化工学报 ›› 2024, Vol. 75 ›› Issue (10): 3639-3650.DOI: 10.11949/0438-1157.20240335
收稿日期:2024-03-25
修回日期:2024-05-23
出版日期:2024-10-25
发布日期:2024-11-04
通讯作者:
刘宪云
作者简介:赵璐(1998—),女,硕士研究生,494006389@qq.com
基金资助:
Lu ZHAO( ), Han WU, Xianyun LIU(
), Han WU, Xianyun LIU( )
)
Received:2024-03-25
Revised:2024-05-23
Online:2024-10-25
Published:2024-11-04
Contact:
Xianyun LIU
摘要:
采用浸渍法将Ru分散在WC-C载体上制备了Ru/WC-C催化剂,测试了其氨硼烷水解制氢反应性能。通过X射线衍射、透射电子显微镜和X射线光电子能谱对催化剂的物相、形貌和表面元素状态进行了表征。结果表明,Ru均匀分散于WC的表面和周围,与WC之间的电子协同效应促进了氨硼烷水解制氢。在298 K,碱性溶液的条件下,氨硼烷水解反应性能与Ru的含量有关,随着Ru含量的增加,产氢时间缩短,Ru的质量分数为2%的2%Ru/WC-C催化剂完全产氢时间为3.5 min,其氢气的产氢速率值为573.0 min-1,反应活化能为45.3 kJ·mol-1。其在中性水溶液中氢气的产氢速率值仍高达136.2 min-1。WC-C载体的应用可有效地促进反应过程中水分子的活化,进而改善Ru催化剂的性能,这为开发高效的氨硼烷水解制氢催化剂提供了新途径。
中图分类号:
赵璐, 吴涵, 刘宪云. 负载型钌催化剂用于氨硼烷水解制氢反应[J]. 化工学报, 2024, 75(10): 3639-3650.
Lu ZHAO, Han WU, Xianyun LIU. Supported ruthenium catalyst for hydrogen generation from ammonia borane hydrolysis[J]. CIESC Journal, 2024, 75(10): 3639-3650.
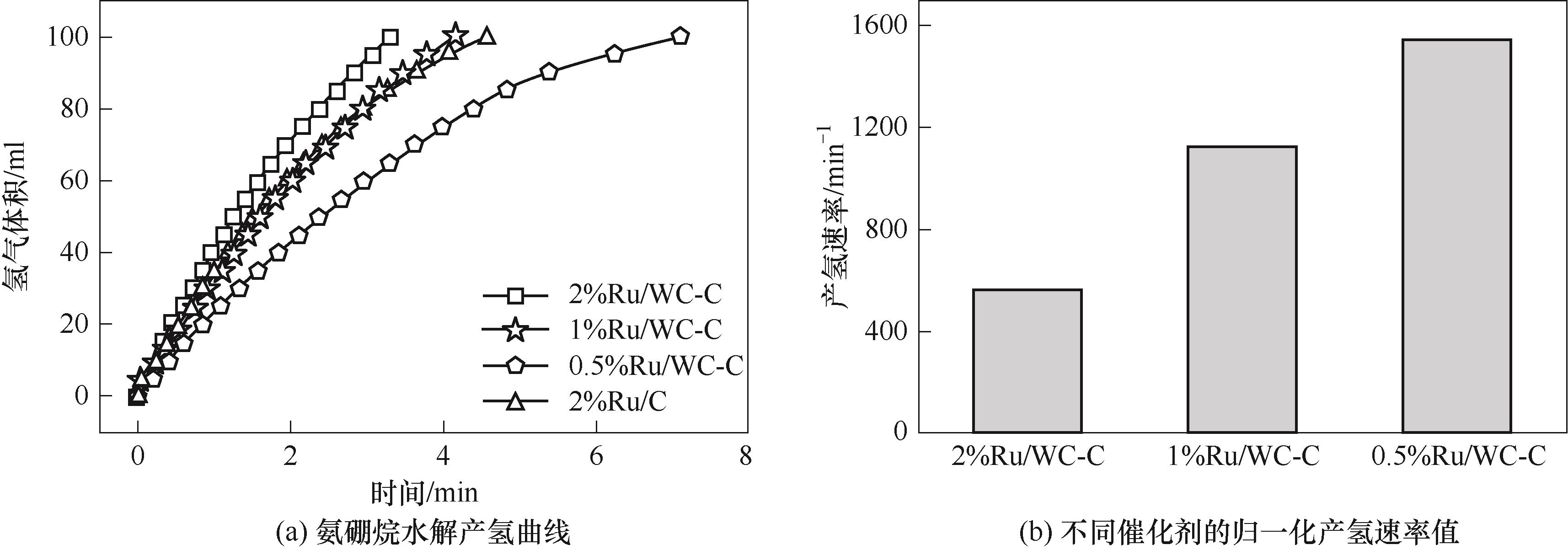
图7 不同Ru质量分数Ru/WC-C催化氨硼烷水解产氢性能
Fig.7 Catalytic activity of hydrogen generation from ammonia borane hydrolysis over Ru/WC-C catalysts with different Ru loading

图10 2%Ru/WC-C催化剂在不同氢氧化钠浓度下的氨硼烷水解产氢性能
Fig.10 Hydrogen generation performance of ammonia borane hydrolysis catalyzed by 2%Ru/WC-C at different NaOH concentrations
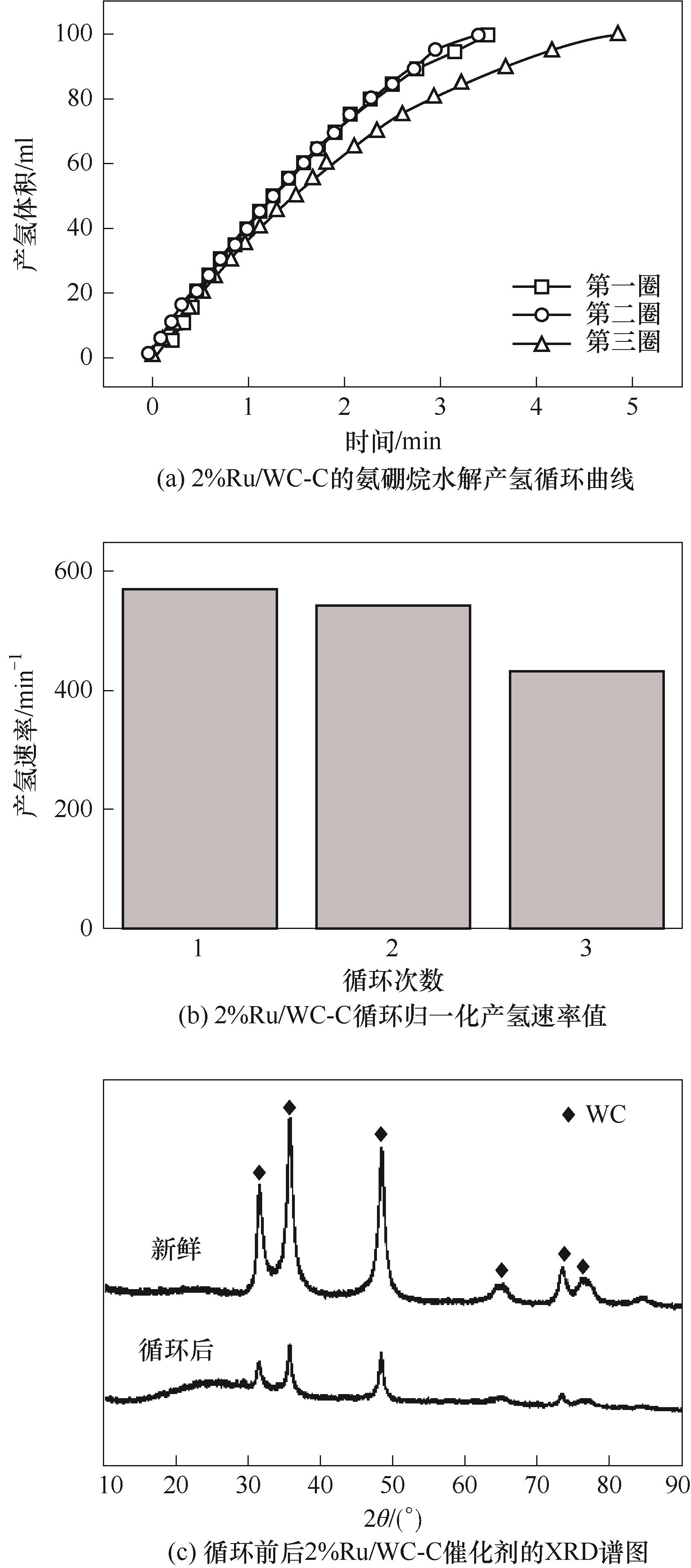
图11 2%Ru/WC-C催化剂氨硼烷水解产氢循环曲线和对应产氢速率值以及循环前后XRD谱图
Fig.11 Cyclic tests of 2%Ru/WC-C catalyst in ammonia borane hydrolysis and corresponding hydrogen production rate values, and XRD patterns of 2%Ru/WC-C before and after cyclic tests
| 1 | Kovač A, Paranos M, Marciuš D. Hydrogen in energy transition: a review[J]. International Journal of Hydrogen Energy, 2021, 46(16): 10016-10035. |
| 2 | Akbayrak S, Özkar S. Ammonia borane as hydrogen storage materials[J]. International Journal of Hydrogen Energy, 2018, 43(40): 18592-18606. |
