化工学报 ›› 2023, Vol. 74 ›› Issue (12): 4749-4763.DOI: 10.11949/0438-1157.20231074
张俊杰1( ), 孙旺1,2(
), 孙旺1,2( ), 高啸天1,2, 乔金硕1,2, 王振华1,2, 孙克宁1,2(
), 高啸天1,2, 乔金硕1,2, 王振华1,2, 孙克宁1,2( )
)
收稿日期:2023-10-17
修回日期:2023-12-03
出版日期:2023-12-25
发布日期:2024-02-19
通讯作者:
孙旺,孙克宁
作者简介:张俊杰(1999—),男,硕士研究生,15801337258@163.com
基金资助:
Junjie ZHANG1( ), Wang SUN1,2(
), Wang SUN1,2( ), Xiaotian GAO1,2, Jinshuo QIAO1,2, Zhenhua WANG1,2, Kening SUN1,2(
), Xiaotian GAO1,2, Jinshuo QIAO1,2, Zhenhua WANG1,2, Kening SUN1,2( )
)
Received:2023-10-17
Revised:2023-12-03
Online:2023-12-25
Published:2024-02-19
Contact:
Wang SUN, Kening SUN
摘要:
随着双碳目标和能源革命的持续推进,氢能作为重要的清洁能源受到广泛关注。在众多的制氢途径中,固体氧化物电解池(solid oxide electrolytic cell, SOEC)电解水制氢被认为是最有前景的制氢途径之一。为更好地服务SOEC电解水制氢的产业化发展,对SOEC技术的研究现状和面临的挑战进行了系统梳理。虽然SOEC电解水制氢具有制氢效率高、环境污染小、余热高效利用等优点,但在长时间稳定运行和大规模集成上还有许多难点问题亟需解决,目前国内SOEC产业化尚在起步阶段,与国外发展水平还有一定的差距。提升材料的稳定性、优化系统控制是SOEC技术的重点发展方向。此外,加快SOEC产业化进程还需要协同发展上下游产业链,进一步降低制备成本。随着技术的成熟,SOEC有望在化工、分布式能源等领域获得广泛的应用。
中图分类号:
张俊杰, 孙旺, 高啸天, 乔金硕, 王振华, 孙克宁. 固体氧化物电解池制氢关键技术及产业化进展[J]. 化工学报, 2023, 74(12): 4749-4763.
Junjie ZHANG, Wang SUN, Xiaotian GAO, Jinshuo QIAO, Zhenhua WANG, Kening SUN. Key technology and industrialization progress of hydrogen production by solid oxide electrolytic cell[J]. CIESC Journal, 2023, 74(12): 4749-4763.
| 电解技术 | 优势 | 劣势 |
|---|---|---|
| AEC | 技术成熟;成本低;稳定性高 | 电流密度低;高浓度碱液 |
| PEMEC | 电流密度大;响应速度快;功率密度高;产品气体纯度高 | 成本高;使用贵金属催化剂 |
| SOEC | 工作温度高;效率高;材料成本低 | 稳定性一般;技术成熟度低 |
表1 典型电解水技术的优缺点
Table 1 Advantages and disadvantages of typical water electrolysis technologies
| 电解技术 | 优势 | 劣势 |
|---|---|---|
| AEC | 技术成熟;成本低;稳定性高 | 电流密度低;高浓度碱液 |
| PEMEC | 电流密度大;响应速度快;功率密度高;产品气体纯度高 | 成本高;使用贵金属催化剂 |
| SOEC | 工作温度高;效率高;材料成本低 | 稳定性一般;技术成熟度低 |

图2 理想电解反应的总能量需求(ΔH)、热能需求(ΔQ)和电能需求(ΔG)随温度变化曲线[2]
Fig.2 Total(ΔH), thermal(ΔQ) and electrical(ΔG) energy demand of an ideal electrolysis process as function of the temperature[2]
| 组件 | 典型材料 | 类型 | 优势 | 问题 |
|---|---|---|---|---|
| 氧电极 | LSM | 钙钛矿 | 与YSZ化学相容性好,催化活性高, 化学稳定性好 | 离子电导率低 |
| LSC | 钙钛矿 | 电导率高 | Co热膨胀系数高,长期运行稳定 性差 | |
| LSCF | 钙钛矿 | 电化学活性高,极化电阻低,稳定性好 | Co热膨胀系数高,与YSZ相容性差 | |
LnBCO (Ln=La, Pr,Nd,Sm,Gd, Y) | 双钙钛矿 | 电子-离子混合导电材料,电导率高, 催化活性好 | — | |
| La2NiO4 | R-P型钙钛矿 | 氧扩散系数高,具有与电解质匹配的 热膨胀系数 | 中温区的电子导电性较低 | |
| 氢电极 | Ni-YSZ | 金属陶瓷 | 催化活性高、价格低 | Ni的团聚、氧化 |
| SFM | 钙钛矿 | 氧化还原稳定性优异 | 导电性差,催化活性低 | |
| 电解质 | YSZ | 锆基氧离子导体 | 优异的离子导电性和力学性能 | 离子电导率会随温度的降低而 大幅减小 |
| GDC | 铈基氧离子导体 | 中低温离子导电性好 | 高温下Ce4+会发生副反应 | |
| 连接体 | Crofer 22 APU | 高铬不锈钢 | 抗氧化性好,热膨胀系数匹配,成本较低 | — |
| 密封材料 | 玻璃陶瓷密封材料 | 化学稳定性好,成本低 | 热循环性能差 |
表2 SOEC各组件典型材料
Table 2 Typical materials for each component of SOEC
| 组件 | 典型材料 | 类型 | 优势 | 问题 |
|---|---|---|---|---|
| 氧电极 | LSM | 钙钛矿 | 与YSZ化学相容性好,催化活性高, 化学稳定性好 | 离子电导率低 |
| LSC | 钙钛矿 | 电导率高 | Co热膨胀系数高,长期运行稳定 性差 | |
| LSCF | 钙钛矿 | 电化学活性高,极化电阻低,稳定性好 | Co热膨胀系数高,与YSZ相容性差 | |
LnBCO (Ln=La, Pr,Nd,Sm,Gd, Y) | 双钙钛矿 | 电子-离子混合导电材料,电导率高, 催化活性好 | — | |
