化工学报 ›› 2022, Vol. 73 ›› Issue (8): 3739-3748.DOI: 10.11949/0438-1157.20220625
收稿日期:2022-05-05
修回日期:2022-06-01
出版日期:2022-08-05
发布日期:2022-09-06
通讯作者:
葛亮,徐铜文
作者简介:杨宏欣 (1997—),男,硕士研究生,yhx524@mail.ustc.edu.cn
基金资助:
Hongxin YANG1( ), Xingya LI1, Liang GE1,2(
), Xingya LI1, Liang GE1,2( ), Tongwen XU1(
), Tongwen XU1( )
)
Received:2022-05-05
Revised:2022-06-01
Online:2022-08-05
Published:2022-09-06
Contact:
Liang GE, Tongwen XU
摘要:
基于超酸催化聚合机理制备了含咔唑片段的聚合物主链结构,并通过调控季铵化反应中N-甲基哌啶单体的用量,得到了不同哌啶离子含量的阴离子选择性分离膜。通过核磁共振氢谱证明了聚合反应和季铵化反应顺利进行。热重和动态机械分析的测试结果表明膜具有优异的热稳定性和力学性能。通过电渗析 (ED) 测试膜的阴离子分离性能,结果显示离子通量和选择性均优于商业膜Neosepta ACS。在NaCl/Na2SO4体系中,QPC-Pip-60的Cl-通量可达3.24 mol·m-2·h-1,选择性可达11.6。在NaOH/Na2WO4碱回收体系下,良好的微相分离结构使得OH-通量可达3.59 mol·m-2·h-1,选择性最高为70。长时间稳定性测试和碱稳定性测试结果表明所制备的系列膜具有优异的循环稳定性和耐碱性能。
中图分类号:
杨宏欣, 李兴亚, 葛亮, 徐铜文. 含哌啶阳离子侧长链型一/二价阴离子选择性分离膜的制备[J]. 化工学报, 2022, 73(8): 3739-3748.
Hongxin YANG, Xingya LI, Liang GE, Tongwen XU. Preparation of mono-/divalent anion permselective membranes with piperidinium-type long side-chain[J]. CIESC Journal, 2022, 73(8): 3739-3748.
| 膜名称 | PC-Br/mmol | N-甲基哌啶/mmol | NMP/ml | 理论IEC/(mmol·g-1) |
|---|---|---|---|---|
| QPC-Pip-60 | 4.71 | 2.83 | 22 | 1.24 |
| QPC-Pip-80 | 4.71 | 3.77 | 22 | 1.59 |
| QPC-Pip-100 | 4.71 | 4.71 | 22 | 1.91 |
表1 季铵化反应的反应物用量
Table 1 The amount of reactants for quaternization reaction
| 膜名称 | PC-Br/mmol | N-甲基哌啶/mmol | NMP/ml | 理论IEC/(mmol·g-1) |
|---|---|---|---|---|
| QPC-Pip-60 | 4.71 | 2.83 | 22 | 1.24 |
| QPC-Pip-80 | 4.71 | 3.77 | 22 | 1.59 |
| QPC-Pip-100 | 4.71 | 4.71 | 22 | 1.91 |

图6 QPC-Pip-x系列膜的IEC、含水率、溶胀度及QPC-Pip-x系列和ACS膜的面电阻和迁移数
Fig.6 The IEC, water uptake and swelling ratio result of QPC-Pip-x series membranes and the surface resistance and transport number of QPC-Pip-x series membranes and ACS membrane
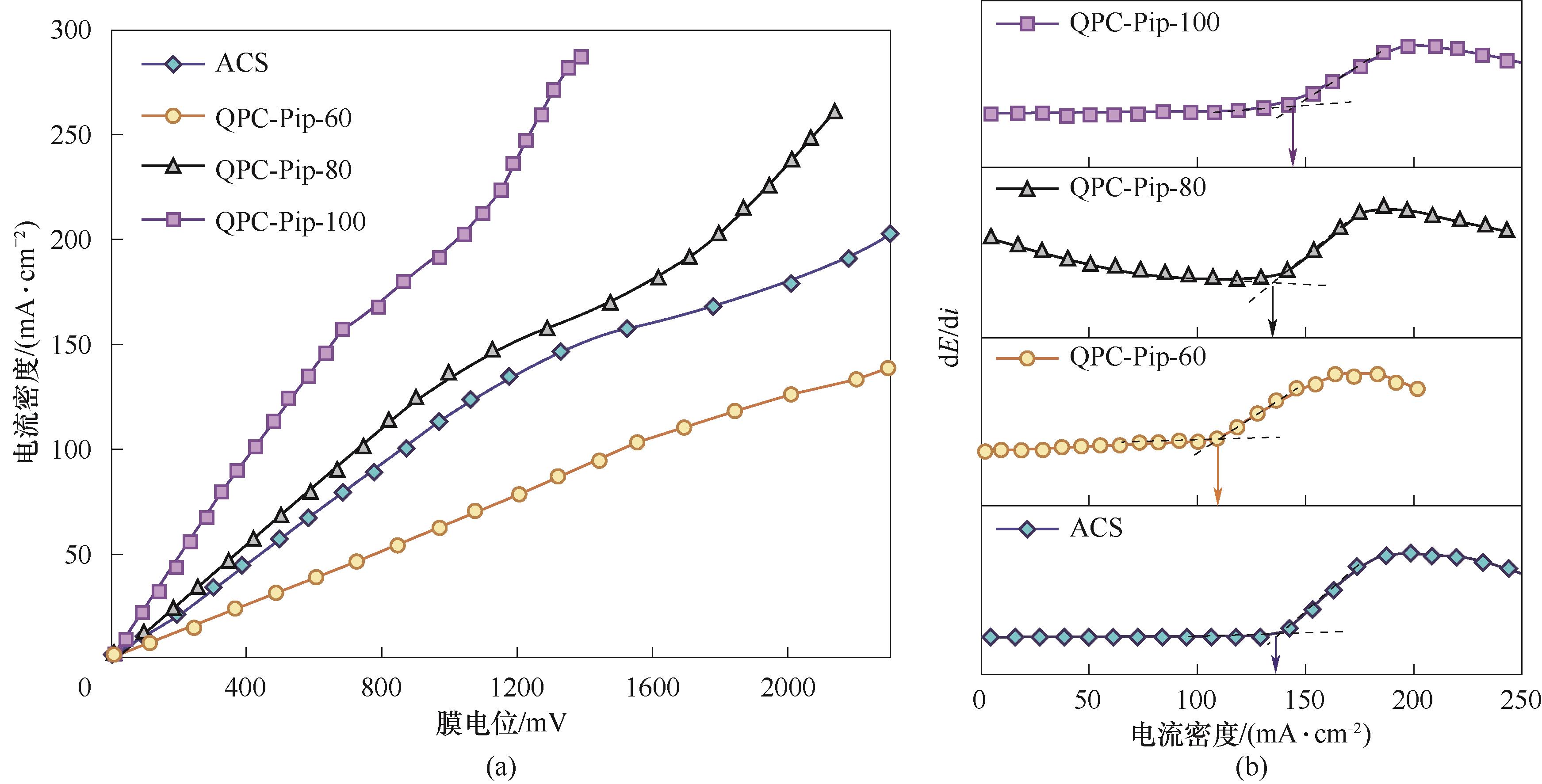
图7 QPC-Pip-x和Neosepta ACS的I-V曲线图及I-V曲线的导数dE/di同电流密度的函数图
Fig.7 The I-V curves of QPC-Pip-x and Neosepta ACS and the derivative of dE/di as a function of current density calculated from the I-V curves
| 膜名称 | 抗拉强度/MPa | 断裂伸长率/% |
|---|---|---|
| QPC-Pip-60 | 32.74 | 9.56 |
| QPC-Pip-80 | 30.49 | 12.84 |
| QPC-Pip-100 | 29.20 | 14.11 |
表2 QPC-Pip-x系列膜的抗拉强度和断裂伸长率
Table 2 The tensile strength and elongation of break of QPC-Pip-x series membrane
| 膜名称 | 抗拉强度/MPa | 断裂伸长率/% |
|---|---|---|
| QPC-Pip-60 | 32.74 | 9.56 |
| QPC-Pip-80 | 30.49 | 12.84 |
| QPC-Pip-100 | 29.20 | 14.11 |