| 3 | 王雨桐, 潘伦, 张香文, 等. 氨硼烷水解制氢研究进展[J]. 化工学报, 2021, 72(1): 180-191. |
| Wang Y T, Pan L, Zhang X W, et al. Research progress of ammonia borane hydrolytic hydrogen production[J]. CIESC Journal, 2021, 72(1): 180-191. | |
| 4 | Yüksel Alpaydın C, Gülbay S K, Ozgur Colpan C. A review on the catalysts used for hydrogen production from ammonia borane[J]. International Journal of Hydrogen Energy, 2020, 45(5): 3414-3434. |
| 5 | Li Y T, Zhang S H, Zheng G P, et al. Ultrafine Ru nanoparticles anchored to porous g-C3N4 as efficient catalysts for ammonia borane hydrolysis[J]. Applied Catalysis A: General, 2020, 595: 117511. |
| 6 | Yao Q L, Shi W M, Feng G, et al. Ultrafine Ru nanoparticles embedded in SiO2 nanospheres: highly efficient catalysts for hydrolytic dehydrogenation of ammonia borane[J]. Journal of Power Sources, 2014, 257: 293-299. |
| 7 | Fan G Y, Liu Q Q, Tang D M, et al. Nanodiamond supported Ru nanoparticles as an effective catalyst for hydrogen evolution from hydrolysis of ammonia borane[J]. International Journal of Hydrogen Energy, 2016, 41(3): 1542-1549. |
| 8 | Zhou Q X, Yang H X, Xu C X. Nanoporous Ru as highly efficient catalyst for hydrolysis of ammonia borane[J]. International Journal of Hydrogen Energy, 2016, 41(30): 12714-12721. |
| 9 | Chen G Z, Wang R Y, Zhao W, et al. Effect of Ru crystal phase on the catalytic activity of hydrolytic dehydrogenation of ammonia borane[J]. Journal of Power Sources, 2018, 396: 148-154. |
| 10 | Li S H, Song X R, Li Y T, et al. Efficient hydrolytic dehydrogenation of ammonia borane over ultrafine Ru nanoparticles supported on biomass-derived porous carbon[J]. International Journal of Hydrogen Energy, 2021, 46(54): 27555-27566. |