| La2NiO4 | R-P型钙钛矿 | 氧扩散系数高,具有与电解质匹配的 热膨胀系数 | 中温区的电子导电性较低 | |
| 氢电极 | Ni-YSZ | 金属陶瓷 | 催化活性高、价格低 | Ni的团聚、氧化 |
| SFM | 钙钛矿 | 氧化还原稳定性优异 | 导电性差,催化活性低 | |
| 电解质 | YSZ | 锆基氧离子导体 | 优异的离子导电性和力学性能 | 离子电导率会随温度的降低而 大幅减小 |
| GDC | 铈基氧离子导体 | 中低温离子导电性好 | 高温下Ce4+会发生副反应 | |
| 连接体 | Crofer 22 APU | 高铬不锈钢 | 抗氧化性好,热膨胀系数匹配,成本较低 | — |
| 密封材料 | 玻璃陶瓷密封材料 | 化学稳定性好,成本低 | 热循环性能差 |
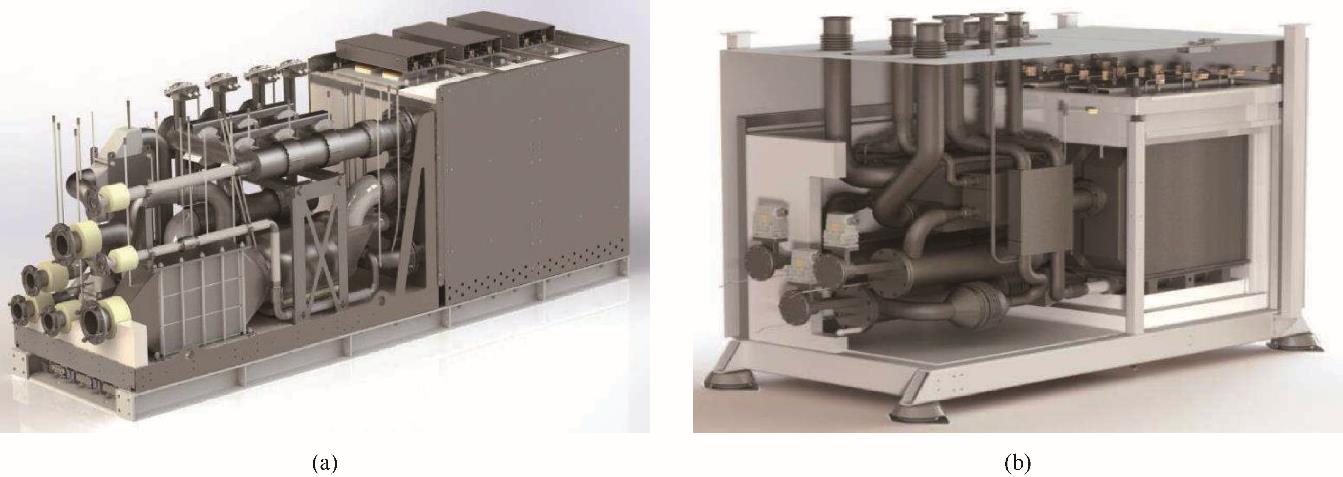
图6 GrInHy Gen. 0 RSOC电堆模块(集成8个电堆)和Gen. 1 RSOC电堆模块(集成24个电堆)[79]
Fig.6 GrInHy Gen. 0 RSOC hotbox with 3 stack modules (8 stacks each) and Gen. 1 hotbox with 1 stack module (24 stacks)[79]
| 1 | Dolle C, Neha N, Coutanceau C. Electrochemical hydrogen production from biomass[J]. Current Opinion in Electrochemistry, 2022, 31: 100841. |
| 2 | Buttler A, Spliethoff H. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: a review[J]. Renewable and Sustainable Energy Reviews, 2018, 82: 2440-2454. |
| 3 | Gambou F, Guilbert D, Zasadzinski M, et al. A comprehensive survey of alkaline electrolyzer modeling: electrical domain and specific electrolyte conductivity[J]. Energies, 2022, 15(9): 3452. |
| 4 | Krishnan S, Koning V, Theodorus de Groot M, et al. Present and future cost of alkaline and PEM electrolyser stacks[J]. International Journal of Hydrogen Energy, 2023, 48(83): 32313-32330. |
| 5 | Jang D, Cho H S, Lee S, et al. Investigation of the operation characteristics and optimization of an alkaline water electrolysis system at high temperature and a high current density[J]. Journal of Cleaner Production, 2023, 424: 138862. |
| 6 | Nuttall L J, Fickett A P, Titterington W A. Hydrogen generation by solid polymer electrolyte water electrolysis[M]//Veziroğlu T N. Hydrogen Energy. Boston, MA: Springer, 1975: 441-455. |
| 7 | Norazahar N, Khan F, Rahmani N, et al. Degradation modelling and reliability analysis of PEM electrolyzer[J]. International Journal of Hydrogen Energy, 2023, 50: 842-856. |
| 8 | Shiva Kumar S, Lim H. An overview of water electrolysis technologies for green hydrogen production[J]. Energy Reports, 2022, 8: 13793-13813. |
| 9 | Baroutaji A, Arjunan A, Robinson J, et al. Additive manufacturing for proton exchange membrane (PEM) hydrogen technologies: merits, challenges, and prospects[J]. International Journal of Hydrogen Energy, 2023, 52: 561-584. |
| 10 | Carmo M, Fritz D L, Mergel J, et al. A comprehensive review on PEM water electrolysis[J]. International Journal of Hydrogen Energy, 2013, 38(12): 4901-4934. |
| 11 | Majumdar A, Haas M, Elliot I, et al. Control and control-oriented modeling of PEM water electrolyzers: a review[J]. International Journal of Hydrogen Energy, 2023, 48(79): 30621-30641. |
| 12 | Hauch A, Küngas R, Blennow P, et al. Recent advances in solid oxide cell technology for electrolysis[J]. Science, 2020, 370(6513): eaba6118. |
