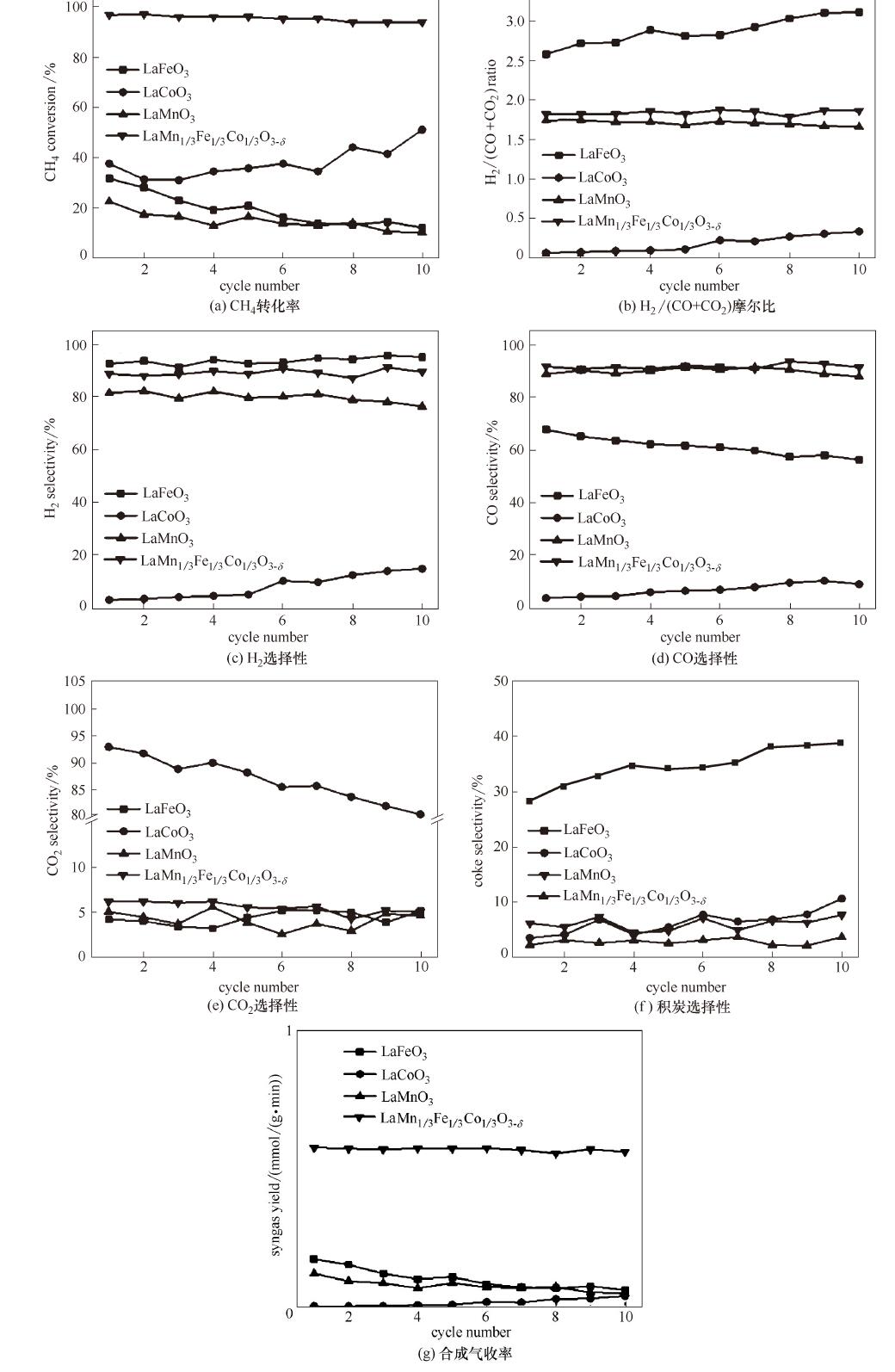
图5 LaMn1-x-yFexCoyO3-δ载氧体900℃下循环稳定性测试结果
Fig.5 Cyclic stability test of LaMn1-x-yFexCoyO3-δ oxygen carriers for continuous redox cycles in CL-POM at 900℃
| 1 | 苏小平, 王力, 杨武, 等. 甲烷化学链重整制合成气的研究进展[J]. 地下水, 2017, 39(4): 198-202. |
| Su X P, Wang L, Yang W. et al. Research progress on chemical looping reforming of methane to syngas [J]. Ground Water, 2017, 39(4): 198-202. | |
| 2 | 高亚娜. NiO / MgO 整体式催化剂上甲烷部分氧化制备合成气[J]. 工业催化, 2014, 22(5): 369-373. |
| Gao Y N. Partial oxidation of methane to synthesis gas over monolithic NiO/MgO catalyst[J]. Industrial Catalysis, 2014, 22(5): 369-373. | |
| 3 | Zhao K, He F, Huang Z, et al. La1-xSrxFeO3 perovskites oxygen carriers for the partial oxidation of methane to syngas[J]. Chinese Journal of Catalysis, 2014, 35(7): 1196-1205. |
| 4 | 向文军, 王佩怡. 天然气部分氧化制合成气的研究进展[J]. 河北化工, 2012, 35(10): 17-20. |
| Xiang W J, Wang P Y. Research progress on preparation of synthetic gas by partial oxidation of natural gas[J]. Hebei Chemical Industry, 2012, 35(10): 17-20. | |
| 5 | Protasova L, Snijkers F. Recent developments in oxygen carrier materials for hydrogen production via chemical looping processes[J]. Fuel, 2016, 181: 75-93. |
| 6 | 黄振, 何方, 赵坤, 等. 基于晶格氧的甲烷化学链重整制合成气[J]. 化学进展, 2012, 24(8): 1599-1609. |
| Huang Z, He F, Zhao K, et al. Synthesis gas production by chemical-looping reforming of methane using lattice oxygen[J]. Progress In Chemistry, 2012, 24(8): 1599-1609. | |
| 7 | 方稳, 李家德, 余长林, 等.稀土在甲烷部分氧化制取合成气中的应用研究[J]. 有色金属科学与工程, 2014, 5(6): 125-131. |
| Fang W, Li J D, Yu C L, et al. Application of rare earth to producing synthesis gas from the partial oxidation of methane[J]. Nonferrous Metals Science and Engineering, 2014, 5(6): 125-131. | |
| 8 | Siriwardane R, Tian H J, Fisher J. Production of pure hydrogen and synthesis gas with Cu-Fe oxygen carriers using combined processes of chemical looping combustion and methane decomposition/reforming[J]. International Journal of Hydrogen Energy, 2015, 40(4): 1698-1708. |
| 9 | Ebrahimi H, Rahmani M. Modeling chemical looping syngas production in a microreactor using perovskite oxygen carriers[J]. International Journal of Hydrogen Energy, 2018, 43(10): 5231-5248. |
| 10 | Zeng L, Cheng Z, Fan J A, et al. Metal oxide redox chemistry for chemical looping processes[J]. Nature Reviews Chemistry, 2018, 2: 349-346. |