| 11 | Du C, Ao Q, Cao N, et al. Facile synthesis of monodisperse ruthenium nanoparticles supported on graphene for hydrogen generation from hydrolysis of ammonia borane[J]. International Journal of Hydrogen Energy, 2015, 40(18): 6180-6187. |
| 12 | 王小燕, 张若凡, 司航, 等. 椰壳炭负载钌催化剂的制备及其催化氨硼烷水解制氢性能[J]. 石油炼制与化工, 2023, 54(7): 64-70. |
| Wang X Y, Zhang R F, Si H, et al. Preparation of coconut shell carbon supported ruthenium as a catalyst for the hydrolytic dehydrogenation of ammonia borane[J]. Petroleum Processing and Petrochemicals, 2023, 54(7): 64-70. | |
| 13 | 邱小魁, 张若凡, 王小燕, 等. 竹茹丝炭负载钌催化剂光催化氨硼烷水解产氢研究[J]. 无机盐工业, 2023, 55(10): 153-158. |
| Qiu X K, Zhang R F, Wang X Y, et al. Research on bamboo shavings carbon supported ruthenium catalysts for hydrogen generation from photocatalytic hydrolysis of ammonia borane[J]. Inorganic Chemicals Industry, 2023, 55(10): 153-158. | |
| 14 | İzgi M S, Onat E, Şahin Ö, et al. Green and active hydrogen production from hydrolysis of ammonia borane by using caffeine carbon quantum dot-supported ruthenium catalyst in methanol solvent by hydrothermal treatment[J]. International Journal of Hydrogen Energy, 2024, 51: 180-192. |
| 15 | 孙海杰, 刘欣改, 陈志浩, 等. Ru/ZrO2催化剂催化氨硼烷水解产氢研究[J]. 广西师范大学学报(自然科学版), 2021, 39(3): 92-101. |
| Sun H J, Liu X G, Chen Z H, et al. Performance of Ru/ZrO2 catalysts for hydrogen generation from catalytic hydrolysis of ammonia borane[J]. Journal of Guangxi Normal University (Natural Science Edition), 2021, 39(3): 92-101. | |
| 16 | 赵红鸽, 张世豪, 郑修成. Ru/WO3纳米催化剂的制备、表征及催化氨硼烷水解性能研究[J]. 信阳师范学院学报(自然科学版), 2022, 35(3): 458-463. |
| Zhao H G, Zhang S H, Zheng X C. Preparation, characterization and catalytic performance of Ru/WO3 nanocatalysts for ammonia borane hydrolysis[J]. Journal of Xinyang Normal University, 2022, 35(3): 458-463. | |
| 17 | 石倩玉, 杨玉美, 王小萌, 等. SBA-15负载钌基纳米粒子催化氨硼烷水解制氢[J]. 中国有色金属学报, 2022, 32(7): 2042-2049. |