| 膜名称 | 电流密度/(mA·cm-2) | 进料溶液 | Cl-通量/(mol·m-2·h-1) | 选择性 | 文献 |
|---|---|---|---|---|---|
| PAES-6C-IM | 5 | 0.05 mol·L-1 NaCl+0.05 mol·L-1 Na2SO4 | 1.26 | 7.1 | [ |
| PQC76/DSA-0.5 | 5 | 0.2 mol·L-1 NaCl+0.2 mol·L-1 Na2SO4 | 1.80 | 10 | [ |
| QPEI/PVA-C10-5 | 20 | 0.05 M NaCl + 0.05 mol·L-1 Na2SO4 | 1.59 | 6.31 | [ |
| 交联两性AEM | 5 | 0.05 mol·L-1 NaCl + 0.05 mol·L-1 Na2SO4 | 1.33 | 12.5 | [ |
| QP-P11-1 | 3.5 | 0.05 mol·L-1 NaCl+0.05 mol·L-1 Na2SO4 | 1.04 | 13 | [ |
| CrPsf-3 | 12 | 0.05 mol·L-1 NaCl+0.05 mol·L-1 Na2SO4 | 2.97 | 5.7 | [ |
| 均相共混-15-AIEM | 2.5 | 0.05 mol·L-1 NaCl+0.05 mol·L-1 Na2SO4 | — | 21.8 | [ |
| CBTS-integrated CCAPMs | 10 | 0.1 mol·L-1 NaCl+0.1 mol·L-1 Na2SO4 | 1.86 | 7.3 | [ |
| AMX-LPDA#DBSA | 8 | 0.05 mol·L-1 NaCl+0.05 mol·L-1 Na2SO4 | 2.47 | 1.4 | [ |
| (PSS/PAH)5PSS AEM | 1.13 | 0.05 mol·L-1 NaCl+0.05 mol·L-1 Na2SO4 | 0.242 | 7.4 | [ |
| QPC-Pip-60 | 10 | 0.1 mol·L-1 NaCl+0.1 mol·L-1 Na2SO4 | 3.24 | 11.6 | 本工作 |
| QPC-Pip-80 | 10 | 0.1 mol·L-1 NaCl+0.1 mol·L-1 Na2SO4 | 3.27 | 11.0 | 本工作 |
| QPC-Pip-100 | 10 | 0.1 mol·L-1 NaCl+0.1 mol·L-1 Na2SO4 | 3.32 | 10.5 | 本工作 |
表3 近期文献中单价阴离子选择性渗透膜在Cl-/WO42-体系下的ED性能结果
Table 3 An overview of the ED performance in Cl-/WO42- system of the monovalent anion permselective membranes in recent literatures
| 膜名称 | 电流密度/(mA·cm-2) | 进料溶液 | Cl-通量/(mol·m-2·h-1) | 选择性 | 文献 |
|---|---|---|---|---|---|
| PAES-6C-IM | 5 | 0.05 mol·L-1 NaCl+0.05 mol·L-1 Na2SO4 | 1.26 | 7.1 | [ |
| PQC76/DSA-0.5 | 5 | 0.2 mol·L-1 NaCl+0.2 mol·L-1 Na2SO4 | 1.80 | 10 | [ |
| QPEI/PVA-C10-5 | 20 | 0.05 M NaCl + 0.05 mol·L-1 Na2SO4 | 1.59 | 6.31 | [ |
| 交联两性AEM | 5 | 0.05 mol·L-1 NaCl + 0.05 mol·L-1 Na2SO4 | 1.33 | 12.5 | [ |
| QP-P11-1 | 3.5 | 0.05 mol·L-1 NaCl+0.05 mol·L-1 Na2SO4 | 1.04 | 13 | [ |
| CrPsf-3 | 12 | 0.05 mol·L-1 NaCl+0.05 mol·L-1 Na2SO4 | 2.97 | 5.7 | [ |
| 均相共混-15-AIEM | 2.5 | 0.05 mol·L-1 NaCl+0.05 mol·L-1 Na2SO4 | — | 21.8 | [ |
| CBTS-integrated CCAPMs | 10 | 0.1 mol·L-1 NaCl+0.1 mol·L-1 Na2SO4 | 1.86 | 7.3 | [ |
| AMX-LPDA#DBSA | 8 | 0.05 mol·L-1 NaCl+0.05 mol·L-1 Na2SO4 | 2.47 | 1.4 | [ |
| (PSS/PAH)5PSS AEM | 1.13 | 0.05 mol·L-1 NaCl+0.05 mol·L-1 Na2SO4 | 0.242 | 7.4 | [ |
| QPC-Pip-60 | 10 | 0.1 mol·L-1 NaCl+0.1 mol·L-1 Na2SO4 | 3.24 | 11.6 | 本工作 |
| QPC-Pip-80 | 10 | 0.1 mol·L-1 NaCl+0.1 mol·L-1 Na2SO4 | 3.27 | 11.0 | 本工作 |
| QPC-Pip-100 | 10 | 0.1 mol·L-1 NaCl+0.1 mol·L-1 Na2SO4 | 3.32 | 10.5 | 本工作 |
