化工学报 ›› 2024, Vol. 75 ›› Issue (2): 520-530.DOI: 10.11949/0438-1157.20231165
收稿日期:2023-11-13
修回日期:2023-12-26
出版日期:2024-02-25
发布日期:2024-04-10
通讯作者:
赵长颖
作者简介:刘志鹏(1997—),男,硕士研究生,liuzhipeng97@sjtu.edu.cn
Zhipeng LIU( ), Changying ZHAO(
), Changying ZHAO( ), Rui WU, Zhihao ZHANG
), Rui WU, Zhihao ZHANG
Received:2023-11-13
Revised:2023-12-26
Online:2024-02-25
Published:2024-04-10
Contact:
Changying ZHAO
摘要:
在电解水制氢的过程中,多孔电极内的孔隙会发生气泡阻塞现象,这会妨碍气体扩散以及电解液在多孔电极内的流动,从而导致电极传质电阻的增加,进而影响电解水制氢的速率和能耗。采用3D金属打印技术制备了LSL-PTL、MMM-PTL和SLS-PTL三种规则的镍铁合金电极扩散层,进行了可视化的水电解实验,定量地记录了不同电流密度下梯度多孔传输层中气液两相流动的变化,包括气泡形态、孔隙含气率和气泡脱离速率等参数,研究扩散层梯度对气液传质过程的影响,并分析了不同电极梯度结构对电解过程中阻抗和过电位的影响。实验结果显示,与SLS-PTL和MMM-PTL相比,LSL-PTL的梯度结构从催化层即逐渐增大孔隙尺寸,始终保持较低的容积含气率,可以加速气泡在扩散层中的迁移,使气液交换更加频繁,有效减小气液传质阻力,并获得更低的传质阻抗和电解过电位,三种梯度电极在相同电流密度下的电解电势关系为
中图分类号:
刘志鹏, 赵长颖, 吴睿, 张智昊. 基于水电解制氢的梯度多孔传输层中气液流动可视化实验研究[J]. 化工学报, 2024, 75(2): 520-530.
Zhipeng LIU, Changying ZHAO, Rui WU, Zhihao ZHANG. Experimental study of gas-liquid flow visualization in gradient porous transport layers based on hydrogen production by water electrolysis[J]. CIESC Journal, 2024, 75(2): 520-530.
| 名称 | 结构 | 具体参数 |
|---|---|---|
| LSL-PTL | 扩散层孔隙尺寸从催化层界面向外递增 | 孔隙边长依次为0.8、1.0和1.2 mm 每层孔隙的深度均为1.8 mm 扩散层孔隙度25.67% |
| MMM-PTL | 扩散层孔隙尺寸从催化层向外各处均匀一致 | 孔隙边长1.01 mm 孔隙深度5.4 mm 扩散层孔隙度25.67% |
| SLS-PTL | 扩散层孔隙尺寸从催化层界面向外递减 | 孔的边长依次为1.2、1.0和0.8 mm 每层孔隙的深度均为1.8 mm 扩散层孔隙度25.67% |
表1 三种不同梯度的多孔传输层详细参数
Table 1 Detailed parameters of three different gradients of the PTL