| 11 | Chen S, Zeng L, Tian H, et al. Enhanced lattice oxygen reactivity over Ni-modified WO3-based redox catalysts for chemical looping partial oxidation of methane[J]. ACS Catalysis, 2017, 7(5): 3548-3559. |
| 12 | Ding H R, Xu Y, Luo C, et al. A novel composite perovskite-based material for chemical-looping steam methane reforming to hydrogen and syngas[J]. Energy Conversion and Management, 2018, 171: 12-19. |
| 13 | Johansson M, Mattisson T, Lyngfelt A. Investigation of Mn3O4 with stabilized ZrO2 for chemical-looping combustion[J]. Chem. Eng. Res. Des., 2006, 84(9): 807-818. |
| 14 | Luo M, Yi Y, Wang S Z. Review of hydrogen production using chemical-looping technology[J]. Renewable and Sustainable Energy Reviews, 2018, 81: 3186-3214. |
| 15 | Zhao K, Zheng A Q, Li H B, et al. Exploration of the mechanism of chemical looping steam methane reforming using double perovskite-type oxides La1.6Sr0.4FeCoO6[J]. Applied Catalysis B: Environmental, 2017, 219: 672-682. |
| 16 | Ran R, Wu X D, Weng D, et al. Oxygen storage capacity and structural properties of Ni-doped LaMnO3 perovskites[J]. Journal of Alloys and Compounds, 2013, 577: 288-294. |
| 17 | Zhao K, He F, Huang Z, et al. Perovskite-type LaFe1- xMnxO3 (x=0, 0.3, 0.5, 0.7, 1.0) oxygen carriers for chemical-looping steam methane reforming: oxidation activity and resistance to carbon formation[J]. Korean J. Chem. Eng, 2017, 34(6): 1651-1660. |
| 18 | Zhao K, He F, Huang Z, et al. Perovskite-type oxides LaFe1-xCoxO3 for chemical looping steam methane reforming to syngas and hydrogen co-production[J]. Applied Energy, 2016, 168: 193-203. |
| 19 | Haribal V P, He F, Mishra A. Iron-doped BaMnO3 for hybrid water splitting and syngas generation[J]. ChemSusChem, 2017, 10(17): 3402-3408. |
| 20 | Lee M, Lim H S, Kim Y, et al. Enhancement of highly-concentrated hydrogen productivity in chemical looping steam methane reforming using Fe-substituted LaCoO3[J]. Energy Conversion and Management, 2020, 207: 112507. |
| 21 | 代小平, 余长春. LaMO3 纳米复合钙钛矿氧载体化学循环重整甲烷制合成气[J]. 催化学报, 2011, 32(8): 1411-1417. |
| Dai X P, Yu C C. Nano-perovskite-based (LaMO3) oxygen carrier for syngas generation by chemical-looping reforming of methane[J]. Chinese Journal of Catalysis, 2011, 32(8): 1411-1417. | |
| 22 | Wang Y J, Zheng Y N, Wang Y H, et al. Evaluation of Fe substitution in perovskite LaMnO3 for the production of high purity syngas and hydrogen[J]. Journal of Power Sources, 2020, 449.227505. |