| 1 | Zaffora A, Culcasi A, Gurreri L, et al. Energy harvesting by waste acid/base neutralization via bipolar membrane reverse electrodialysis[J]. Energies, 2020, 13(20): 5510. |
| 2 | Al-Amshawee S, Yunus M Y B M, Azoddein A A M, et al. Electrodialysis desalination for water and wastewater: a review[J]. Chemical Engineering Journal 2020, 380: 122231. |
| 3 | Zhao Y, Tang K, Liu H, et al. An anion exchange membrane modified by alternate electro-deposition layers with enhanced monovalent selectivity[J]. Journal of Membrane Science, 2016, 520: 262-271. |
| 4 | Li C, Wang G, Yu D, et al. Cross-linked anion exchange membranes with hydrophobic side-chains for anion separation[J]. Journal of Membrane Science, 2019, 581: 150-157. |
| 5 | Ge L, Wu B, Yu D, et al. Monovalent cation perm-selective membranes (MCPMs): new developments and perspectives[J]. Chinese Journal of Chemical Engineering, 2017, 25(11): 1606-1615. |
| 6 | Vaselbehagh M, Karkhanechi H, Takagi R, et al. Surface modification of an anion exchange membrane to improve the selectivity for monovalent anions in electrodialysis — experimental verification of theoretical predictions[J]. Journal of Membrane Science, 2015, 490: 301-310. |
| 7 | Afsar N U, Li X, Zhu Y, et al. In-situ interfacial polymerization endows surface enrichment of —COOH groups on anion exchange membranes for efficient Cl-/ S O 4 2 - separation[J]. Journal of Polymer Science, 2021.DOI:10.1002/pol.20210735 . |
| 8 | Zhao Y, Zhu J, Ding J, et al. Electric-pulse layer-by-layer assembled of anion exchange membrane with enhanced monovalent selectivity[J]. Journal of Membrane Science, 2018, 548: 81-90. |
| 9 | Li J, Yuan S, Wang J, et al. Mussel-inspired modification of ion exchange membrane for monovalent separation[J]. Journal of Membrane Science, 2018, 553: 139-150. |
| 10 | Lejarazu-Larrañaga A, Zhao Y, Molina S, et al. Alternating current enhanced deposition of a monovalent selective coating for anion exchange membranes with antifouling properties[J]. Separation and Purification Technology, 2019, 229: 115807. |
| 11 | Fujimoto C, Kim D S, Hibbs M, et al. Backbone stability of quaternized polyaromatics for alkaline membrane fuel cells[J]. Journal of Membrane Science, 2012, 423: 438-449. |
| 12 | Mohanty A D, Tignor S E, Krause J A, et al. Systematic alkaline stability study of polymer backbones for anion exchange membrane applications[J]. Macromolecules, 2016, 49(9): 3361-3372. |
