化工学报 ›› 2024, Vol. 75 ›› Issue (11): 3870-3882.DOI: 10.11949/0438-1157.20240610
收稿日期:2024-06-03
修回日期:2024-07-25
出版日期:2024-11-25
发布日期:2024-12-26
通讯作者:
李先锋
作者简介:鲁文静(1991—),女,博士,项目研究员,luwenjing@dicp.ac.cn
基金资助:Received:2024-06-03
Revised:2024-07-25
Online:2024-11-25
Published:2024-12-26
Contact:
Xianfeng LI
摘要:
液流电池储能技术具有安全性高、效率高、寿命长、环境友好等特点,适用于大规模储能和分布式储能。离子传导膜是液流电池的关键材料之一,其性质和成本直接影响着液流电池的性能和成本。复合离子传导膜选择层和多孔支撑层的组成和结构可以分别调控,可以兼具高选择性、高传导性和高稳定性,目前已经在液流电池中得到广泛的研究和应用。此外,通过对复合膜选择层结构的调控,其选择性和传导性等性能可以得到进一步优化,从而提升液流电池的性能和寿命。本综述基于液流电池用复合膜的研究进展,总结不同的基于多孔支撑层的多孔复合膜选择层的结构调控策略,为液流电池用离子传导膜的进一步发展提供理论指导。
中图分类号:
鲁文静, 李先锋. 液流电池多孔复合离子传导膜研究进展[J]. 化工学报, 2024, 75(11): 3870-3882.
Wenjing LU, Xianfeng LI. Research process of porous composite ion conducting membranes for flow batteries[J]. CIESC Journal, 2024, 75(11): 3870-3882.
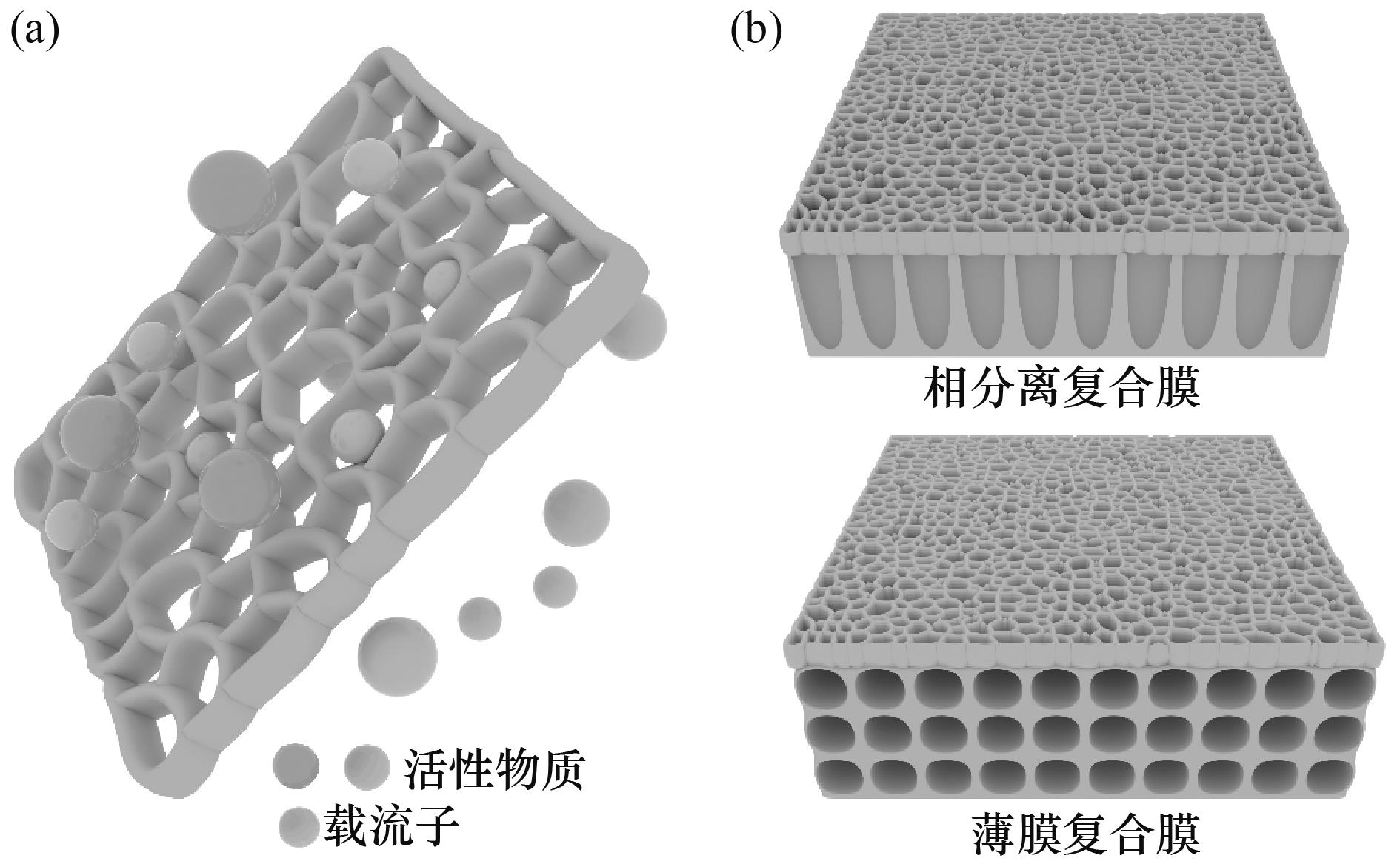
图1 典型的FB用多孔膜示意图(a)[16];FB用复合膜示意图(b)[16]
Fig.1 Schematic diagram of a typical porous membrane in FBs (a)[16]; Schematic diagram of composite membranes in FBs (b)[16]
| 聚合物 | 基底 | 制备方法 | 选择层 结构 | 选择层 厚度 | 体系 | 电流密度/(mA·cm-2) | 能量效率/% | 循环次数/时间 | 文献 |
|---|---|---|---|---|---|---|---|---|---|
| 多巴胺涂覆PA | 多孔PES膜 | 界面聚合 | — | 120 nm | VFB | 80 | 约80 | 158次 | [ |
| MEPBr/Nafion | 多孔Daramic膜 | 刮涂 | 致密 | — | ZBFB | 40 | 85.31 | >120次 | [ |
| PFSA/FSA | 多孔PE膜 | 刮涂 | 致密 | 约20 μm | VFB | 60 | 约85 | 约100次 | [ |
| PBI | PFSA离子交换膜 | 浸涂 | 致密 | 约4 μm | VFB | 140 | 86 | 960次 | [ |
| PBI | PBI静电纺丝纳米纤维 | 溶剂辅助 | 致密 | 7 μm | VFB | 80 | 约86 | 200次 | [ |
| PBI | 多孔聚丙烯膜 | 层压 | 致密 | 1 μm | VFB | 120 | 80 | 2860次 | [ |
| Nafion | 多孔PES/SPEEK膜 | 喷涂 | 致密 | 约30 μm | VFB | 80 | 86.5 | 100次 | [ |
| L-PSQ | Celgard 2400 | 浸涂 | 致密 | 约1.7 μm | 非水系V(acac)3体系 | 约1.4 | 43.7 | 约50次 | [ |
| PFSA | 多孔聚碳酸酯膜 | 朗缪尔-布洛杰特 | 层状 | 42 nm | VFB | 200 | 约74 | 800次 | [ |
| PBI | 多孔PVDF膜 | 喷涂 | 层状 | 4 μm | VFB | 80 | 85.1 | 20次 | [ |
| PDDA/U | Celgard 2400 | 刮涂 | 致密 | 45 μm | 非水系V(acac)3体系 | 0.5 | 42.5 | — | [ |
| PANI | Nafion 115 | 浸涂 | 致密 | 2 μm | VFB | 50 | 91 | 约100次 | [ |
| Py | 多孔PES膜 | 界面聚合 | — | 60 nm | VFB | 80 | 81 | 93次 | [ |
| PBI | 多孔Nafion膜 | 喷涂 | 致密 | — | VFB | 20 | 约78 | — | [ |
| PDDA/PSS | PTFE/SPEEK | 层层自组装 | 层状 | — | VFB | 80 | 约82 | 80次 | [ |
| PBI | 多孔PE膜 | 浸涂 | 致密 | — | VFB | 300 | 78.7 | >500次 | [ |
| SPEEK | 多孔陶瓷膜 | 旋涂 | 致密 | <10 nm | AZIFB | 80 | 80.2 | >1000 h | [ |
| PA | 多孔PES/SPEEK膜 | 界面聚合 | — | 约180 nm | VFB | 260 | >80 | 1000次 | [ |
| PIM⑪ | 多孔PAN膜 | 旋涂 | 多孔 | 约0.4 μm | 2,6-DHAQ/Fe(CN)6 | 40 | — | 400次 | [ |
| PIM | 多孔PAN膜 | 旋涂 | 多孔 | 4 μm | 2,6-DHAQ/Fe(CN)6 | 80 | 约65 | 3390次 | [ |
| PEG/Nafion | 多孔Daramic膜 | 刮涂 | 致密 | — | ZBFB | 160 | 约63 | >85次 | [ |
表1 典型的FB用聚合物基TFCM汇总
Table 1 Summary of several typical polymer-based TFCMs in FBs
| 聚合物 | 基底 | 制备方法 | 选择层 结构 | 选择层 厚度 | 体系 | 电流密度/(mA·cm-2) | 能量效率/% | 循环次数/时间 | 文献 |
|---|---|---|---|---|---|---|---|---|---|
| 多巴胺涂覆PA | 多孔PES膜 | 界面聚合 | — | 120 nm | VFB | 80 | 约80 | 158次 | [ |
| MEPBr/Nafion | 多孔Daramic膜 | 刮涂 | 致密 | — | ZBFB | 40 | 85.31 | >120次 | [ |
| PFSA/FSA | 多孔PE膜 | 刮涂 | 致密 | 约20 μm | VFB | 60 | 约85 | 约100次 | [ |
| PBI | PFSA离子交换膜 | 浸涂 | 致密 | 约4 μm | VFB | 140 | 86 | 960次 | [ |