| Shi Q Y, Yang Y M, Wang X M, et al. SBA-15 supported ruthenium-based nanoparticles for catalytic hydrolysis of ammonia borane to generate hydrogen[J]. The Chinese Journal of Nonferrous Metals, 2022, 32(7): 2042-2049. | |
| 18 | 孙海杰, 刘欣改, 陈志浩, 等. 二氧化硅负载钌催化剂催化氨硼烷水解产氢研究[J]. 无机盐工业, 2020, 52(5): 81-85. |
| Sun H J, Liu X G, Chen Z H, et al. Research on Ru/SiO2 catalysts for hydrogen generation from catalytic hydrolysis of ammonia borane[J]. Inorganic Chemicals Industry, 2020, 52(5): 81-85. | |
| 19 | Ge Y Z, Qin X T, Li A W, et al. Maximizing the synergistic effect of CoNi catalyst on α-MoC for robust hydrogen production[J]. Journal of the American Chemical Society, 2021, 143(2): 628-633. |
| 20 | Zhou D J, Huang X Y, Wen H, et al. Ru-Fe nanoalloys supported on N-doped carbon as efficient catalysts for hydrogen generation from ammonia borane[J]. Sustainable Energy and Fuels, 2020, 4(7): 3677-3686. |
| 21 | Wang P C, Wang C L, Yang Y, et al. RuP nanoparticles supported on N, O codoped porous hollow carbon for efficient hydrogen oxidation reaction[J]. Advanced Materials Interfaces, 2022, 9(9): 2102193. |
| 22 | Song H Q, Cheng Y J, Li B J, et al. Carbon dots and RuP2 nanohybrid as an efficient bifunctional catalyst for electrochemical hydrogen evolution reaction and hydrolysis of ammonia borane[J]. ACS Sustainable Chemistry and Engineering, 2020, 8(9): 3995-4002. |
| 23 | He Y T, Peng Y M, Wang Y, et al. Air-engaged fabrication of nitrogen-doped carbon skeleton as an excellent platform for ultrafine well-dispersed RuNi alloy nanoparticles toward efficient hydrolysis of ammonia borane[J]. Fuel, 2021, 297: 120750. |
| 24 | Ma Y Y, Lang Z L, Yan L K, et al. Highly efficient hydrogen evolution triggered by a multi-interfacial Ni/WC hybrid electrocatalyst[J]. Energy & Environmental Science, 2018, 11(8): 2114-2123. |
| 25 | Jiang R F, Meng J Y, Yang S L, et al. Ru nanoclusters confined in N, O-codoped porous carbon as robust catalysts for hydrolytic dehydrogenation of NH3BH3 [J]. Applied Surface Science, 2022, 606: 154795. |