| 13 | Miyanishi S, Yamaguchi T. Analysis of the degradation mechanism of the polyarylene ether anion-exchange membrane for alkaline fuel cell and water-splitting cell applications[J]. New Journal of Chemistry, 2017, 41(16): 8036-8044. |
| 14 | Varcoe J R, Slade R C T. Prospects for alkaline anion-exchange membranes in low temperature fuel cells[J]. Fuel cells, 2005, 5(2): 187-200. |
| 15 | Merle G, Wessling M, Nijmeijer K. Anion exchange membranes for alkaline fuel cells: a review[J]. Journal of Membrane Science, 2011, 377(1/2): 1-35. |
| 16 | Couture G, Alaaeddine A, Boschet F, et al. Polymeric materials as anion-exchange membranes for alkaline fuel cells [J]. Progress in Polymer Science, 2011, 36(11): 1521-1557. |
| 17 | Diaz A M, Zolotukhin M G, Fomine S, et al. A novel, one-pot synthesis of novel 3F, 5F, and 8F aromatic polymers [J]. Macromolecular Rapid Communications, 2007, 28(2): 183-187. |
| 18 | Lee W H, Mohanty A D, Bae C. Fluorene-based hydroxide ion conducting polymers for chemically stable anion exchange membrane fuel cells[J]. ACS Macro Letters, 2015, 4(4): 453-457. |
| 19 | Hibbs M R, Fujimoto C H, Cornelius C J. Synthesis and characterization of p o l y ( p h e n y l e n e ) - b a s e d anion exchange membranes for alkaline fuel cells[J]. Macromolecules, 2009, 42(21): 8316-8321. |
| 20 | Rao A, Thankamony R L, Kim H J, et al. Imidazolium-functionalized poly(arylene ether sulfone) block copolymer as an anion exchange membrane for alkaline fuel cell[J]. Polymer, 2013, 54(1): 111-119. |
| 21 | Xue J, Liu X, Zhang J, et al. Poly(phenylene oxide)s incorporating N-spirocyclic quaternary ammonium cation/cation strings for anion exchange membranes[J]. Journal of Membrane Science, 2019, 595: 117507. |
| 22 | Wang X, Lin C, Gao Y, et al. Anion exchange membranes with twisted poly(terphenylene) backbone: effect of the N-cyclic cations[J]. Journal of Membrane Science, 2021, 635: 119525. |
| 23 | Zhang F, Li T, Chen W, et al. Electron-donating C-NH2 link backbone for highly alkaline and mechanical stable anion exchange membranes[J]. ACS Applied Materials & Interfaces, 2021, 13(8): 10490-10499. |
| 24 | Tanaka N, Sawada S, Yamaki T, et al. Improvement of HI concentration performance for hydrogen production iodine-sulfur process using crosslinked cation-exchange membrane[J]. Chemical Engineering Science, 2021, 237: 116575. |