| 13 | Shen F Y, Wang R F, Tucker M C. Long term durability test and post mortem for metal-supported solid oxide electrolysis cells[J]. Journal of Power Sources, 2020, 474: 228618. |
| 14 | Nechache A, Hody S. Alternative and innovative solid oxide electrolysis cell materials: a short review[J]. Renewable and Sustainable Energy Reviews, 2021, 149: 111322. |
| 15 | Choe C, Cheon S, Gu J, et al. Critical aspect of renewable syngas production for power-to-fuel via solid oxide electrolysis: integrative assessment for potential renewable energy source[J]. Renewable and Sustainable Energy Reviews, 2022, 161: 112398. |
| 16 | Khan M A, Zhao H B, Zou W W, et al. Recent progresses in electrocatalysts for water electrolysis[J]. Electrochemical Energy Reviews, 2018, 1(4): 483-530. |
| 17 | Xu Y H, Cai S S, Chi B, et al. Technological limitations and recent developments in a solid oxide electrolyzer cell: a review[J]. International Journal of Hydrogen Energy, 2023, 50: 548-591. |
| 18 | Shiva Kumar S, Himabindu V. Hydrogen production by PEM water electrolysis — a review[J]. Materials Science for Energy Technologies, 2019, 2(3): 442-454. |
| 19 | Rashid M M, Al Mesfer M K, Naseem H, et al. Hydrogen production by water electrolysis: a review of alkaline water electrolysis, PEM water electrolysis and high temperature water electrolysis[J]. International Journal of Engineering and Advanced Technology, 2015, 4(3): 80-93. |
| 20 | Singh K, Kannan R, Thangadurai V. Perspective of perovskite-type oxides for proton conducting solid oxide fuel cells[J]. Solid State Ionics, 2019, 339: 114951. |
| 21 | Shen M H, Ai F J, Ma H L, et al. Progress and prospects of reversible solid oxide fuel cell materials[J]. iScience, 2021, 24(12): 103464. |
| 22 | Adler S B. Factors governing oxygen reduction in solid oxide fuel cell cathodes[J]. Chemical Reviews, 2004, 104(10): 4791-4843. |
| 23 | 杨晓幸, 苗鹤, 袁金良. 可逆固体氧化物燃料电池氧电极材料的研究进展[J]. 化工进展, 2021, 40(9): 4904-4917. |
| Yang X X, Miao H, Yuan J L. Research progress on oxygen electrode materials for reversible solid oxide fuel cells[J]. Chemical Industry and Engineering Progress, 2021, 40(9): 4904-4917. | |
| 24 | Petrov A N, Kononchuk O F, Andreev A V, et al. Crystal structure, electrical and magnetic properties of La1 - x Sr x CoO3 - y [J]. Solid State Ionics, 1995, 80(3/4): 189-199. |
| 25 | Sharma V I, Yildiz B. Degradation mechanism in La0.8Sr0.2CoO3 as contact layer on the solid oxide electrolysis cell anode[J]. Journal of the Electrochemical Society, 2010, 157(3): B441. |
| 26 | Tietz F, Sebold D, Brisse A, et al. Degradation phenomena in a solid oxide electrolysis cell after 9000 h of operation[J]. Journal of Power Sources, 2013, 223: 129-135. |
| 27 | Tian Y F, Li J, Liu Y Y, et al. Preparation and properties of PrBa0.5Sr0.5Co1.5Fe0.5O5+ δ as novel oxygen electrode for reversible solid oxide electrochemical cell[J]. International Journal of Hydrogen Energy, 2018, 43(28): 12603-12609. |