| PBI | PBI静电纺丝纳米纤维 | 溶剂辅助 | 致密 | 7 μm | VFB | 80 | 约86 | 200次 | [ |
| PBI | 多孔聚丙烯膜 | 层压 | 致密 | 1 μm | VFB | 120 | 80 | 2860次 | [ |
| Nafion | 多孔PES/SPEEK膜 | 喷涂 | 致密 | 约30 μm | VFB | 80 | 86.5 | 100次 | [ |
| L-PSQ | Celgard 2400 | 浸涂 | 致密 | 约1.7 μm | 非水系V(acac)3体系 | 约1.4 | 43.7 | 约50次 | [ |
| PFSA | 多孔聚碳酸酯膜 | 朗缪尔-布洛杰特 | 层状 | 42 nm | VFB | 200 | 约74 | 800次 | [ |
| PBI | 多孔PVDF膜 | 喷涂 | 层状 | 4 μm | VFB | 80 | 85.1 | 20次 | [ |
| PDDA/U | Celgard 2400 | 刮涂 | 致密 | 45 μm | 非水系V(acac)3体系 | 0.5 | 42.5 | — | [ |
| PANI | Nafion 115 | 浸涂 | 致密 | 2 μm | VFB | 50 | 91 | 约100次 | [ |
| Py | 多孔PES膜 | 界面聚合 | — | 60 nm | VFB | 80 | 81 | 93次 | [ |
| PBI | 多孔Nafion膜 | 喷涂 | 致密 | — | VFB | 20 | 约78 | — | [ |
| PDDA/PSS | PTFE/SPEEK | 层层自组装 | 层状 | — | VFB | 80 | 约82 | 80次 | [ |
| PBI | 多孔PE膜 | 浸涂 | 致密 | — | VFB | 300 | 78.7 | >500次 | [ |
| SPEEK | 多孔陶瓷膜 | 旋涂 | 致密 | <10 nm | AZIFB | 80 | 80.2 | >1000 h | [ |
| PA | 多孔PES/SPEEK膜 | 界面聚合 | — | 约180 nm | VFB | 260 | >80 | 1000次 | [ |
| PIM⑪ | 多孔PAN膜 | 旋涂 | 多孔 | 约0.4 μm | 2,6-DHAQ/Fe(CN)6 | 40 | — | 400次 | [ |
| PIM | 多孔PAN膜 | 旋涂 | 多孔 | 4 μm | 2,6-DHAQ/Fe(CN)6 | 80 | 约65 | 3390次 | [ |
| PEG/Nafion | 多孔Daramic膜 | 刮涂 | 致密 | — | ZBFB | 160 | 约63 | >85次 | [ |
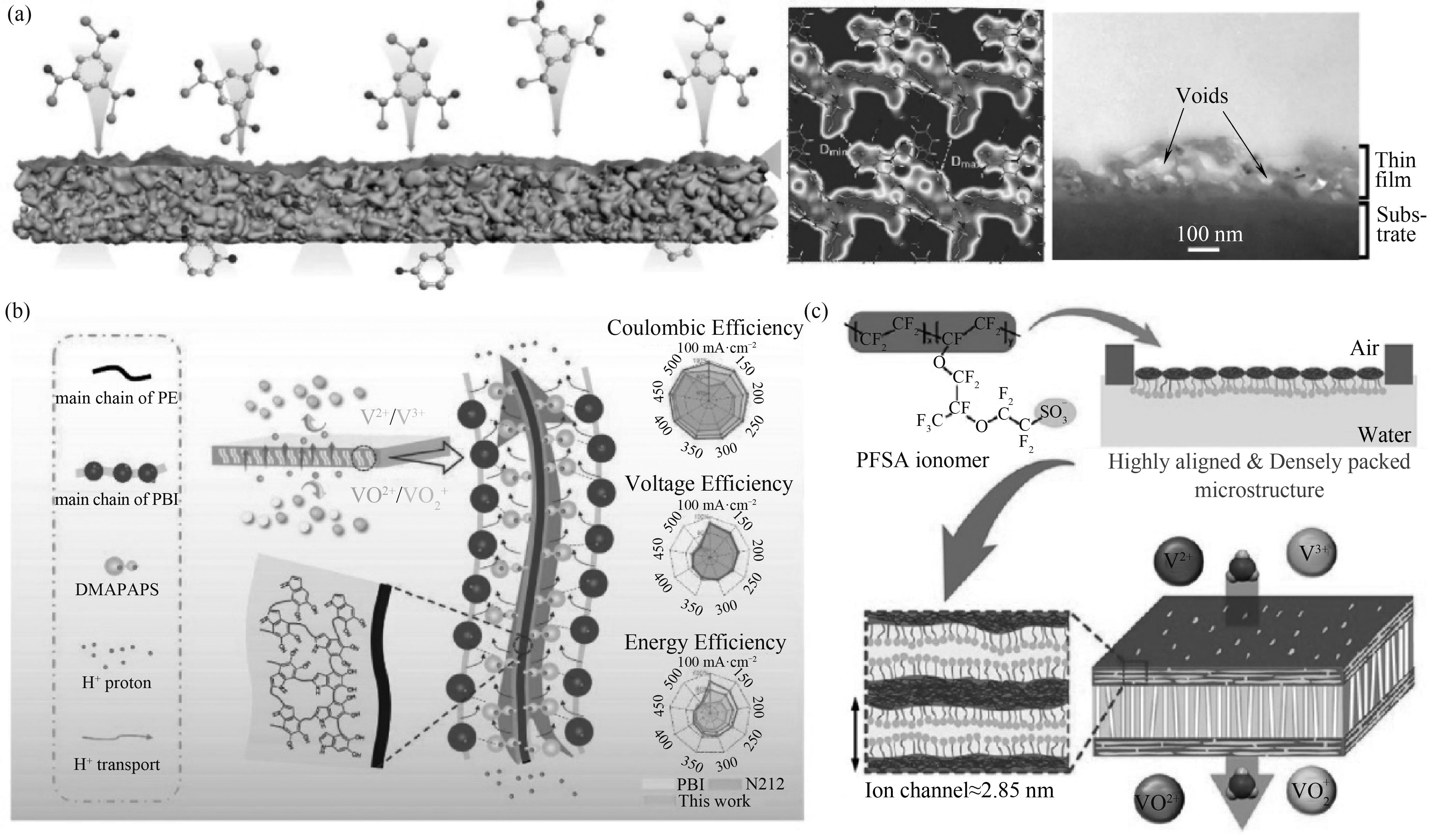
图2 PA基TFCM示意图及其交联PA骨架和横截面形貌图(a)[29];复合膜中PBI传导性聚合物与PE基底间的两性离子界面示意图(b)[35];超薄PFSA离聚物膜示意图(c)[39]
Fig.2 Schematic diagram of PA-based TFCM and its cross-linked PA framework and its cross-section morphology (a)[29]; Schematic illustration of the zwitterionic interface between PBI conductive polymer and PE substrate in the composite membrane (b)[35]; Schematic diagram of the ultrathin PFSA ionomer membrane (c)[39]
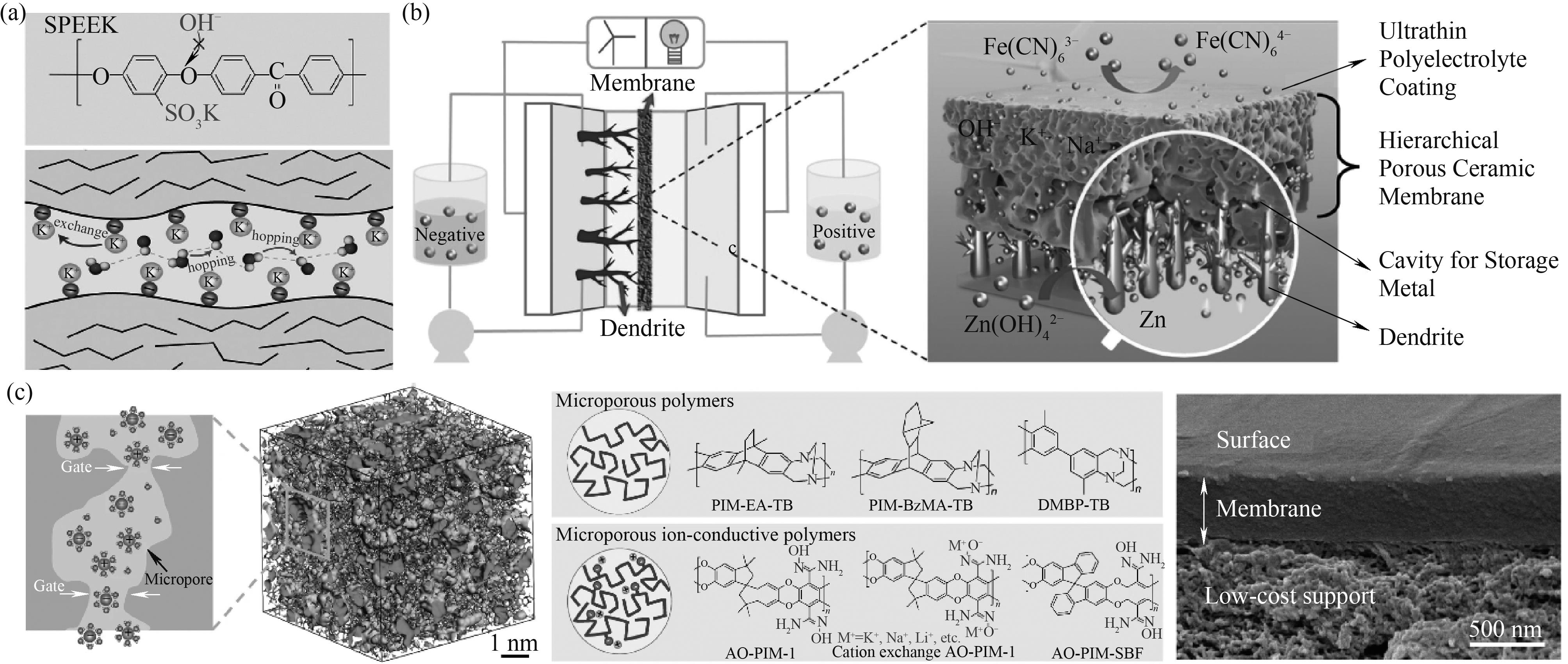
图3 SPEEK的化学结构和碱性介质中SPEEK膜内的离子迁移示意图(a)[56];水系AZIFB和层状多孔陶瓷膜抑制锌枝晶形成的示意图(b)[43];微孔膜内相互贯通的亚纳米空隙和微孔聚合物的大孔结构示意图以及基于PIMs的TFCM的横截面图(c)[47]
Fig.3 Chemical structure of SPEEK and schematic diagram of ion transport in SPEEK membranes in alkaline media (a)[56]; Schematic diagrams of an aqueous AZIFB and a hierarchical porous ceramic composite membrane for inhibiting zinc dendrite formation (b)[43]; Schematic diagrams of interconnected sub-nanometre-sized cavities in microporous membranes, the macromolecular structures of microporous polymers and the cross-sectional image of a TFCM based on PIMs (c)[47]
| 聚合物/纳米颗粒 | 基底 | 制备方法 | 厚度 | 体系 | 电流密度/ (mA·cm-2) | 能量效率/% | 循环次数 | 文献 |
|---|---|---|---|---|---|---|---|---|
| PVDF/MOF(UiO-66) | 多孔Daramic膜 | 刮涂/原位生长 | 约1.5 μm | ZIFB | 80 | >81 | 50次 | [ |
| LDHs纳米片 | 多孔PES/SPEEK膜 | 原位垂直生长 | 2 μm | AZIFB | 260 | 80 | 800次 | [ |
| Nafion/LDHs | 多孔PES/SPEEK膜 | 喷涂 | 约15 μm | AZIFB | 200 | 82.36 | 400次 | [ |
| N-CNTs | 多孔PES/SPEEK膜 | 部分嵌入法 | 约2 μm | AZIFB | 200 | 80.38 | >100次 | [ |
| PA/ZSM-35 | 多孔PES/SPEEK膜 | 原位界面聚合 | 约3 μm | VFB | 180 | 约82 | 1000次 | [ |
| GO纳米片 | Nafion 212膜 | 旋涂 | 约100 nm | VFB | 80 | 约85 | >180次 | [ |
| Nafion/BNNSs | 多孔PES/SPEEK膜 | 喷涂 | 约3.5 μm | AZIFB | 200 | >80 | 约200次 | [ |
| PDDA/PSS | Nafion-NdZr复合膜 | 层层自组装 | — | VFB | 40 | 约76 | 200次 | [ |
| GO/Nafion | Nafion | 旋涂 | 442 nm | VFB | 80 | 约77 | >200次 | [ |