| 25 | Du X, Wang Z, Zhang H, et al. Prepared poly(aryl piperidinium) anion exchange membranes for acid recovery to improve dialysis coefficients and selectivity[J]. Journal of Membrane Science, 2021, 619: 118805. |
| 26 | Chen N, Wang H H, Kim S P, et al. Poly (fluorenyl aryl piperidinium) membranes and ionomers for anion exchange membrane fuel cells[J]. Nature Communications, 2021, 12(1): 2367. |
| 27 | 杨珊珊, 姚宇洋, 董云迪, 等. 基于二苯并-18-冠-6基体改性的K+选择性离子交换膜的制备及性能研究[J]. 化工学报, 2022, 73(4): 1781-1793. |
| Yang S S, Yao Y Y, Dong Y D, et al. Preparation and performance of ion exchange membrane with K+ selectivity based on dibenzo-18-crown-6 modification[J]. CIESC Journal, 2022, 73(4): 1781-1793. | |
| 28 | Olvera L I, Guzmán-Gutiérrez M T, Zolotukhin M G, et al. Novel high molecular weight aromatic fluorinated polymers from one-pot, metal-free step polymerizations[J]. Macromolecules, 2013, 46(18): 7245-7256. |
| 29 | Zhu Y, Ding L, Liang X, et al. Beneficial use of rotatable-spacer side-chains in alkaline anion exchange membranes for fuel cells[J]. Energy & Environmental Science, 2018, 11(12): 3472- 3479. |
| 30 | Irfan M, Ge L, Wang Y, et al. Hydrophobic side chains impart anion exchange membranes with high monovalent-divalent anion selectivity in electrodialysis[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(4): 4429-4442. |
| 31 | Belloň T, Polezhaev P, Vobecká L, et al. Experimental observation of phenomena developing on ion-exchange systems during current-voltage curve measurement[J]. Journal of Membrane Science, 2019, 572: 607-618. |
| 32 | Xiao X, Shehzad M A, Yasmin A, et al. Anion permselective membranes with chemically-bound carboxylic polymer layer for fast anion separation[J]. Journal of Membrane Science, 2020, 614: 118553. |
| 33 | Li M, Li W, Zhang X, et al. Polyvinyl alcohol-based monovalent anion selective membranes with excellent permselectivity in selectrodialysis[J]. Journal of Membrane Science, 2021, 620: 118889. |
| 34 | Liao J, Yu X, Pan N, et al. Amphoteric ion-exchange membranes with superior mono-/bi-valent anion separation performance for electrodialysis applications[J]. Journal of Membrane Science, 2019, 577: 153-164. |
| 35 | Nightingale J E R. Phenomenological theory of ion solvation. Effective radii of hydrated ions[J]. The Journal of Physical Chemistry, 1959, 63(9): 1381-1387. |