| 28 | Wang Y, Li W Y, Ma L, et al. Degradation of solid oxide electrolysis cells: phenomena, mechanisms, and emerging mitigation strategies—a review[J]. Journal of Materials Science & Technology, 2020, 55: 35-55. |
| 29 | Park J H, Kim K J, Jung C H, et al. Boosting the performance of solid oxide electrolysis cells via incorporation of Gd3+ and Nd3+ double-doped ceria[J]. Fuel Cells, 2020, 20(6): 712-717. |
| 30 | Zheng J E, Wang X Y, Yu J, et al. Enhanced performance of a Ba0.5Sr0.5Co0.8Fe0.2O3– δ based oxygen electrode for solid oxide electrolysis cells by decorating with Ag particles[J]. Materials Research Express, 2021, 8(3): 035502. |
| 31 | Tan Y, Wang A, Jia L C, et al. High-performance oxygen electrode for reversible solid oxide cells with power generation and hydrogen production at intermediate temperature[J]. International Journal of Hydrogen Energy, 2017, 42(7): 4456-4464. |
| 32 | 勾匀婕, 李广东, 王振华, 等. 固体氧化物电解池技术的应用前景与挑战[J]. 石油化工高等学校学报, 2022, 35(6): 28-37. |
| Gou Y J, Li G D, Wang Z H, et al. Application prospect and challenge of solid oxide electrolytic cell technology[J]. Journal of Petrochemical Universities, 2022, 35(6): 28-37. | |
| 33 | Jiang S P, Chan S H. A review of anode materials development in solid oxide fuel cells[J]. Journal of Materials Science, 2004, 39(14): 4405-4439. |
| 34 | Nakajo A, Cocco A P, DeGostin M B, et al. Evolution of 3-D transport pathways and triple-phase boundaries in the Ni-YSZ hydrogen electrode upon fuel cell or electrolysis cell operation[J]. ECS Transactions, 2017, 78(1): 3205-3215. |
| 35 | Tsekouras G, Irvine J T S. The role of defect chemistry in strontium titanates utilised for high temperature steam electrolysis[J]. Journal of Materials Chemistry, 2011, 21(25): 9367-9376. |
| 36 | Jin C, Yang C H, Zhao F, et al. La0.75Sr0.25Cr0.5Mn0.5O3 as hydrogen electrode for solid oxide electrolysis cells[J]. International Journal of Hydrogen Energy, 2011, 36(5): 3340-3346. |
| 37 | Liu S B, Liu Q X, Luo J L. The excellence of La(Sr)Fe(Ni)O3 as an active and efficient cathode for direct CO2 electrochemical reduction at elevated temperatures[J]. Journal of Materials Chemistry A, 2017, 5(6): 2673-2680. |
| 38 | Hosoi K, Hagiwara H, Ida S, et al. La0.8Sr0.2FeO3– δ as fuel electrode for solid oxide reversible cells using LaGaO3-based oxide electrolyte[J]. The Journal of Physical Chemistry C, 2016, 120(29): 16110-16117. |
| 39 | Zhang L J, Wang Z H, Cao Z Q, et al. High activity oxide Pr0.3Sr0.7Ti0.3Fe0.7O3- δ as cathode of SOEC for direct high-temperature steam electrolysis[J]. International Journal of Hydrogen Energy, 2017, 42(17): 12104-12110. |
| 40 | Deka D J, Gunduz S, Kim J, et al. Hydrogen production from water in a solid oxide electrolysis cell: effect of Ni doping on lanthanum strontium ferrite perovskite cathodes[J]. Industrial & Engineering Chemistry Research, 2019, 58(50): 22497-22505. |