| GO | 多孔PES膜 | 旋涂 | 约4 nm | VFB | 40 | 约81 | 300次 | [ |
| PFSA-g-GO | 多孔PC膜 | LB法 | 亚-20 nm | VFB | 200 | 78 | 700次 | [ |
| 分子筛纳米片 | 多孔PES/SPEEK膜 | 表面偏析过程 | — | AZIFB | 80 | 约81.9 | >600次 | [ |
| ZSM-35 /Nafion | 多孔PES/SPEEK膜 | 喷涂 | 约8 μm | VFB | 200 | >81 | >100次 | [ |
表2 典型的FB用无机纳米颗粒基TFCM汇总
Table 2 Summary of several typical nanoparticle-based TFCMs in FBs
| 聚合物/纳米颗粒 | 基底 | 制备方法 | 厚度 | 体系 | 电流密度/ (mA·cm-2) | 能量效率/% | 循环次数 | 文献 |
|---|---|---|---|---|---|---|---|---|
| PVDF/MOF(UiO-66) | 多孔Daramic膜 | 刮涂/原位生长 | 约1.5 μm | ZIFB | 80 | >81 | 50次 | [ |
| LDHs纳米片 | 多孔PES/SPEEK膜 | 原位垂直生长 | 2 μm | AZIFB | 260 | 80 | 800次 | [ |
| Nafion/LDHs | 多孔PES/SPEEK膜 | 喷涂 | 约15 μm | AZIFB | 200 | 82.36 | 400次 | [ |
| N-CNTs | 多孔PES/SPEEK膜 | 部分嵌入法 | 约2 μm | AZIFB | 200 | 80.38 | >100次 | [ |
| PA/ZSM-35 | 多孔PES/SPEEK膜 | 原位界面聚合 | 约3 μm | VFB | 180 | 约82 | 1000次 | [ |
| GO纳米片 | Nafion 212膜 | 旋涂 | 约100 nm | VFB | 80 | 约85 | >180次 | [ |
| Nafion/BNNSs | 多孔PES/SPEEK膜 | 喷涂 | 约3.5 μm | AZIFB | 200 | >80 | 约200次 | [ |
| PDDA/PSS | Nafion-NdZr复合膜 | 层层自组装 | — | VFB | 40 | 约76 | 200次 | [ |
| GO/Nafion | Nafion | 旋涂 | 442 nm | VFB | 80 | 约77 | >200次 | [ |
| GO | 多孔PES膜 | 旋涂 | 约4 nm | VFB | 40 | 约81 | 300次 | [ |
| PFSA-g-GO | 多孔PC膜 | LB法 | 亚-20 nm | VFB | 200 | 78 | 700次 | [ |
| 分子筛纳米片 | 多孔PES/SPEEK膜 | 表面偏析过程 | — | AZIFB | 80 | 约81.9 | >600次 | [ |
| ZSM-35 /Nafion | 多孔PES/SPEEK膜 | 喷涂 | 约8 μm | VFB | 200 | >81 | >100次 | [ |
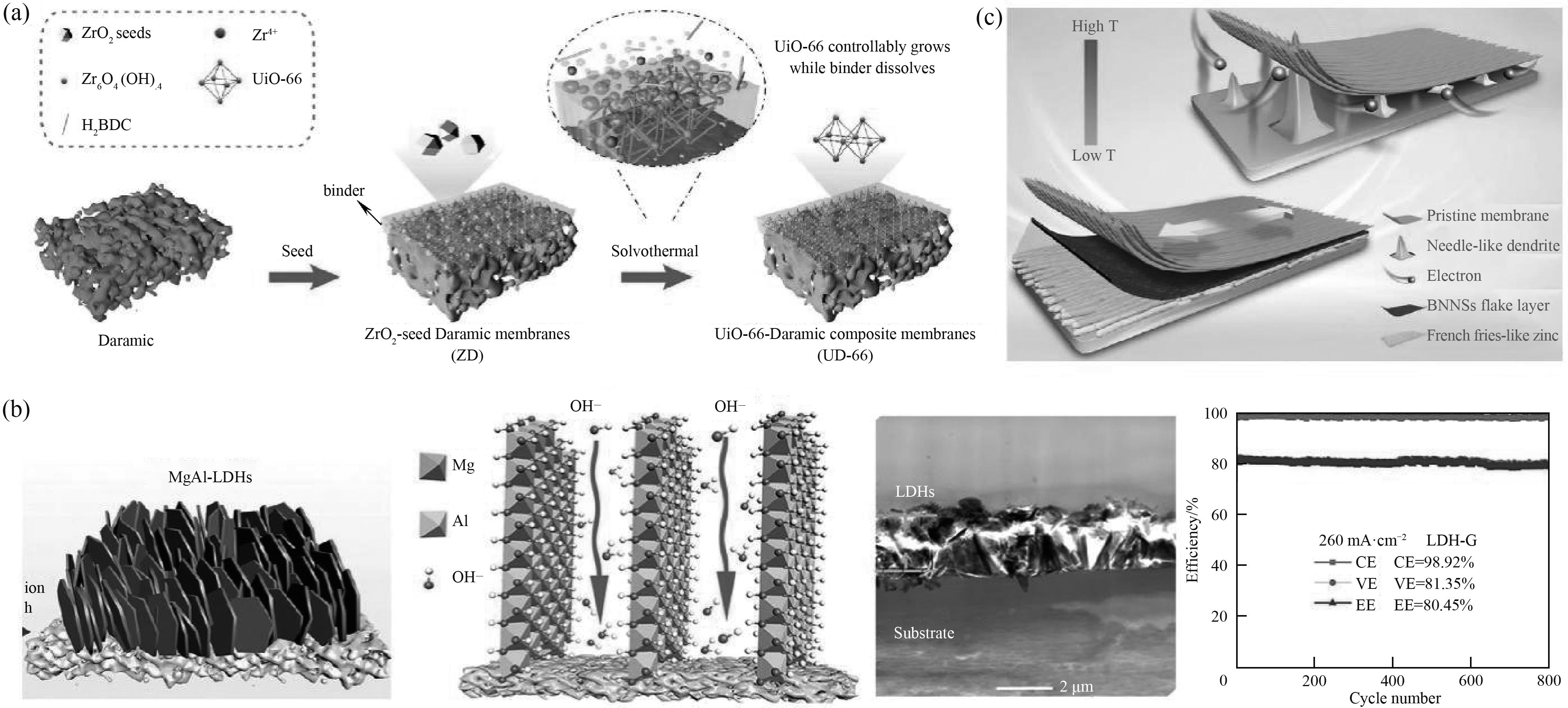
图4 黏结剂调控的限制二次生长法示意图(a)[57];LDH-G及OH-在LDH层间通道定向传输示意图、LDH-G的横截面形貌以及LDH-G组装的AZIFB在260 mA cm-2电流密度下的性能(b)[58];BNNSs纳米片层对锌沉积的协同效应(热导率和机械强度)示意图(c)[63]
Fig.4 Schematic illustration of the binder-controlled restrained second-growth method (a)[57]; Schematics diagrams of LDH-G, the directional hydroxide ions transport in the interlayer channels of LDHs, the cross-section morphology of LDH-G and the performance of LDH-G in an AZIFB at 260 mA cm-2 (b) [58]; Schematic diagram of the synergistic effect (thermal conductivity and mechanical strength) of BNNSs flake layer on zinc deposition (c)[63]
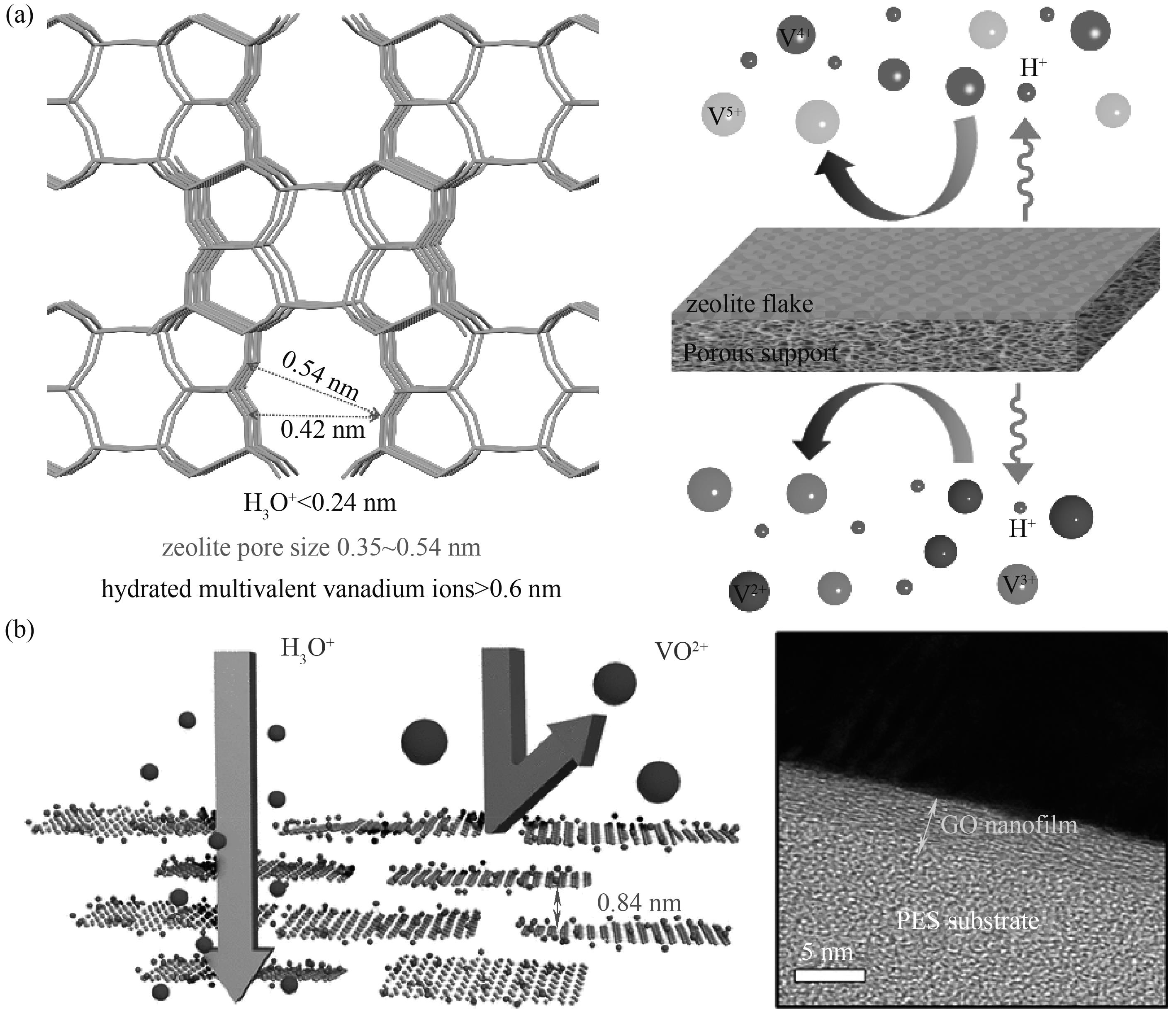
图5 VFB用由分子筛片层和多孔膜基底组成的复合膜的设计原理(a)[50];GO选择层示意图和GO/PES复合膜的横截面图(b)[66]
Fig.5 Design principle of a VFB with a porous membrane bearing a zeolite flake layer (a)[50]; Schematic diagram of the GO selective layer and the cross-section image of GO/PES membrane (b)[66]
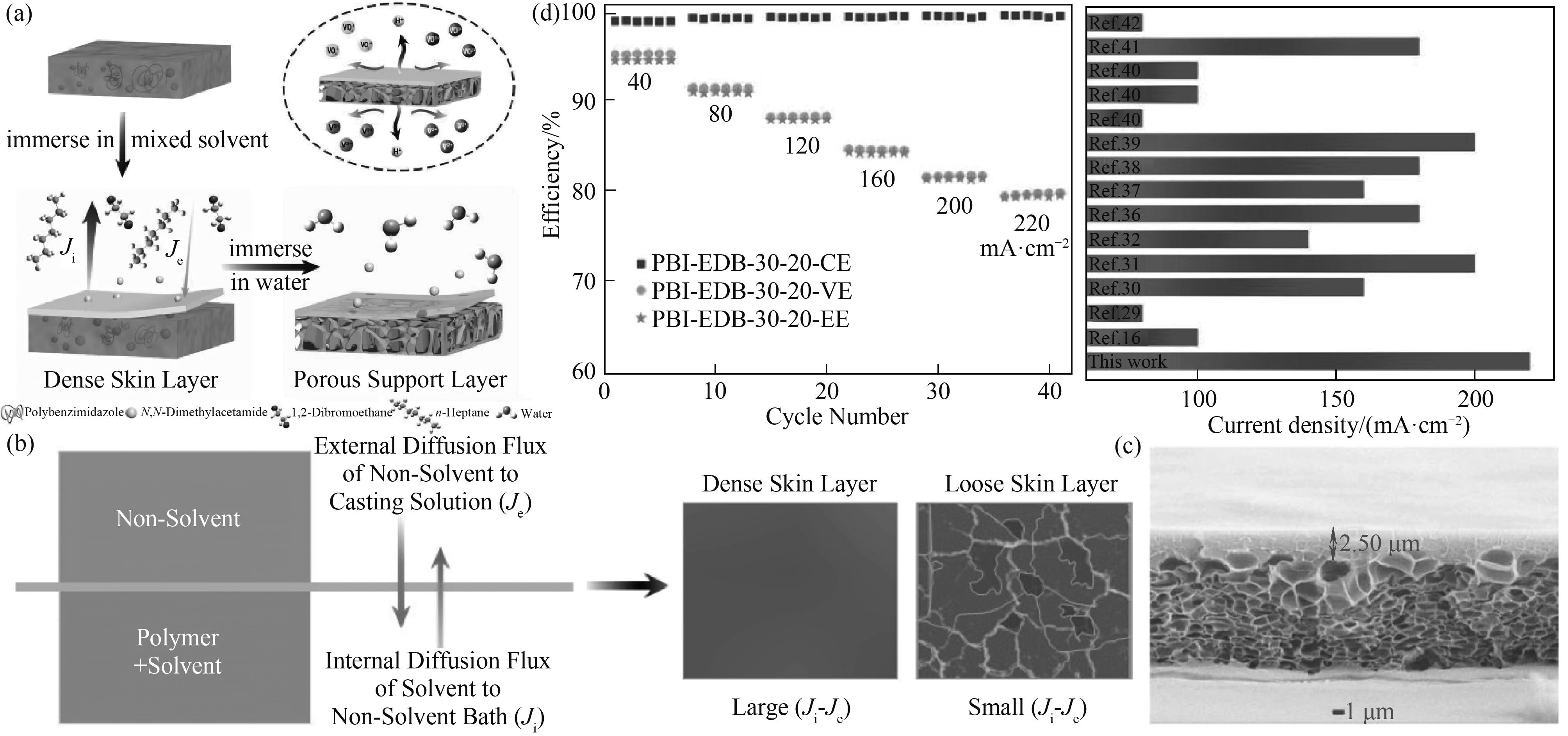
图6 两步NIPS法示意图(a);两步NIPS法的成膜机理(b);所制备膜的横截面形貌(c);所制备膜在不同电流密度下的VFB性能及其与文献报道值的比较(能量效率>80%)(d)[71]
Fig.6 Schematic diagram of the two-step NIPS method (a); The formation mechanism of membranes prepared by the two-step non-solvent induced phase separation method (b); The cross-section morphology of the as-prepared membrane (c); VFB performances of the as-prepared membrane at different current densities and the performance comparison with the reported papers (Energy efficiency >80%) (d)[71]
| 1 | Zhao Z M, Liu X H, Zhang M Q, et al. Development of flow battery technologies using the principles of sustainable chemistry[J]. Chemical Society Reviews, 2023, 52(17): 6031-6074. |