| 23 | Zhu X, Li K Z, Neal L M, et al. Perovskites as geo-inspired oxygen storage materials for chemical looping and three-way catalysis: a perspective[J]. ACS Catalysis, 2018, 8(9): 8213-8236. |
| 24 | Shannon R D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides[J]. Acta Crystallographica Section A, 1976, 32(5): 751-767. |
| 25 | Jia Y Q. Crystal radii and effective ionic radii of the rare earth ions[J]. Journal of Solid State Chemistry, 1991, 95(1): 184-187. |
| 26 | Zhao K, Chen J, Li H B, et al. Effects of Co-substitution on the reactivity of double perovskite oxides LaSrFe2-xCoxO6 for the chemical-looping steam methane reforming[J]. Journal of the Energy Institute, 2019, 92(3): 594-603. |
| 27 | Zhao K, Chen J, Li H B, et al. Investigation of the relationship between electronic properties and reactivity of 3DOM LaFe1-xCoxO3 for methane reforming to produce syngas[J]. International Journal of Energy Research, 2019, 43(13): 7120-7134. |
| 28 | He F, Li X A, Zhao K, et al. The use of La1-xSrxFeO3 perovskite-type oxides as oxygen carriers in chemical-looping reforming of methane[J]. Fuel, 2013, 108: 465-473. |
| 29 | Zhao K, Shen Y, Huang Z, et al. Different oxidation routes for lattice oxygen recovery of double-perovskite type oxides LaSrFeCoO6 as oxygen carriers for chemical looping steam methane reforming[J]. Journal of Energy Chemistry, 2017, 26(3): 501-509. |
| 30 | Zhang C H, Guo Y L, Guo Y, et al. LaMnO3 perovskite oxides prepared by different methods for catalytic oxidation of toluene[J]. Applied Catalysis B: Environmental, 2014, 148/149: 490-498. |
| 31 | Zhao K, Li L W, Zheng A Q, et al. Synergistic improvements in stability and performance of the double perovskite-type oxides La2-xSrxFeCoO6 for chemical looping steam methane reforming[J]. Applied Energy, 2017, 197: 393-404. |
| 32 | Ding H R, Luo C, Li X S, et al. Development of BaSrCo-based perovskite for chemical-looping steam methane reforming: a study on synergistic effects of A-site elements and CeO2 support[J]. Fuel, 2019, 253: 311-319. |
| 33 | Zhang R J, Cao Y, Li H B. The role of CuO modified La0.7Sr0.3FeO3 perovskite on intermediate-temperature partial oxidation of methane via chemical looping scheme[J]. International Journal of Hydrogen Energy, 2020, 45(7): 4073-4083. |