| 41 | Xu Y H, Huang L W, Zhang Y K, et al. Enhanced steam electrolysis with exsolved iron nanoparticles in perovskite cathode[J]. International Journal of Hydrogen Energy, 2022, 47(58): 24287-24296. |
| 42 | Lee J G, Jeon O S, Ryu K H, et al. Effects of 8 mol% yttria-stabilized zirconia with copper oxide on solid oxide fuel cell performance[J]. Ceramics International, 2015, 41(6): 7982-7988. |
| 43 | Colomer M T, Guglieri C, Díaz-Moreno S, et al. Effect of titania doping and sintering temperature on titanium local environment and electrical conductivity of YSZ[J]. Journal of Alloys and Compounds, 2016, 689: 512-524. |
| 44 | Li H, Kon A, Chang C H, et al. Fast firing of bismuth doped yttria-stabilized zirconia for enhanced densification and ionic conductivity[J]. Journal of the Ceramic Society of Japan, 2016, 124(4): 370-374. |
| 45 | Kim J, Jun A, Gwon O, et al. Hybrid-solid oxide electrolysis cell: a new strategy for efficient hydrogen production[J]. Nano Energy, 2018, 44: 121-126. |
| 46 | Zhu S Y, Wang Y L, Rao Y Y, et al. Chemically-induced mechanical unstability of samaria-doped ceria electrolyte for solid oxide electrolysis cells[J]. International Journal of Hydrogen Energy, 2014, 39(24): 12440-12447. |
| 47 | Nolan M. Formation of Ce3+ at the cerium dioxide (1 1 0) surface by doping[J]. Chemical Physics Letters, 2010, 492(1/2/3): 115-118. |
| 48 | Sala E M, Mazzanti N, Mogensen M B, et al. Current understanding of ceria surfaces for CO2 reduction in SOECs and future prospects — a review[J]. Solid State Ionics, 2022, 375: 115833. |
| 49 | Li M M, Hou J, Fan Y, et al. Interface modification of Ru-CeO2 co-infiltrated SFM electrode and construction of SDC/YSZ bilayer electrolyte for direct CO2 electrolysis[J]. Electrochimica Acta, 2022, 426: 140771. |
| 50 | Preininger M, Stoeckl B, Subotić V, et al. Characterization and performance study of commercially available solid oxide cell stacks for an autonomous system[J]. Energy Conversion and Management, 2020, 203: 112215. |
| 51 | Falk-Windisch H, Claquesin J, Sattari M, et al. Co- and Ce/Co-coated ferritic stainless steel as interconnect material for intermediate temperature solid oxide fuel cells[J]. Journal of Power Sources, 2017, 343: 1-10. |
| 52 | Talic B, Molin S, Wiik K, et al. Comparison of iron and copper doped manganese cobalt spinel oxides as protective coatings for solid oxide fuel cell interconnects[J]. Journal of Power Sources, 2017, 372: 145-156. |
| 53 | Frangini S, Della Seta L, Paoletti C. Effect of additive particle size on the CuO-accelerated formation of LaFeO3 perovskite conversion coatings in molten carbonate baths[J]. Surface and Coatings Technology, 2019, 374: 513-520. |
| 54 | Tulyaganov D U, Reddy A A, Kharton V V, et al. Aluminosilicate-based sealants for SOFCs and other electrochemical applications — a brief review[J]. Journal of Power Sources, 2013, 242: 486-502. |