| 2 | Chen Z Q, Liu Y F, Yu W T, et al. Cost evaluation and sensitivity analysis of the alkaline zinc-iron flow battery system for large-scale energy storage applications[J]. Journal of Energy Storage, 2021, 44: 103327. |
| 3 | Michel Myures X, Suresh S, Arthanareeswaran G. Construction of thermal, chemical and mechanically stable ion exchange membranes with improved ion selectivity for vanadium redox flow batteries applications[J]. Journal of Power Sources, 2024, 591: 233818. |
| 4 | Liu J, Xiao J, Yang J H, et al. The TWh challenge: next generation batteries for energy storage and electric vehicles[J]. Next Energy, 2023, 1(1): 100015. |
| 5 | Xie C X, Wang C, Xu Y, et al. Reversible multielectron transfer I-/IO3 - cathode enabled by a hetero-halogen electrolyte for high-energy-density aqueous batteries[J]. Nature Energy, 2024, 9: 714-724. |
| 6 | Zhang C K, Yuan Z Z, Li X F. Designing better flow batteries: an overview on Fifty Years’ research[J]. ACS Energy Letters, 2024, 9: 3456-3473. |
| 7 | Wu J, Wang Y X, Wu Y L, et al. Freestanding covalent organic framework membranes with enhanced proton perm-selectivity for flow batteries[J]. Journal of Membrane Science, 2023, 687: 122091. |
| 8 | Tang L Y, Li T Y, Lu W J, et al. Reversible solid bromine complexation into Ti3C2T x MXene carriers: a highly active electrode for bromine-based flow batteries with ultralow self-discharge[J]. Energy & Environmental Science, 2024, 17(9): 3136-3145. |
| 9 | Zhi L P, Liao C Y, Xu P C, et al. An artificial bridge between the anode and the anolyte enabled by an organic ligand for sustainable zinc-based flow batteries[J]. Energy & Environmental Science, 2024, 17(2): 717-726. |
| 10 | Yu D L, Zhi L P, Zhang F F, et al. Scalable alkaline zinc-iron/nickel hybrid flow battery with energy density up to 200 W·h·L-1 [J]. Advanced Materials, 2023, 35(7): 2209390. |
| 11 | Chen Z, Li T Y, Xie C X, et al. A Neutral zinc-iron flowbattery with long lifespan and high power density[J]. ACS Energy Letters, 2024, 9(7): 3426-3432. |
| 12 | Tang L Y, Lu W J, Li X F. Electrolytes for bromine-based flow batteries: challenges, strategies, and prospects[J]. Energy Storage Materials, 2024, 70: 103532. |
| 13 | Kellamis C J, Wainright J S. A zinc–iodine hybrid flow battery with enhanced energy storage capacity[J]. Journal of Power Sources, 2024, 589: 233753. |
| 14 | Khor A, Leung P, Mohamed M R, et al. Review of zinc-based hybrid flow batteries: from fundamentals to applications[J]. Materials Today Energy, 2018, 8: 80-108. |
| 15 | Yuan Z Z, Yin Y B, Xie C X, et al. Advanced materials for zinc-based flow battery: development and challenge[J]. Advanced Materials, 2019, 31: 1902025. |
| 16 | Lu W J, Li X F. Advanced membranes boost the industrialization of flow battery[J]. Accounts of Materials Research, 2023, 4(8): 681-692. |
| 17 | Zhi L P, Yuan Z Z, Li X F. Recent development and prospect of membranes for alkaline zinc-iron flow battery[J]. Advanced Membranes, 2022, 2: 100029. |
| 18 | Lu W J, Yuan Z Z, Zhao Y Y, et al. Porous membranes in secondary battery technologies[J]. Chemical Society Reviews, 2017, 46(8): 2199-2236. |