| 26 | Guan S Y, An L L, Aahraf S, et al. Oxygen vacancy excites Co3O4 nanocrystals embedded into carbon nitride for accelerated hydrogen generation[J]. Applied Catalysis B: Environmental, 2020, 269: 118775. |
| 27 | Cui C C, Liu Y Y, Mehdi S, et al. Enhancing effect of Fe-doping on the activity of nano Ni catalyst towards hydrogen evolution from NH3BH3 [J]. Applied Catalysis B: Environmental, 2020, 265: 118612. |
| 28 | Jin H Y, Chen J Y, Mao S T, et al. Transition metal induced the contraction of tungsten carbide lattice as superior hydrogen evolution reaction catalyst[J]. ACS Applied Materials and Interfaces, 2018, 10(26): 22094-22101. |
| 29 | Wang Y T, Pan L, Chen Y X, et al. Mo-doped Ni-based catalyst for remarkably enhancing catalytic hydrogen evolution of hydrogen-storage materials[J]. International Journal of Hydrogen Energy, 2020, 45: 15560-15570. |
| 30 | Hou C C, Li Q, Wang C J, et al. Ternary Ni-Co-P nanoparticles and their hybrids with graphene as noble-metal-free catalysts to boost the hydrolytic dehydrogenation of ammonia-borane[J]. Energy & Environmental Science, 2017, 8(10): 1770-1776. |
| 31 | Yang S L, Zhu Y, Liu J X, et al. Highly efficient catalytic hydrolysis of NH3BH3 over Ru nanoparticles anchored to chitosan-h-BN composite[J]. International Journal of Hydrogen Energy, 2020, 45: 18708-18718. |
| 32 | 孙海杰, 刘欣改, 陈志浩, 等. 羟基磷灰石负载Ru催化氨硼烷产氢性能研究[J]. 江西师范大学学报(自然科学版), 2020, 44(4): 424-428. |
| Sun H J, Liu X G, Chen Z H, et al. The performance of Ru/HAP catalysts for hydrogen generation from catalytic hydrolysis of ammonia borane[J]. Journal of Jiangxi Normal University( Natural Science), 2020, 44(4): 424-428. | |