| 55 | Wang S F, Lu H C, Liu Y X, et al. Characteristics of glass sealants for intermediate-temperature solid oxide fuel cell applications[J]. Ceramics International, 2017, 43: S613-S620. |
| 56 | Rodríguez-López S, Wei J, Laurenti K C, et al. Mechanical properties of solid oxide fuel cell glass-ceramic sealants in the system BaO/SrO-MgO-B2O3-SiO2 [J]. Journal of the European Ceramic Society, 2017, 37(11): 3579-3594. |
| 57 | 郭祥, 乔金硕, 王振华, 等. 碳燃料固体氧化物燃料电池结构研究进展[J]. 化工学报, 2023, 74(1): 290-302. |
| Guo X, Qiao J S, Wang Z H, et al. Progress of structure for carbon-fueled solid oxide fuel cells[J]. CIESC Journal, 2023, 74(1): 290-302. | |
| 58 | Yao Y, Ma Y, Wang C P, et al. A cofuel channel microtubular solid oxide fuel/electrolysis cell[J]. Applied Energy, 2022, 327: 120010. |
| 59 | Wang B, Li T, Xiao R, et al. Study on the 4-channel micro-monolithic design with geometry control for reversible solid oxide cell[J]. Separation and Purification Technology, 2023, 315: 123732. |
| 60 | Zhang Z, Guan C Z, Xie L D, et al. Design and analysis of a novel opposite trapezoidal flow channel for solid oxide electrolysis cell stack[J]. Energies, 2022, 16(1): 159. |
| 61 | Peters R, Frank M, Tiedemann W, et al. Long-term experience with a 5/15 kW-class reversible solid oxide cell system[J]. Journal of the Electrochemical Society, 2021, 168(1): 014508. |
| 62 | Choi Y, Byun S, Seo D W, et al. New design and performance evaluation of 1 kW-class reversible solid oxide electrolysis-fuel cell stack using flat-tubular cells[J]. Journal of Power Sources, 2022, 542: 231744. |
| 63 | Kotisaari M, Thomann O, Montinaro D, et al. Evaluation of a SOE stack for hydrogen and syngas production: a performance and durability analysis[J]. Fuel Cells, 2017, 17(4): 571-580. |
| 64 | Aicart J, Wuillemin Z, Gervasoni B, et al. Performance evaluation of a 4-stack solid oxide module in electrolysis mode[J]. International Journal of Hydrogen Energy, 2022, 47(6): 3568-3579. |
| 65 | Skafte T L, Rizvandi O B, Smitshuysen A L, et al. Electrothermally balanced operation of solid oxide electrolysis cells[J]. Journal of Power Sources, 2022, 523: 231040. |
| 66 | Sun Y, Hu X F, Gao J, et al. Solid oxide electrolysis cell under real fluctuating power supply with a focus on thermal stress analysis[J]. Energy, 2022, 261: 125096. |
| 67 | Hauch A, Traulsen M L, Küngas R, et al. CO2 electrolysis — gas impurities and electrode overpotential causing detrimental carbon deposition[J]. Journal of Power Sources, 2021, 506: 230108. |
| 68 | Lu B W, Zhang Z J, Zhang Z, et al. Control strategy of solid oxide electrolysis cell operating temperature under real fluctuating renewable power[J]. Energy Conversion and Management, 2024, 299: 117852. |
| 69 | Cui T C, Xiao G P, Yan H J, et al. Numerical simulation and analysis of the thermal stresses of a planar solid oxide electrolysis cell[J]. International Journal of Green Energy, 2023, 20(4): 432-444. |