| 19 | Xu W Y, Xu J P, Yi Z Y, et al. Zwitterionic channels within covalent organic frameworks facilitate proton-selective transport for flow battery membrane[J]. Chemical Engineering Science, 2024, 299: 120468. |
| 20 | Ban T, Wang Z H, Wang Y F, et al. Phosphoric acid pre-swelling strategy constructing acid-doped fluoropoly(aryl pyridinium) membranes to enable high-performance vanadium flow batteries[J]. Chemical Engineering Journal, 2024, 494: 153231. |
| 21 | Peng K, Tang G G, Zhang C, et al. Progress and prospects of pH-neutral aqueous organic redox flow batteries: electrolytes and membranes[J]. Journal of Energy Chemistry, 2024, 96: 89-109. |
| 22 | Wang F R, Ai F, Lu Y C. Ion selective membrane for redox flow battery, what’s next?[J]. Next Energy, 2023, 1(3): 100053. |
| 23 | Yang R, Zhang S Y, Zhu Y. A high performance, stable anion exchange membrane for alkaline redox flow batteries[J]. Journal of Power Sources, 2024, 594: 233974. |
| 24 | Meng X Y, Peng Q W, Peng L M, et al. In situ growth of covalent organic framework on graphene oxide nanosheet enable proton-selective transport in flow battery membrane[J]. Journal of Power Sources, 2024, 609: 234690. |
| 25 | Zhang H Z, Zhang H M, Li X F, et al. Nanofiltration (NF) membranes: the next generation separators for all vanadium redox flow batteries (VRBs)?[J]. Energy & Environmental Science, 2011, 4(5): 1676-1679. |
| 26 | Dai Q, Zhao Z M, Shi M Q, et al. Ion conductive membranes for flow batteries: design and ions transport mechanism[J]. Journal of Membrane Science, 2021, 632: 119355. |
| 27 | Wu J E, Dai Q, Zhang H M, et al. Recent development in composite membranes for flow batteries[J]. ChemSusChem, 2020, 13(15): 3805-3819. |
| 28 | Teng X G, Guo Y Y, Liu D L, et al. A polydopamine-coated polyamide thin film composite membrane with enhanced selectivity and stability for vanadium redox flow battery[J]. Journal of Membrane Science, 2020, 601: 117906. |
| 29 | Dai Q, Liu Z Q, Huang L, et al. Thin-film composite membrane breaking the trade-off between conductivity and selectivity for a flow battery[J]. Nature Communications, 2020, 11: 13. |
| 30 | Geng K, Tang H Y, Li Y, et al. A facile strategy for disentangling the conductivity and selectivity dilemma enables advanced composite membrane for vanadium flow batteries[J]. Journal of Membrane Science, 2020, 607: 118177. |
| 31 | Wan Y H, Sun J, Jiang H R, et al. A highly-efficient composite polybenzimidazole membrane for vanadium redox flow battery[J]. Journal of Power Sources, 2021, 489: 229502. |
| 32 | Gubler L, Vonlanthen D, Schneider A, et al. Composite membranes containing a porous separator and a polybenzimidazole thin film for vanadium redox flow batteries[J]. Journal of the Electrochemical Society, 2020, 167(10): 100502. |
| 33 | Lee W, Jung M, Serhiichuk D, et al. Layered composite membranes based on porous PVDF coated with a thin, dense PBI layer for vanadium redox flow batteries[J]. Journal of Membrane Science, 2019, 591: 117333. |