| 33 | Wang C L, Tuninetti J, Wang Z, et al. Hydrolysis of ammonia-borane over Ni/ZIF-8 nanocatalyst: high efficiency, mechanism, and controlled hydrogen release[J]. Journal of the American Chemical Society, 2017, 139(33): 11610-11615. |
| 34 | Li Y T, Zhang X L, Peng Z K, et al. Hierarchical porous g-C3N4 coupled ultrafine RuNi alloys as extremely active catalysts for the hydrolytic dehydrogenation of ammonia borane[J]. ACS Sustainable Chemistry and Engineering, 2020, 8(22): 8458-8468. |
| [1] | 胡术刚, 田国庆, 刘文娟, 徐广飞, 刘华清, 张建, 王艳龙. 纳米零价铁的制备及氧化还原技术的应用进展[J]. 化工学报, 2024, 75(9): 3041-3055. |
| [2] | 王军锋, 张俊杰, 张伟, 王家乐, 双舒炎, 张亚栋. 液相放电等离子体分解甲醇制氢:电极配置的优化[J]. 化工学报, 2024, 75(9): 3277-3286. |
| [3] | 罗欣怡, 徐强, 佘永璐, 聂腾飞, 郭烈锦. 光电分解水制氢气泡动力学特性及其传质机理研究[J]. 化工学报, 2024, 75(9): 3083-3093. |
| [4] | 曹佳蕾, 孙立岩, 曾德望, 尹凡, 高子翔, 肖睿. 双流化床化学链制氢反应器的数值模拟[J]. 化工学报, 2024, 75(8): 2865-2874. |
| [5] | 丁家琦, 刘海涛, 赵普, 朱香凝, 王晓放, 谢蓉. 煤炭超临界水制氢反应器内多相流场智能滚动预测研究[J]. 化工学报, 2024, 75(8): 2886-2896. |
| [6] | 白炳林, 杜燊, 李明佳, 张传琪. 基于水相剥离的单壁碳纳米管薄膜透光和导电特性[J]. 化工学报, 2024, 75(7): 2680-2687. |
| [7] | 吴哲明, 张碧云, 郑仁朝. 腈水解酶立体选择性改造及其合成布瓦西坦[J]. 化工学报, 2024, 75(7): 2633-2643. |
| [8] | 赵璐璐, 唐二军, 邢旭腾, 刘少杰, 褚晓萌, 呼娜, 张泽. POSS改性氧化石墨烯对涂层防腐和疏水性能的影响[J]. 化工学报, 2024, 75(5): 1977-1986. |
| [9] | 司友明, 郑凌峰, 陈鹏忠, 樊江莉, 彭孝军. 新型锑氧簇光刻胶的性能与机理研究[J]. 化工学报, 2024, 75(4): 1705-1717. |
| [10] | 徐安冉, 刘凯, 王娜, 赵振宇, 李洪, 高鑫. 强吸波催化剂协同微波能强化果糖脱水制5-羟甲基糠醛[J]. 化工学报, 2024, 75(4): 1565-1577. |
| [11] | 严孝清, 赵瑛, 张宇哲, 欧鸿辉, 黄起中, 胡华贵, 杨贵东. 五重孪晶铜纳米线@聚吡咯制备及其电催化硝酸盐还原制氨[J]. 化工学报, 2024, 75(4): 1519-1532. |
| [12] | 杨玉维, 李敏, 要智颖, 孙沁林, 刘洋, 葛丹, 孙冰冰. 类器官芯片在纳米药物递送系统研究中的应用及前景[J]. 化工学报, 2024, 75(4): 1209-1221. |
| [13] | 王沛, 段睿明, 张广儒, 金万勤. 光热驱动的膜分离生物甲烷制氢过程建模与仿真分析[J]. 化工学报, 2024, 75(3): 967-973. |
| [14] | 刘志鹏, 赵长颖, 吴睿, 张智昊. 基于水电解制氢的梯度多孔传输层中气液流动可视化实验研究[J]. 化工学报, 2024, 75(2): 520-530. |
| [15] | 曹宇, 张国辉, 高昂, 杜心宇, 周静, 蔡永茂, 余璇, 于晓明. 二维MXene材料在太阳能电池和金属离子电池中的研究进展[J]. 化工学报, 2024, 75(2): 412-428. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号