| 名称 | 结构 | 具体参数 |
|---|---|---|
| LSL-PTL | 扩散层孔隙尺寸从催化层界面向外递增 | 孔隙边长依次为0.8、1.0和1.2 mm 每层孔隙的深度均为1.8 mm 扩散层孔隙度25.67% |
| MMM-PTL | 扩散层孔隙尺寸从催化层向外各处均匀一致 | 孔隙边长1.01 mm 孔隙深度5.4 mm 扩散层孔隙度25.67% |
| SLS-PTL | 扩散层孔隙尺寸从催化层界面向外递减 | 孔的边长依次为1.2、1.0和0.8 mm 每层孔隙的深度均为1.8 mm 扩散层孔隙度25.67% |
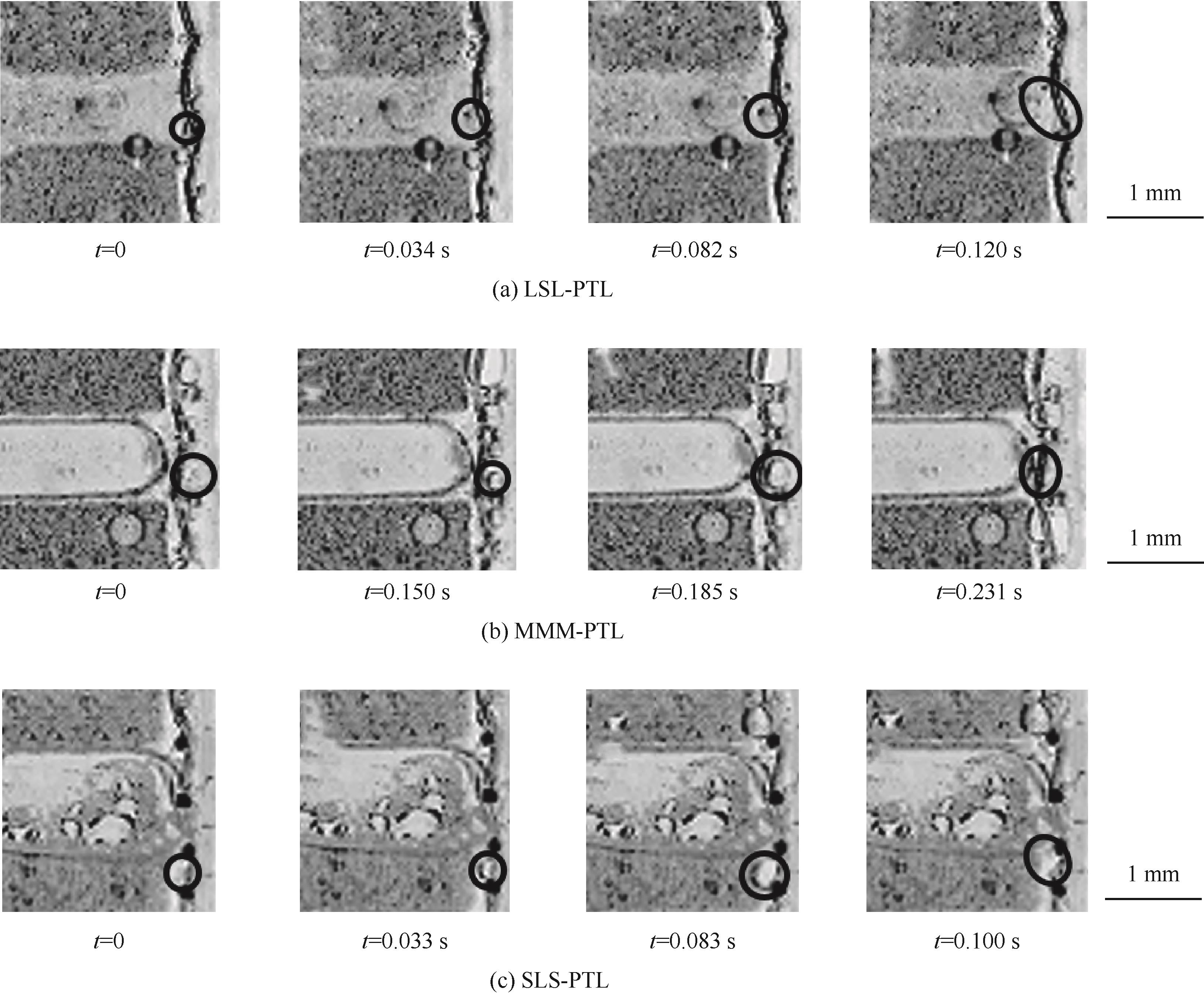
图4 0.1 A/cm2电流密度下催化层与扩散层界面处气泡生成与脱离
Fig.4 Bubble generation and detachment at the interface of catalytic and diffusion layers at 0.1 A/cm2 current density
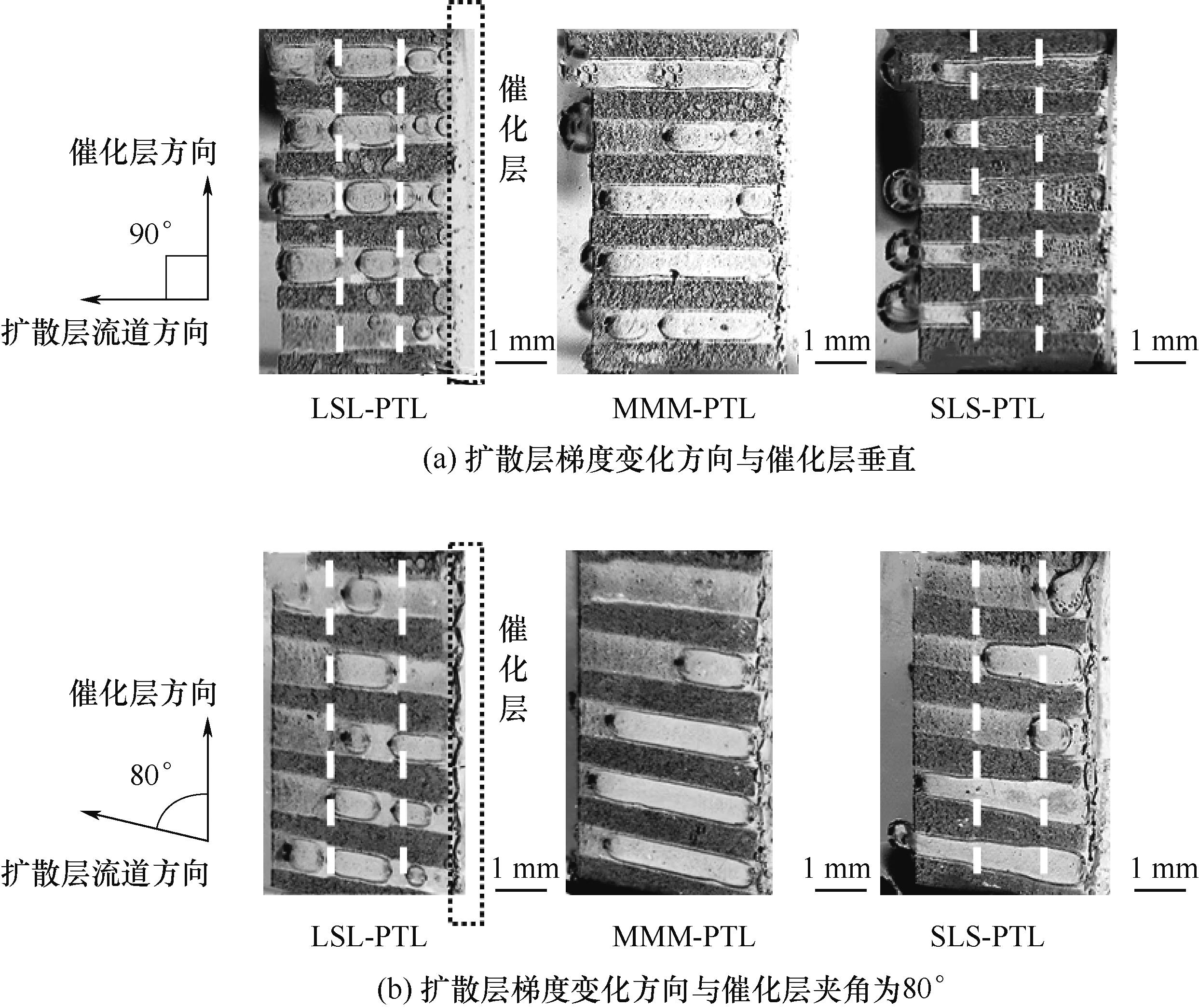
图5 LSL-PTL、MMM-PTL、SLS-PTL在0.1 A/cm2的电流下HER产生的气泡在多孔传输层中的形态(白色虚线为扩散层中不同梯度孔隙分界)
Fig.5 Morphology of bubbles generated by HER in the porous transport layer at a current of 0.1 A/cm2 for LSL-PTL, MMM-PTL, and SLS-PTL (the white dashed line represents the boundary between pores with different gradients in the diffusion layer)
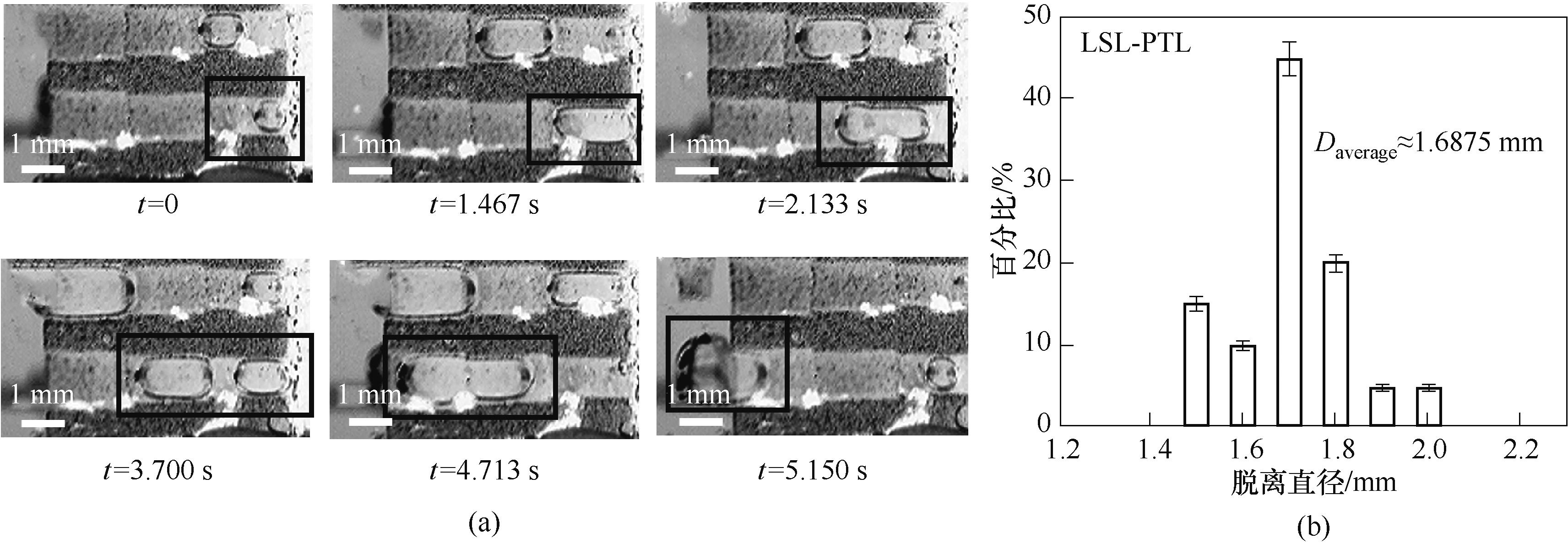
图6 0.1 A/cm2电流密度下LSL-PTL扩散层内气泡形态和位置随时间变化(a)及气泡脱离直径分布情况(b)
Fig.6 (a) Sets of photographs demonstrating the typical bubble removal processes releasing from LSL-PTL at 0.1 A/cm2 current density; (b) Distribution of the diameters of hydrogen bubbles that detached from LSL-PTL at 0.1 A/cm2 current density
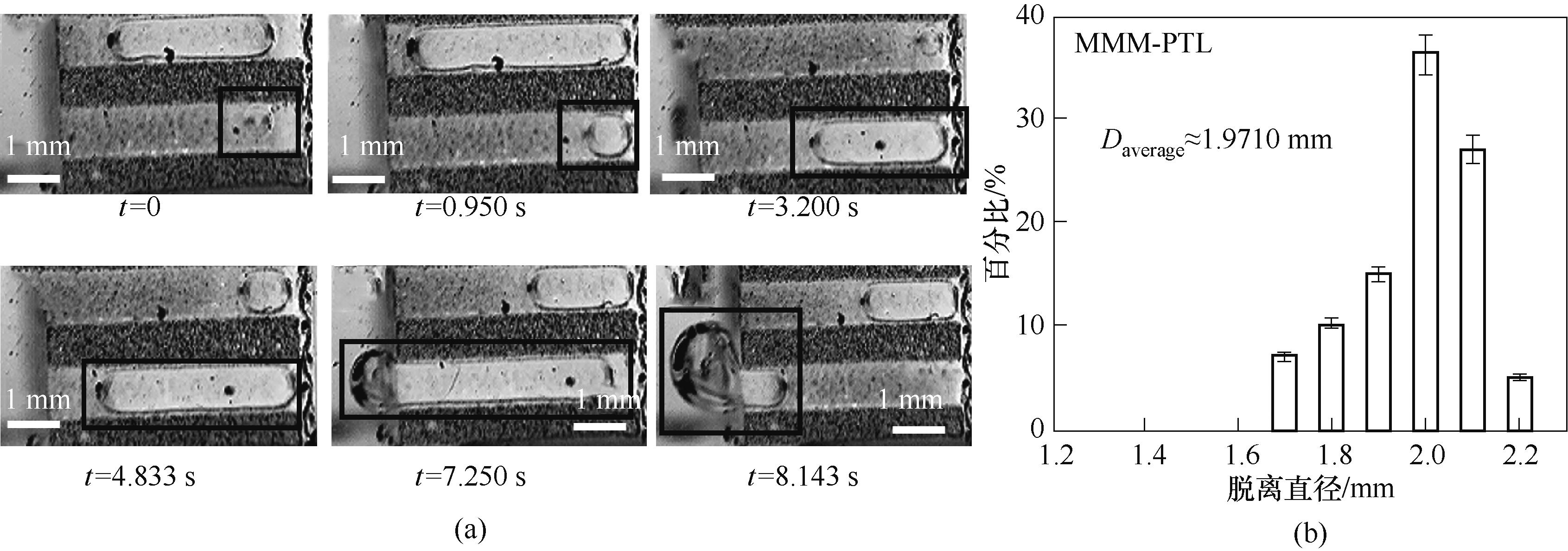
图7 0.1 A/cm2电流密度下MMM-PTL扩散层内气泡形态和位置随时间变化(a)及气泡脱离直径分布情况(b)
Fig.7 (a) Sets of photographs demonstrating the typical bubble removal processes releasing from MMM-PTL at 0.1 A/cm2 current density; (b) Distribution of the diameters of hydrogen bubbles that detached from MMM -PTL at 0.1 A/cm2 current density