| 34 | Jung M, Lee W, Nambi Krishnan N, et al. Porous-Nafion/PBI composite membranes and Nafion/PBI blend membranes for vanadium redox flow batteries[J]. Applied Surface Science, 2018, 450: 301-311. |
| 35 | Zhang D H, Zhang X H, Luan C, et al. Zwitterionic interface engineering enables ultrathin composite membrane for high-rate vanadium flow battery[J]. Energy Storage Materials, 2022, 49: 471-480. |
| 36 | Hua L, Lu W J, Li T Y, et al. A highly selective porous composite membrane with bromine capturing ability for a bromine-based flow battery[J]. Materials Today Energy, 2021, 21: 100763. |
| 37 | Thong P T, Ajeya K V, Dhanabalan K, et al. A coupled-layer ion-conducting membrane using composite ionomer and porous substrate for application to vanadium redox flow battery[J]. Journal of Power Sources, 2022, 521: 230912. |
| 38 | Li Y, Li X F, Cao J Y, et al. Composite porous membranes with an ultrathin selective layer for vanadium flow batteries[J]. Chemical Communications, 2014, 50(35): 4596-4599. |
| 39 | Kim J Q, So S, Kim H T, et al. Highly ordered ultrathin perfluorinated sulfonic acid ionomer membranes for vanadium redox flow battery[J]. ACS Energy Letters, 2021, 6(1): 184-192. |
| 40 | Lu W J, Li T Y, Yuan C G, et al. Advanced porous composite membrane with ability to regulate zinc deposition enables dendrite-free and high-areal capacity zinc-based flow battery[J]. Energy Storage Materials, 2022, 47: 415-423. |
| 41 | Mehboob S, Lee J Y, Ahn J H, et al. Perfect capacity retention of all-vanadium redox flow battery using Nafion/polyaniline composite membranes[J]. Journal of Industrial and Engineering Chemistry, 2023, 121: 348-357. |
| 42 | Teng X G, Wang M R, Li G W, et al. Polypyrrole thin film composite membrane prepared via interfacial polymerization with high selectivity for vanadium redox flow battery[J]. Reactive and Functional Polymers, 2020, 157: 104777. |
| 43 | Huang K, Mu F Y, Hou X X, et al. Porous ceramic metal-based flow battery composite membrane[J]. Angewandte Chemie International Edition, 2024, 63(19): e202401558. |
| 44 | Jung J, Won J, Hwang S S. Highly selective composite membranes using ladder-like structured polysilsesquioxane for a non-aqueous redox flow battery[J]. Journal of Membrane Science, 2020, 595: 117520. |
| 45 | Cho E, Won J. Novel composite membrane coated with a poly(diallyldimethylammonium chloride)/urushi semi-interpenetrating polymer network for non-aqueous redox flow battery application[J]. Journal of Power Sources, 2016, 335: 12-19. |
| 46 | Teng X G, Yu C, Wu X F, et al. PTFE/SPEEK/PDDA/PSS composite membrane for vanadium redox flow battery application[J]. Journal of Materials Science, 2018, 53(7): 5204-5215. |
| 47 | Tan R, Wang A Q, Malpass-Evans R, et al. Hydrophilic microporous membranes for selective ion separation and flow-battery energy storage[J]. Nature Materials, 2020, 19(2): 195-202. |
| 48 | Tan R, Wang A Q, Ye C C, et al. Thin film composite membranes with regulated crossover and water migration for long-life aqueous redox flow batteries[J]. Advanced Science, 2023, 10(20): 2206888. |