| 70 | Reytier M, Cren J, Petitjean M, et al. Development of a cost-efficient and performing high temperature steam electrolysis stack[J]. ECS Transactions, 2013, 57(1): 3151-3160. |
| 71 | Chatroux A, Reytier M, Di Iorio S, et al. A packaged and efficient SOEC system demonstrator[J]. ECS Transactions, 2015, 68(1): 3519-3526. |
| 72 | Reytier M, Di Iorio S, Chatroux A, et al. Stack performances in high temperature steam electrolysis and co-electrolysis[J]. International Journal of Hydrogen Energy, 2015, 40(35): 11370-11377. |
| 73 | Saarinen V, Pennanen J, Kotisaari M, et al. Design, manufacturing, and operation of movable 2 × 10 kW size rSOC system[J]. Fuel Cells, 2021, 21(5): 477-487. |
| 74 | Posdziech O, Schwarze K, Brabandt J. Efficient hydrogen production for industry and electricity storage via high-temperature electrolysis[J]. International Journal of Hydrogen Energy, 2019, 44(35): 19089-19101. |
| 75 | Mermelstein J, Posdziech O. Development and demonstration of a novel reversible SOFC system for utility and micro grid energy storage[J]. Fuel Cells, 2017, 17(4): 562-570. |
| 76 | Bi L, Boulfrad S, Traversa E. Steam electrolysis by solid oxide electrolysis cells (SOECs) with proton-conducting oxides[J]. Chemical Society Reviews, 2014, 43(24): 8255-8270. |
| 77 | Schwarze K, Posdziech O, Kroop S, et al. Green industrial hydrogen via reversible high-temperature electrolysis[J]. ECS Transactions, 2017, 78(1): 2943-2952. |
| 78 | Schwarze K, Posdziech O, Mermelstein J, et al. Operational results of an 150/30 kW RSOC system in an industrial environment[J]. Fuel Cells, 2019, 19(4): 374-380. |
| 79 | Posdziech O, Geißler T, Schwarze K, et al. System development and demonstration of large-scale high-temperature electrolysis[J]. ECS Transactions, 2019, 91(1): 2537-2546. |
| 80 | Patcharavorachot Y, Chatrattanawet N, Arpornwichanop A, et al. Comparative energy, economic, and environmental analyses of power-to-gas systems integrating SOECs in steam-electrolysis and co-electrolysis and methanation[J]. Thermal Science and Engineering Progress, 2023, 42: 101873. |
| 81 | Küngas R, Blennow P, Heiredal-Clausen T, et al. eCOs — a commercial CO2 electrolysis system developed by haldor topsoe[J]. ECS Transactions, 2017, 78(1): 2879-2884. |
| 82 | Lehtinen T, Noponen M. Solid oxide electrolyser demonstrator development at elcogen[J]. ECS Transactions, 2021, 103(1): 1939-1944. |
| 83 | Sunfire. Renewable hydrogen project “multiplhy”: world's largest high-temperature electrolyzer from sunfire successfully installed [EB/OL]. (2023-04-11)[2023-06-04]. . |
| 84 | Lin M, Haussener S. An integrated concentrated solar fuel generator utilizing a tubular solid oxide electrolysis cell as solar absorber[J]. Journal of Power Sources, 2018, 400: 592-604. |