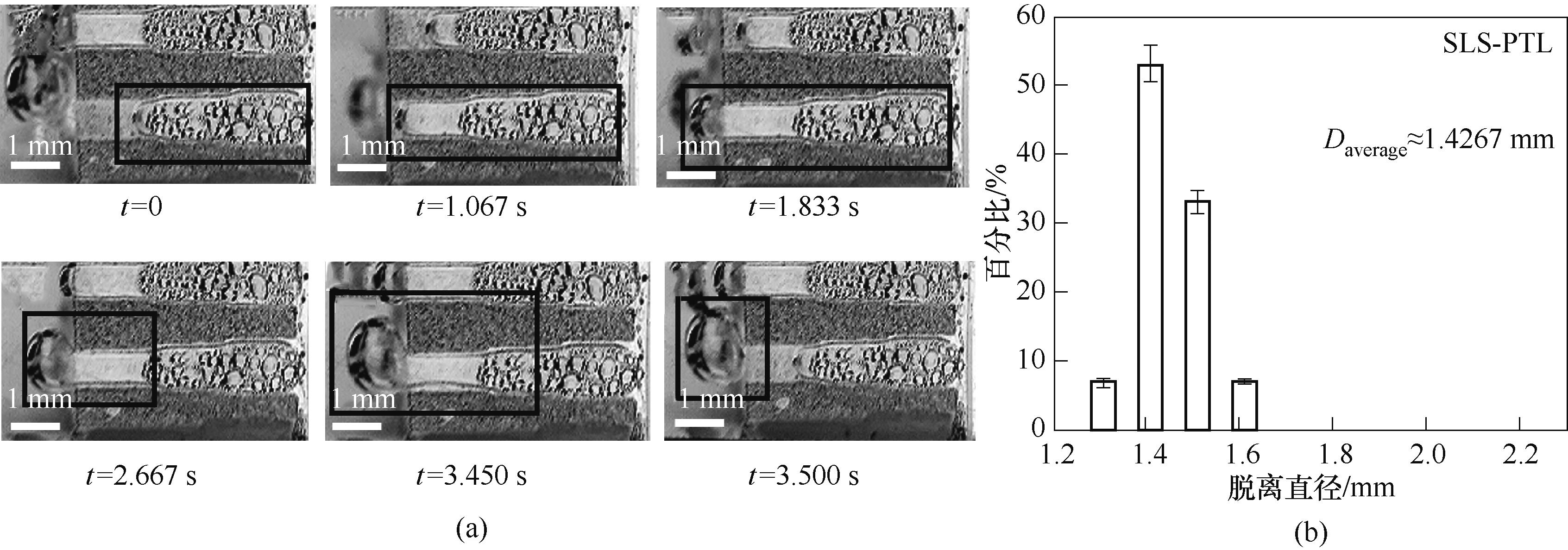
图8 0.1 A/cm2电流密度下SLS-PTL扩散层内气泡形态和位置随时间变化(a)及气泡脱离直径分布情况(b)
Fig.8 (a) Sets of photographs demonstrating the typical bubble removal processes releasing from SLS-PTL at 0.1 A/cm2 current density; (b) Distribution of the diameters of hydrogen bubbles that detached from SLS-PTL at 0.1 A/cm2 current density
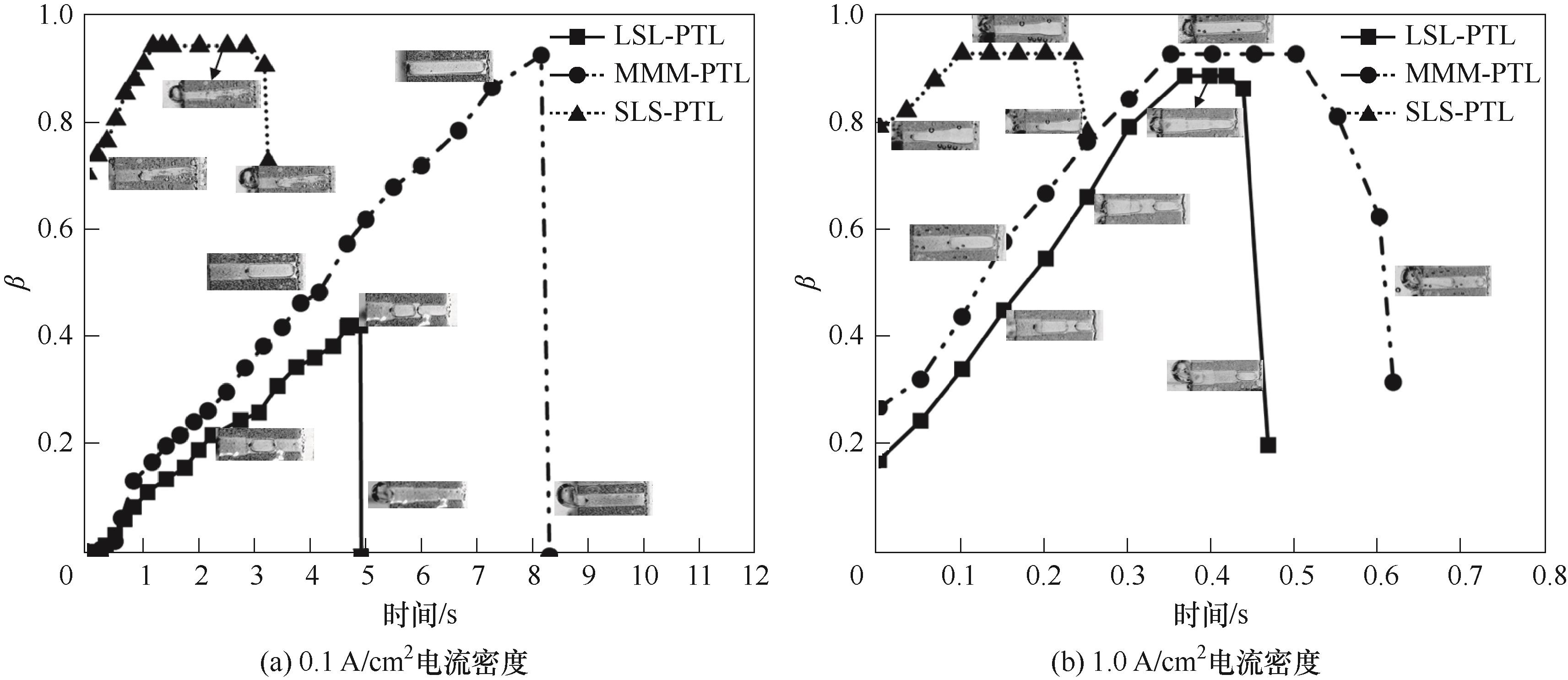
图9 扩散层中一个脱离周期内的气泡演化过程和容积含气率变化
Fig.9 Volumetric gas content in the diffusion layer during one detachment cycle versus time at different current densities
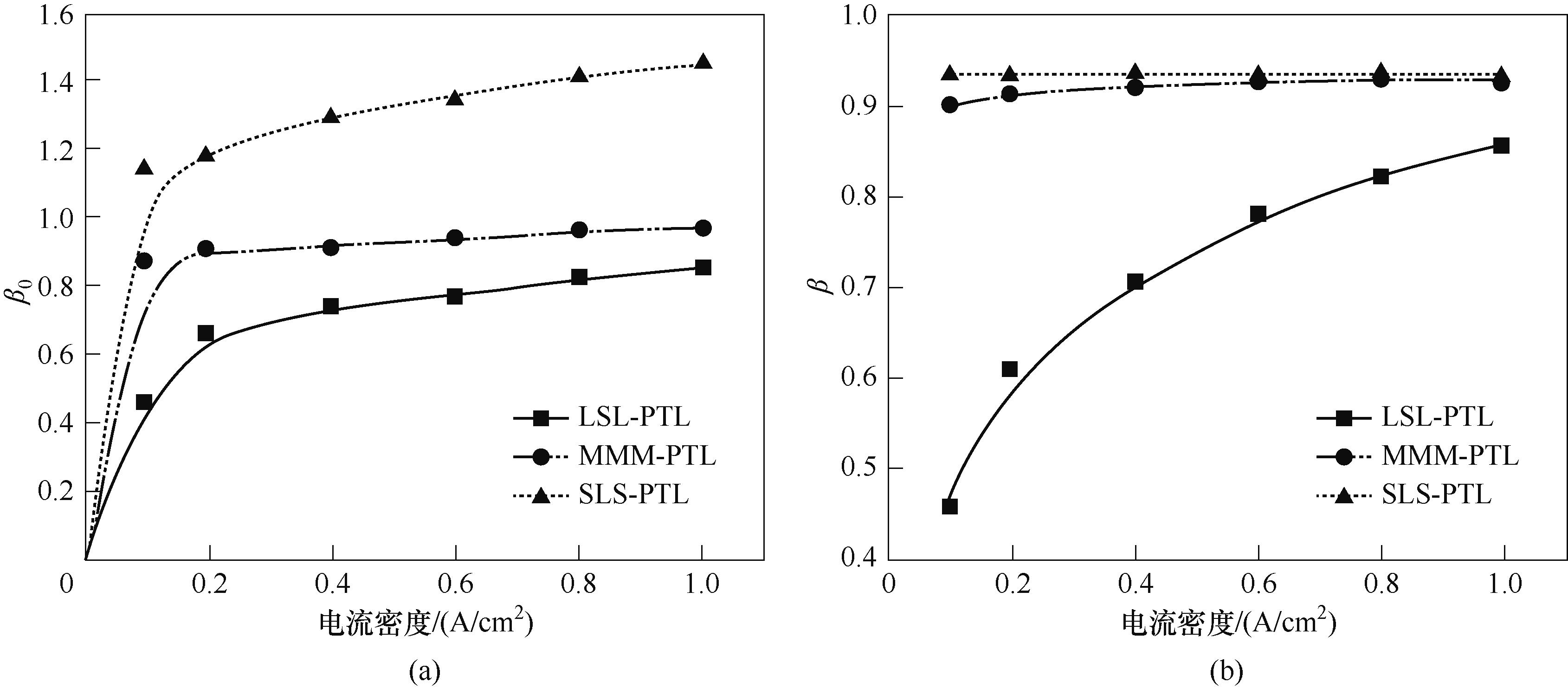
图11 不同电流密度条件下气泡脱离时刻的临界β0及气泡脱离时刻的最大容积含气率β
Fig.11 Maximum β0 at the moment of bubble detachment and maximum volumetric gas content β at the moment of bubble detachment for different current density conditions
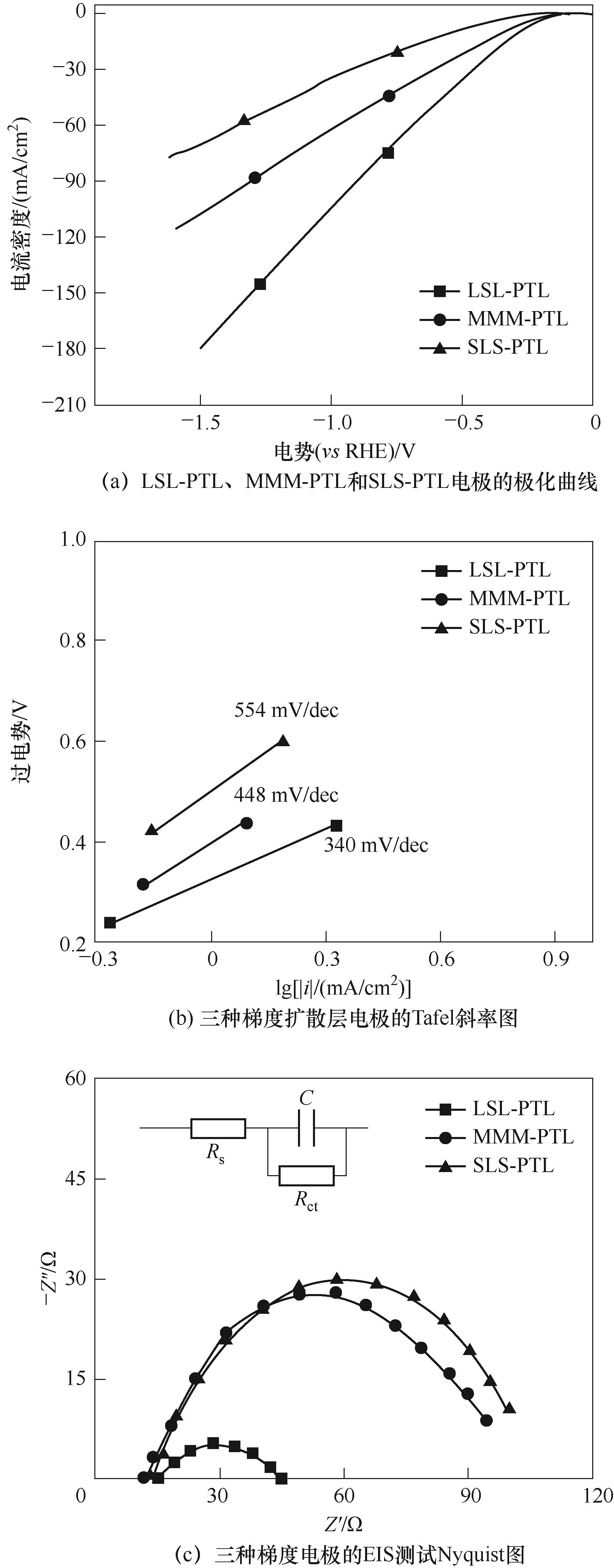
图13 LSL-PTL、MMM-PTL和SLS-PTL电极的极化曲线(a)、Tafel斜率图(b)、EIS测试Nyquist图(c)
Fig.13 (a) Polarization curve of LSL-PTL, MMM-PTL and SLS-PTL electrodes; (b) Tafel plots for LSL-PTL, MMM-PTL and SLS-PTL electrodes; (c) EIS Nyquist plots of LSL-PTL, MMM-PTL and SLS-PTL electrodes
| 1 | 何钰江, 刘会灯, 王皓宇, 等. “双碳”目标下氢能发展体系构建和产业创新布局展望[J]. 电工电能新技术, 2023, 42(9): 65-76. |
| He Y J, Liu H D, Wang H Y, et al. Prospects for construction of hydrogen energy development system and industrial innovation layout under “double carbon” goal[J]. Advanced Technology of Electrical Engineering and Energy, 2023, 42(9): 65-76. | |