| 49 | Xia Y S, Cao H Y, Xu F, et al. Polymeric membranes with aligned zeolite nanosheets for sustainable energy storage[J]. Nature Sustainability, 2022, 5: 1080-1091. |
| 50 | Yuan Z Z, Zhu X X, Li M R, et al. A Highly ion-selective zeolite flake layer on porous membranes for flow battery applications[J]. Angewandte Chemie International Edition, 2016, 55(9): 3058-3062. |
| 51 | Yuan Z Z, Duan Y Q, Zhang H Z, et al. Advanced porous membranes with ultra-high selectivity and stability for vanadium flow batteries[J]. Energy & Environmental Science, 2016, 9(2): 441-447. |
| 52 | Hampson E, Duburg J C, Casella J, et al. A simple approach to balancing conductivity and capacity fade in vanadium redox flow batteries by the tunable pretreatment of polybenzimidazole membranes[J]. Chemical Engineering Journal, 2024, 485: 149930. |
| 53 | Wang J Q, Xu W Y, Xu F, et al. A polybenzimidazole-covalent organic framework hybrid membrane with highly efficient proton-selective transport channels for vanadium redox flow battery[J]. Journal of Membrane Science, 2024, 695: 122470. |
| 54 | Gao S J, Wang D, Fang W X, et al. Ultrathin membranes: a new opportunity for ultrafast and efficient separation[J]. Advanced Materials Technologies, 2020, 5(4): 1901069. |
| 55 | Yuan Z Z, Li X F, Hu J B, et al. Degradation mechanism of sulfonated poly(ether ether ketone) (SPEEK) ion exchange membranes under vanadium flow battery medium[J]. Physical Chemistry Chemical Physics, 2014, 16(37): 19841-19847. |
| 56 | Yuan Z Z, Liang L X, Dai Q, et al. Low-cost hydrocarbon membrane enables commercial-scale flow batteries for long-duration energy storage[J]. Joule, 2022, 6(4): 884-905. |
| 57 | Wu J E, Dai Q, Zhang H M, et al. A defect-free MOF composite membrane prepared via in situ binder-controlled restrained second-growth method for energy storage device[J]. Energy Storage Materials, 2021, 35: 687-694. |
| 58 | Hu J, Yuan C G, Zhi L P, et al. In situ defect-free vertically aligned layered double hydroxide composite membrane for high areal capacity and long-cycle zinc-based flow battery[J]. Advanced Functional Materials, 2021, 31(31): 2102167. |
| 59 | Hu J, Tang X M, Dai Q, et al. Layered double hydroxide membrane with high hydroxide conductivity and ion selectivity for energy storage device[J]. Nature Communications, 2021, 12(1): 3409. |
| 60 | Kong D X, Yuan C G, Zhi L P, et al. N-CNTs-based composite membrane engineered by a partially embedded strategy: a facile route to high-performing zinc-based flow batteries[J]. Advanced Functional Materials, 2023, 33(34): 2301448. |
| 61 | Dai Q, Lu W J, Zhao Y Y, et al. Advanced scalable zeolite “ions-sieving” composite membranes with high selectivity[J]. Journal of Membrane Science, 2020, 595: 117569. |
| 62 | Zhang D S, Wang Q, Peng S S, et al. An interface-strengthened cross-linked graphene oxide/Nafion212 composite membrane for vanadium flow batteries[J]. Journal of Membrane Science, 2019, 587: 117189. |
| 63 | Hu J, Yue M, Zhang H M, et al. A boron nitride nanosheets composite membrane for a long-life zinc-based flow battery[J]. Angewandte Chemie International Edition, 2020, 59(17): 6715-6719. |