| 34 | Tang M C, Liu K, Roddick D M, et al. Enhanced lattice oxygen reactivity over Fe2O3/Al2O3 redox catalyst for chemical-looping dry (CO2) reforming of CH4: synergistic La-Ce effect[J]. Journal of Catalysis, 2018, 368: 38-52. |
| 35 | Neal L M, Shafiefarhood A, Li F X. Dynamic methane partial oxidation using a Fe2O3@La0.8Sr0.2FeO3-δ core-shell redox catalyst in the absence of gaseous oxygen[J]. ACS Catalysis, 2014, 4(10): 3560-3569. |
| 36 | Shafiefarhood A, Hamill J C, Neal L M. Methane partial oxidation using FeOx@La0.8Sr0.2FeO3-δ core-shell catalyst-transient pulse studies[J]. Phys. Chem. Chem. Phys, 2015, 17(46): 31297-31307. |
| 37 | Li T Y, Jayathilake R S, Taylor D D, et al. Structural studies of the Perovskite series La1-xSrxCoO3-δ during chemical looping with methane[J]. Chemical Communications, 2019, 55(34): 4929-4932. |
| [1] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [2] | 傅予, 刘兴翀, 王瀚雨, 李海敏, 倪亚飞, 邹文静, 雷月, 彭永姗. F3EACl修饰层对钙钛矿太阳能电池性能提升的研究[J]. 化工学报, 2023, 74(8): 3554-3563. |
| [3] | 刘晓洋, 喻健良, 侯玉洁, 闫兴清, 张振华, 吕先舒. 螺旋微通道对掺氢甲烷爆轰传播的影响[J]. 化工学报, 2023, 74(7): 3139-3148. |
| [4] | 牛超, 沈胜强, 杨艳, 潘泊年, 李熠桥. 甲烷BOG喷射器流动过程计算与性能分析[J]. 化工学报, 2023, 74(7): 2858-2868. |
| [5] | 周小文, 杜杰, 张战国, 许光文. 基于甲烷脉冲法的Fe2O3-Al2O3载氧体还原特性研究[J]. 化工学报, 2023, 74(6): 2611-2623. |
| [6] | 葛泽峰, 吴雨青, 曾名迅, 查振婷, 马宇娜, 侯增辉, 张会岩. 灰化学成分对生物质气化特性的影响规律[J]. 化工学报, 2023, 74(5): 2136-2146. |
| [7] | 胡晗, 杨亮, 李春晓, 刘道平. 天然烟浸滤液水合物法储甲烷动力学研究[J]. 化工学报, 2023, 74(3): 1313-1321. |
| [8] | 彭晓婉, 郭笑楠, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8浆液法分离CH4/N2的双吸收-吸附塔工艺流程建模与模拟[J]. 化工学报, 2023, 74(2): 784-795. |
| [9] | 孙嘉辰, 裴春雷, 陈赛, 赵志坚, 何盛宝, 巩金龙. 化学链低碳烷烃氧化脱氢技术进展[J]. 化工学报, 2023, 74(1): 205-223. |
| [10] | 李鑫, 曾少娟, 彭奎霖, 袁磊, 张香平. CO2电催化还原制合成气研究进展及趋势[J]. 化工学报, 2023, 74(1): 313-329. |
| [11] | 廖珊珊, 张少刚, 陶骏骏, 刘家豪, 汪金辉. 竖直射流火撞击障碍管道数值模拟分析[J]. 化工学报, 2022, 73(9): 4226-4234. |
| [12] | 张婉晨, 陈晓阳, 吕秋秋, 钟秦, 朱腾龙. Co掺杂SrTi0.3Fe0.7O3-δ 阳极SOFC在化工副产气燃料下的性能及稳定性[J]. 化工学报, 2022, 73(9): 4079-4086. |
| [13] | 袁妮妮, 郭拓, 白红存, 何育荣, 袁永宁, 马晶晶, 郭庆杰. 化学链燃烧过程Fe2O3/Al2O3载氧体表面CH4反应:ReaxFF-MD模拟[J]. 化工学报, 2022, 73(9): 4054-4061. |
| [14] | 沈嘉辉, 王侃宏, 郁达伟, 胡大洲, 魏源送. 游离氨调理污泥厌氧消化优化产甲烷过程与强化有机物释放[J]. 化工学报, 2022, 73(9): 4147-4155. |
| [15] | 王悦琳, 晁伟, 蓝晓程, 莫志朋, 佟淑环, 王铁峰. 合成气生物发酵法制乙醇的研究进展[J]. 化工学报, 2022, 73(8): 3448-3460. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号