| 2 | Schmidt O, Gambhir A, Staffell I, et al. Future cost and performance of water electrolysis: an expert elicitation study[J]. International Journal of Hydrogen Energy, 2017, 42(52): 30470-30492. |
| 3 | Buttler A, Spliethoff H. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: a review[J]. Renewable and Sustainable Energy Reviews, 2018, 82: 2440-2454. |
| 4 | Klose C, Saatkamp T, Münchinger A, et al. Water electrolyzers: all-hydrocarbon MEA for PEM water electrolysis combining low hydrogen crossover and high efficiency[J]. Advanced Energy Materials, 2020, 10(14): 207006. |
| 5 | Kibsgaard J, Chorkendorff I. Considerations for the scaling-up of water splitting catalysts[J]. Nature Energy, 2019, 4(6): 430-433. |
| 6 | Siracusano S, Di Blasi A, Baglio V, et al. Optimization of components and assembling in a PEM electrolyzer stack[J]. International Journal of Hydrogen Energy, 2011, 36(5): 3333-3339. |
| 7 | Yuan S, Zhao C F, Cai X Y, et al. Bubble evolution and transport in PEM water electrolysis: mechanism, impact, and management[J]. Progress in Energy and Combustion Science, 2023, 96: 101075. |
| 8 | Kang Z Y, Mo J K, Yang G Q, et al. Investigation of thin/well-tunable liquid/gas diffusion layers exhibiting superior multifunctional performance in low-temperature electrolytic water splitting[J]. Energy & Environmental Science, 2017, 10(1): 166-175. |
| 9 | Wan L, Xu Z A, Xu Q, et al. Overall design of novel 3D-ordered MEA with drastically enhanced mass transport for alkaline electrolyzers[J]. Energy & Environmental Science, 2022, 15(5): 1882-1892. |