| 64 | Hossain S I, Aziz M A, Shanmugam S. Ultrahigh ion-selective and durable nafion-ndzr composite layer membranes for all-vanadium redox flow batteries[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(4): 1998-2007. |
| 65 | Su L, Zhang D S, Peng S S, et al. Orientated graphene oxide/Nafion ultra-thin layer coated composite membranes for vanadium redox flow battery[J]. International Journal of Hydrogen Energy, 2017, 42(34): 21806-21816. |
| 66 | Park S C, Lee T H, Moon G H, et al. Sub-5 nm graphene oxide nanofilm with exceptionally high H+/V selectivity for vanadium redox flow battery[J]. ACS Applied Energy Materials, 2019, 2(7): 4590-4596. |
| 67 | Lee J, Kim J Q, Ko H, et al. Sub-20 nm ultrathin perfluorosulfonic acid-grafted graphene oxide composite membranes for vanadium redox flow batteries[J]. Journal of Membrane Science, 2023, 688: 122150. |
| 68 | Hou X X, Huang K, Xia Y S, et al. Fish-scale-like nano-porous membrane based on zeolite nanosheets for long stable zinc-based flow battery[J]. AIChE Journal, 2022, 68(9): e17738. |
| 69 | Zhou H, Su Y, Chen X R, et al. Plasma modification of substrate with poly(methylhydrosiloxane) for enhancing the interfacial stability of PDMS/PAN composite membrane[J]. Journal of Membrane Science, 2016, 520: 779-789. |
| 70 | Sang C, Zhang S Y, Si Z H, et al. Design of PDMS/PAN composite membranes with ultra-interfacial stability via layer integration[J]. Materials Horizons, 2024, doi: 10.1039/D4MH00483C . |
| 71 | Shi M Q, Dai Q, Li F, et al. Membranes with well-defined selective layer regulated by controlled solvent diffusion for high power density flow battery[J]. Advanced Energy Materials, 2020, 10(34): 2001382. |
| [1] | 李匡奚, 于佩潜, 王江云, 魏浩然, 郑志刚, 冯留海. 微气泡旋流气浮装置内流动分析与结构优化[J]. 化工学报, 2024, 75(S1): 223-234. |
| [2] | 谢慧慧, 姜佳鑫, 王鑫, 李正, 郭鑫, 吕欣然, 王凌云, 刘杨. 深共晶溶剂聚合物包覆膜传输分离铂、钯的研究[J]. 化工学报, 2024, 75(S1): 235-243. |
| [3] | 邱知, 谭明. 聚离子液体膜的制备及其在低钠高钾健康酱油中的应用[J]. 化工学报, 2024, 75(S1): 244-250. |
| [4] | 刘律, 刘洁茹, 范亮亮, 赵亮. 基于层流效应的被动式颗粒分离微流控方法研究[J]. 化工学报, 2024, 75(S1): 67-75. |
| [5] | 胡俭, 姜静华, 范生军, 刘建浩, 邹海江, 蔡皖龙, 王沣浩. 中深层U型地埋管换热器取热特性研究[J]. 化工学报, 2024, 75(S1): 76-84. |
| [6] | 唐昊, 胡定华, 李强, 张轩畅, 韩俊杰. 抗加速度双切线弧流道内气泡动力学行为数值与可视化研究[J]. 化工学报, 2024, 75(9): 3074-3082. |
| [7] | 李彦熹, 王晔春, 谢向东, 王进芝, 王江, 周煜, 潘盈秀, 丁文涛, 郭烈锦. 蜗壳式多通道气液旋流分离器结构优化及分离特性研究[J]. 化工学报, 2024, 75(8): 2875-2885. |
| [8] | 罗小平, 侯云天, 范一杰. 逆流相分离结构微细通道流动沸腾传热与均温性[J]. 化工学报, 2024, 75(7): 2474-2485. |
| [9] | 张颂红, 赵欣怡, 楼小玲, 沈绍传, 贠军贤. 阳离子交换纳晶胶分离乳过氧化物酶的研究[J]. 化工学报, 2024, 75(7): 2574-2582. |
| [10] | 秦晓巧, 谭宏博, 温娜. 储能式低温空分系统热力学与经济性分析[J]. 化工学报, 2024, 75(7): 2409-2421. |
| [11] | 周文轩, 刘珍, 张福建, 张忠强. 高通量-高截留率时间维度膜法水处理机理研究[J]. 化工学报, 2024, 75(7): 2583-2593. |
| [12] | 张香港, 常玉龙, 汪华林, 江霞. 废弃秸秆等生物质低能耗非相变秒级干燥[J]. 化工学报, 2024, 75(7): 2433-2445. |
| [13] | 霍宗伟, 牛亚宾, 潘艳秋. 油水膜分离中高黏度油滴行为研究和影响因素分析[J]. 化工学报, 2024, 75(6): 2262-2273. |
| [14] | 张祎琪, 谭雪松, 李吾环, 张权, 苗长林, 庄新姝. 温和条件下乙二醇苯醚高效分离回收甘蔗渣组分[J]. 化工学报, 2024, 75(6): 2274-2282. |
| [15] | 许茹枫, 陈煜成, 高丹, 焦静雨, 高栋, 王海彬, 姚善泾, 林东强. 离子交换层析分离单抗电荷异质体的模型辅助过程优化[J]. 化工学报, 2024, 75(5): 1903-1911. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号