| 85 | Houaijia A, Breuer S, Thomey D, et al. Solar hydrogen by high-temperature electrolysis: flowsheeting and experimental analysis of a tube-type receiver concept for superheated steam production[J]. Energy Procedia, 2014, 49: 1960-1969. |
| 86 | Schiller G, Lang M, Szabo P, et al. Solar heat integrated solid oxide steam electrolysis for highly efficient hydrogen production[J]. Journal of Power Sources, 2019, 416: 72-78. |
| 87 | Zhang Q Q, Chang Z S, Fu M K, et al. Thermal performance analysis of an integrated solar reactor using solid oxide electrolysis cells (SOEC) for hydrogen production[J]. Energy Conversion and Management, 2022, 264: 115762. |
| 88 | Zhang Q Q, Chang Z S, Fu M K, et al. Thermal and electrochemical performance analysis of an integrated solar SOEC reactor for hydrogen production[J]. Applied Thermal Engineering, 2023, 229: 120603. |
| 89 | Nechache A, Hody S. Test and evaluation of an hybrid storage solution for buildings, based on a reversible high-temperature electrolyzer[J]. ECS Transactions, 2019, 91(1): 2485-2494. |
| 90 | Lamagna M, Nastasi B, Groppi D, et al. Techno-economic assessment of reversible solid oxide cell integration to renewable energy systems at building and district scale[J]. Energy Conversion and Management, 2021, 235: 113993. |
| 91 | Tallgren J, Himanen O, Noponen M. Experimental characterization of low temperature solid oxide cell stack[J]. ECS Transactions, 2017, 78(1): 3103-3111. |
| [1] | 黄琮琪, 吴一梅, 陈建业, 邵双全. 碱性电解水制氢装置热管理系统仿真研究[J]. 化工学报, 2023, 74(S1): 320-328. |
| [2] | 胡超, 董玉明, 张伟, 张红玲, 周鹏, 徐红彬. 浓硫酸活化五氧化二钒制备高浓度全钒液流电池正极电解液[J]. 化工学报, 2023, 74(S1): 338-345. |
| [3] | 蒋祎璠, 刘蕾, 赵耀, 代彦军. UVLED光学元件液冷散热系统性能研究[J]. 化工学报, 2023, 74(S1): 154-160. |
| [4] | 陈哲文, 魏俊杰, 张玉明. 超临界水煤气化耦合SOFC发电系统集成及其能量转化机制[J]. 化工学报, 2023, 74(9): 3888-3902. |
| [5] | 康飞, 吕伟光, 巨锋, 孙峙. 废锂离子电池放电路径与评价研究[J]. 化工学报, 2023, 74(9): 3903-3911. |
| [6] | 尹刚, 李伊惠, 何飞, 曹文琦, 王民, 颜非亚, 向禹, 卢剑, 罗斌, 卢润廷. 基于KPCA和SVM的铝电解槽漏槽事故预警方法[J]. 化工学报, 2023, 74(8): 3419-3428. |
| [7] | 郑玉圆, 葛志伟, 韩翔宇, 王亮, 陈海生. 中高温钙基材料热化学储热的研究进展与展望[J]. 化工学报, 2023, 74(8): 3171-3192. |
| [8] | 文兆伦, 李沛睿, 张忠林, 杜晓, 侯起旺, 刘叶刚, 郝晓刚, 官国清. 基于自热再生的隔壁塔深冷空分工艺设计及优化[J]. 化工学报, 2023, 74(7): 2988-2998. |
| [9] | 张蒙蒙, 颜冬, 沈永峰, 李文翠. 电解液类型对双离子电池阴阳离子储存行为的影响[J]. 化工学报, 2023, 74(7): 3116-3126. |
| [10] | 李贵贤, 曹阿波, 孟文亮, 王东亮, 杨勇, 周怀荣. 耦合固体氧化物电解槽的CO2制甲醇过程设计与评价研究[J]. 化工学报, 2023, 74(7): 2999-3009. |
| [11] | 邵远哲, 赵忠盖, 刘飞. 基于共同趋势模型的非平稳过程质量相关故障检测方法[J]. 化工学报, 2023, 74(6): 2522-2537. |
| [12] | 胡南, 陶德敏, 杨照岚, 王学兵, 张向旭, 刘玉龙, 丁德馨. 铁炭微电解与硫酸盐还原菌耦合修复铀尾矿库渗滤水的研究[J]. 化工学报, 2023, 74(6): 2655-2667. |
| [13] | 周小文, 杜杰, 张战国, 许光文. 基于甲烷脉冲法的Fe2O3-Al2O3载氧体还原特性研究[J]. 化工学报, 2023, 74(6): 2611-2623. |
| [14] | 李勇, 高佳琦, 杜超, 赵亚丽, 李伯琼, 申倩倩, 贾虎生, 薛晋波. Ni@C@TiO2核壳双重异质结的构筑及光热催化分解水产氢[J]. 化工学报, 2023, 74(6): 2458-2467. |
| [15] | 贠程, 王倩琳, 陈锋, 张鑫, 窦站, 颜廷俊. 基于社团结构的化工过程风险演化路径深度挖掘[J]. 化工学报, 2023, 74(4): 1639-1650. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号