| 10 | De Angelis S, Schuler T, Charalambous M A, et al. Unraveling two-phase transport in porous transport layer materials for polymer electrolyte water electrolysis[J]. Journal of Materials Chemistry A, 2021, 9(38): 22102-22113. |
| 11 | Iwata R, Zhang L N, Wilke K L, et al. Bubble growth and departure modes on wettable/non-wettable porous foams in alkaline water splitting[J]. Joule, 2021, 5(4): 887-900. |
| 12 | Schuler T, Schmidt T J, Büchi F N. Polymer electrolyte water electrolysis: correlating performance and porous transport layer structure(part Ⅱ): Electrochemical performance analysis[J]. Journal of the Electrochemical Society, 2019, 166(10): F555-F565. |
| 13 | 唐英伦, 杨小涛, 赵军, 等. 叠层扩散层对质子交换膜电解池性能影响的实验研究[J]. 工程热物理学报, 2023, 44(2): 304-310. |
| Tang Y L, Yang X T, Zhao J, et al. Experimental study on the effects of stacked diffusion layer on the performance of proton-exchange membrane electrolysis cells[J]. Journal of Engineering Thermophysics, 2023, 44(2): 304-310. | |
| 14 | Yang Y, Li J, Yang Y R, et al. Gradient porous electrode-inducing bubble splitting for highly efficient hydrogen evolution[J]. Applied Energy, 2022, 307: 118278. |
| 15 | Huang B R, Wang X C, Li W Z, et al. Accelerating gas escape in anion exchange membrane water electrolysis by gas diffusion layers with hierarchical grid gradients[J]. Angewandte Chemie, 2023, 135(33): e2023042. |