| 36 | Liao J, Yu X, Chen Q, et al. Monovalent anion selective anion-exchange membranes with imidazolium salt-terminated side-chains: investigating the effect of hydrophobic alkyl spacer length[J]. Journal of Membrane Science, 2020, 599: 117818. |
| 37 | Zhang H, Ding R, Zhang Y, et al. Stably coating loose and electronegative thin layer on anion exchange membrane for efficient and selective monovalent anion transfer[J]. Desalination, 2017, 410: 55-65. |
| 38 | Goel P, E B, Mandal P, et al. Di-quaternized graphene oxide based multi-cationic cross-linked monovalent selective anion exchange membrane for electrodialysis[J]. Separation and Purification Technology, 2021, 276: 119361. |
| 39 | Liao J, Chen Q, Pan N, et al. Amphoteric blend ion-exchange membranes for separating monovalent and bivalent anions in electrodialysis[J]. Separation and Purification Technology, 2020, 242: 116793. |
| 40 | Ahmad M, Tang C, Yang L, et al. Layer-by-layer modification of aliphatic polyamide anion-exchange membranes to increase Cl-/ S O 4 2 - selectivity[J]. Journal of Membrane Science, 2019, 578: 209-219. |
| [1] | 邵苛苛, 宋孟杰, 江正勇, 张旋, 张龙, 高润淼, 甄泽康. 水平方向上冰中受陷气泡形成和分布实验研究[J]. 化工学报, 2023, 74(S1): 161-164. |
| [2] | 吴延鹏, 李晓宇, 钟乔洋. 静电纺丝纳米纤维双疏膜油性细颗粒物过滤性能实验分析[J]. 化工学报, 2023, 74(S1): 259-264. |
| [3] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [4] | 赵亚欣, 张雪芹, 王荣柱, 孙国, 姚善泾, 林东强. 流穿模式离子交换层析去除单抗聚集体[J]. 化工学报, 2023, 74(9): 3879-3887. |
| [5] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [6] | 何松, 刘乔迈, 谢广烁, 王斯民, 肖娟. 高浓度水煤浆管道气膜减阻两相流模拟及代理辅助优化[J]. 化工学报, 2023, 74(9): 3766-3774. |
| [7] | 胡建波, 刘洪超, 胡齐, 黄美英, 宋先雨, 赵双良. 有机笼跨细胞膜易位行为的分子动力学模拟研究[J]. 化工学报, 2023, 74(9): 3756-3765. |
| [8] | 齐聪, 丁子, 余杰, 汤茂清, 梁林. 基于选择吸收纳米薄膜的太阳能温差发电特性研究[J]. 化工学报, 2023, 74(9): 3921-3930. |
| [9] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| [10] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [11] | 张佳怡, 何佳莉, 谢江鹏, 王健, 赵鹬, 张栋强. 渗透汽化技术用于锂电池生产中N-甲基吡咯烷酮回收的研究进展[J]. 化工学报, 2023, 74(8): 3203-3215. |
| [12] | 张贲, 王松柏, 魏子亚, 郝婷婷, 马学虎, 温荣福. 超亲水多孔金属结构驱动的毛细液膜冷凝及传热强化[J]. 化工学报, 2023, 74(7): 2824-2835. |
| [13] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| [14] | 韩奎奎, 谭湘龙, 李金芝, 杨婷, 张春, 张永汾, 刘洪全, 于中伟, 顾学红. 四通道中空纤维MFI分子筛膜用于二甲苯异构体分离[J]. 化工学报, 2023, 74(6): 2468-2476. |
| [15] | 蔡斌, 张效林, 罗倩, 党江涛, 左栗源, 刘欣梅. 导电薄膜材料的研究进展[J]. 化工学报, 2023, 74(6): 2308-2321. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号