| 16 | Van Pham C, Escalera-López D, Mayrhofer K, et al. Essentials of high performance water electrolyzers—from catalyst layer materials to electrode engineering[J]. Advanced Energy Materials, 2021, 11(44): 2101998. |
| 17 | Angulo A, van der Linde P, Gardeniers H, et al. Influence of bubbles on the energy conversion efficiency of electrochemical reactors[J]. Joule, 2020, 4(3): 555-579. |
| 18 | Chatenet M, Pollet B G, Dekel D R, et al. Water electrolysis: from textbook knowledge to the latest scientific strategies and industrial developments[J]. Chemical Society Reviews, 2022, 51(11): 4583-4762. |
| 19 | Razmjooei F, Morawietz T, Taghizadeh E, et al. Increasing the performance of an anion-exchange membrane electrolyzer operating in pure water with a nickel-based microporous layer[J]. Joule, 2021, 5(7): 1776-1799. |
| 20 | Xu Q C, Oener S Z, Lindquist G, et al. Integrated reference electrodes in anion-exchange-membrane electrolyzers: impact of stainless-steel gas-diffusion layers and internal mechanical pressure[J]. ACS Energy Letters, 2021, 6(2): 305-312. |
| 21 | Azarafza A, Ismail M S, Rezakazemi M, et al. Comparative study of conventional and unconventional designs of cathode flow fields in PEM fuel cell[J]. Renewable and Sustainable Energy Reviews, 2019, 116: 109420. |
| 22 | Wang M Y, Wang Z, Gong X Z, et al. The intensification technologies to water electrolysis for hydrogen production—a review[J]. Renewable and Sustainable Energy Reviews, 2014, 29: 573-588. |
| 23 | Zhao X, Ren H, Luo L. Gas bubbles in electrochemical gas evolution reactions[J]. Langmuir, 2019, 35(16): 5392-5408. |
| 24 | Song X Y, Li W Q, Liu X J, et al. Oxygen vacancies enable the visible light photoactivity of chromium-implanted TiO2 nanowires[J]. Journal of Energy Chemistry, 2021, 55: 154-161. |
| 25 | Park J E, Kang S Y, Oh S H, et al. High-performance anion-exchange membrane water electrolysis[J]. Electrochimica Acta, 2019, 295: 99-106. |
| 26 | Taqieddin A, Nazari R, Rajic L, et al. Review—physicochemical hydrodynamics of gas bubbles in two phase electrochemical systems[J]. Journal of the Electrochemical Society, 2017, 164(13): E448-E459. |
| 27 | Huang R L, Zhao C Y, Xu Z G. Investigation of bubble behavior in gradient porous media under pool boiling conditions[J]. International Journal of Multiphase Flow, 2018, 103: 85-93. |
| 28 | Siracusano S, Trocino S, Briguglio N, et al. Electrochemical impedance spectroscopy as a diagnostic tool in polymer electrolyte membrane electrolysis[J]. Materials, 2018, 11(8): 1368. |
| 29 | Xu X, Fu G W, Wang Y X, et al. Highly efficient all-3D-printed electrolyzer toward ultrastable water electrolysis[J]. Nano Letters, 2023, 23(2): 629-636. |
| 30 | Wang L, Huang X L, Jiang S S, et al. Increasing gas bubble escape rate for water splitting with nonwoven stainless steel fabrics[J]. ACS Applied Materials & Interfaces, 2017, 9(46): 40281-40289. |
| 31 | Sun Q Q, Dong Y J, Wang Z L, et al. Synergistic nanotubular copper-doped nickel catalysts for hydrogen evolution reactions[J]. Small, 2018, 14(14): 1704137. |
| [1] | 詹小斌, 王会彬, 蒋亚龙, 史铁林. 声共振混合器高黏度流体混合的功耗特性研究[J]. 化工学报, 2024, 75(2): 531-542. |
| [2] | 刘起超, 张世博, 周云龙, 李昱庆, 陈聪, 冉议文. 起伏振动水平管气液两相流型及转变机理[J]. 化工学报, 2024, 75(2): 493-504. |
| [3] | 李乃良, 刘常松, 杜雪平, 张一帆, 韩东太. 基于Hurst指数的严重段塞流多尺度分形特性[J]. 化工学报, 2024, 75(2): 484-492. |
| [4] | 崔怡洲, 李成祥, 翟霖晓, 刘束玉, 石孝刚, 高金森, 蓝兴英. 亚毫米气泡和常规尺寸气泡气液两相流流动与传质特性对比[J]. 化工学报, 2024, 75(1): 197-210. |
| [5] | 江河, 袁俊飞, 王林, 邢谷雨. 均流腔结构对微细通道内相变流动特性影响的实验研究[J]. 化工学报, 2023, 74(S1): 235-244. |
| [6] | 黄琮琪, 吴一梅, 陈建业, 邵双全. 碱性电解水制氢装置热管理系统仿真研究[J]. 化工学报, 2023, 74(S1): 320-328. |
| [7] | 袁佳琦, 刘政, 黄锐, 张乐福, 贺登辉. 泡状入流条件下旋流泵能量转换特性研究[J]. 化工学报, 2023, 74(9): 3807-3820. |
| [8] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [9] | 邢雷, 苗春雨, 蒋明虎, 赵立新, 李新亚. 井下微型气液旋流分离器优化设计与性能分析[J]. 化工学报, 2023, 74(8): 3394-3406. |
| [10] | 高金明, 郭玉娇, 鄂承林, 卢春喜. 一种封闭罩内顺流多旋臂气液分离器的分离特性研究[J]. 化工学报, 2023, 74(7): 2957-2966. |
| [11] | 周小文, 杜杰, 张战国, 许光文. 基于甲烷脉冲法的Fe2O3-Al2O3载氧体还原特性研究[J]. 化工学报, 2023, 74(6): 2611-2623. |
| [12] | 李勇, 高佳琦, 杜超, 赵亚丽, 李伯琼, 申倩倩, 贾虎生, 薛晋波. Ni@C@TiO2核壳双重异质结的构筑及光热催化分解水产氢[J]. 化工学报, 2023, 74(6): 2458-2467. |
| [13] | 江锦波, 彭新, 许文烜, 门日秀, 刘畅, 彭旭东. 泵出型螺旋槽油气密封泄漏特性及参数影响研究[J]. 化工学报, 2023, 74(6): 2538-2554. |
| [14] | 刘起超, 周云龙, 陈聪. 起伏振动垂直上升管气液两相流截面含气率分析与计算[J]. 化工学报, 2023, 74(6): 2391-2403. |
| [15] | 董鑫, 单永瑞, 刘易诺, 冯颖, 张建伟. 非牛顿流体气泡羽流涡特性数值模拟研究[J]. 化工学报, 2023, 74(5): 1950-1964. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号