• •
杨远平1( ), 马佳琪1, 司桐2, 王翔3, 李水清2(
), 马佳琪1, 司桐2, 王翔3, 李水清2( )
)
收稿日期:2025-09-30
修回日期:2025-11-13
出版日期:2025-12-05
通讯作者:
李水清
作者简介:杨远平(1990—),男,博士,讲师,yangyuanping1@163.com
基金资助:
Yuanping YANG1( ), Jiaqi MA1, Tong SI2, Xiang WANG3, Shuiqing LI2(
), Jiaqi MA1, Tong SI2, Xiang WANG3, Shuiqing LI2( )
)
Received:2025-09-30
Revised:2025-11-13
Online:2025-12-05
Contact:
Shuiqing LI
摘要:
利用切向旋流管状燃烧机理实验台,结合光学诊断与反应动力学分析,系统研究了CH4、CO和H2三种典型燃料掺氨富燃条件下NO x 排放特性及生成机理。实验结果表明,相比于掺混甲烷,CO的引入能够更好地降低吹熄极限和托举临界值,改善火焰稳定性。然而,活性燃料的掺混燃烧导致NO x 排放呈数量级增加,尤其在CO/NH3混燃体系,掺氨比0.6时NO x 排放高于4500 (体积分数×10-6,@3.5%O2),远超CH4和H2混燃体系。OH*和NH2*化学自发光以及N主要转化路径的动力学分析表明,富燃条件下,NO x 生成主要来源于NH i 路径,OH和NH i 自由基在NO生成与消耗中起枢纽作用,CO掺氨燃烧体系中,高OH浓度增强了NH i 自由基的氧化,进而导致NO x 排放高;同时不同燃料掺混条件下氨脱氢反应及NH i 还原反应的差异同样影响氮的转化路径。
中图分类号:
杨远平, 马佳琪, 司桐, 王翔, 李水清. 不同特征燃料宽范围掺氨富燃污染物排放特性及动力学分析[J]. 化工学报, DOI: 10.11949/0438-1157.20251092.
Yuanping YANG, Jiaqi MA, Tong SI, Xiang WANG, Shuiqing LI. Impact of fuel properties on pollutant emissions and reaction Kinetics in ammonia-blended fuel-rich combustion[J]. CIESC Journal, DOI: 10.11949/0438-1157.20251092.
| ENH3 | VNH3 (L/min) | VCH4 (L/min) | Vair (L/min) |
|---|---|---|---|
| 0.0 | 0.00 | 1.68 | 14.55 |
| 0.2 | 0.85 | 1.34 | 14.36 |
| 0.4 | 1.70 | 1.01 | 14.26 |
| 0.6 | 2.55 | 0.67 | 14.08 |
| 0.8 | 3.39 | 0.34 | 13.95 |
| 1.0 | 4.24 | 0.00 | 13.77 |
表1 CH4/NH3/Air燃烧体系流量参数设置
Table 1 Flow parameters of CH4/NH3/Air flame with varied ENH3
| ENH3 | VNH3 (L/min) | VCH4 (L/min) | Vair (L/min) |
|---|---|---|---|
| 0.0 | 0.00 | 1.68 | 14.55 |
| 0.2 | 0.85 | 1.34 | 14.36 |
| 0.4 | 1.70 | 1.01 | 14.26 |
| 0.6 | 2.55 | 0.67 | 14.08 |
| 0.8 | 3.39 | 0.34 | 13.95 |
| 1.0 | 4.24 | 0.00 | 13.77 |
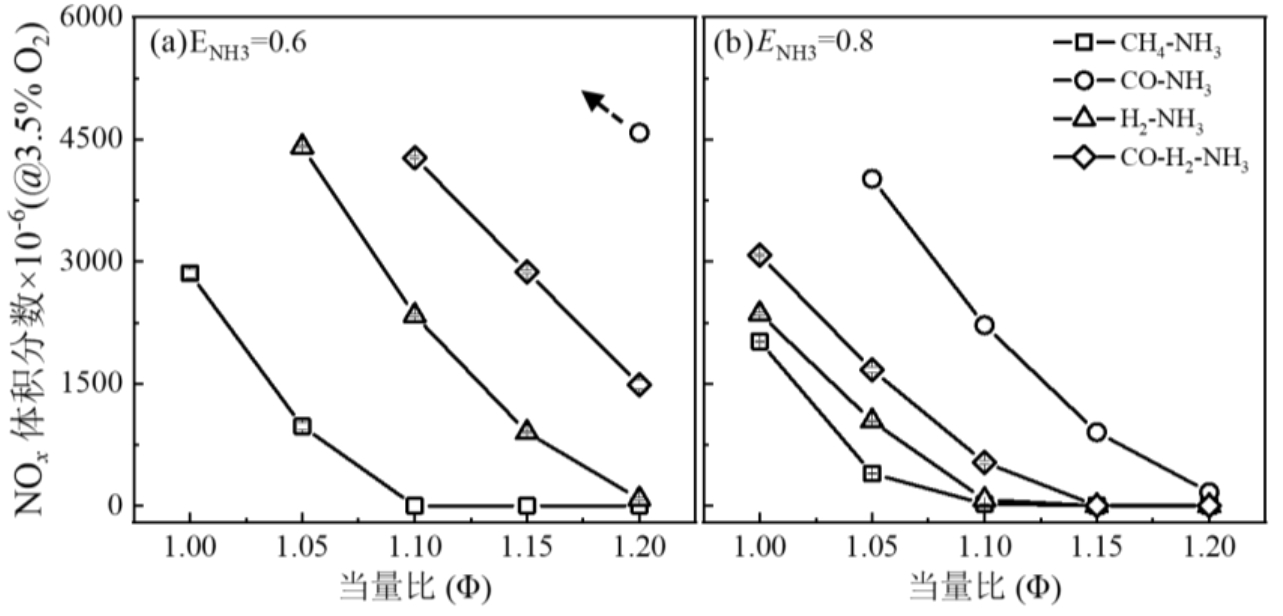
图7 富燃条件下不同特征燃料掺氨燃烧NO x 排放特性,(a) ENH3=0.6, (b) ENH3=0.8注:ENH3=0.6(a) and 0.8(b)
Fig.7 NO x emission characteristics of ammonia co-combustion with different representative fuels under fuel-rich conditions,
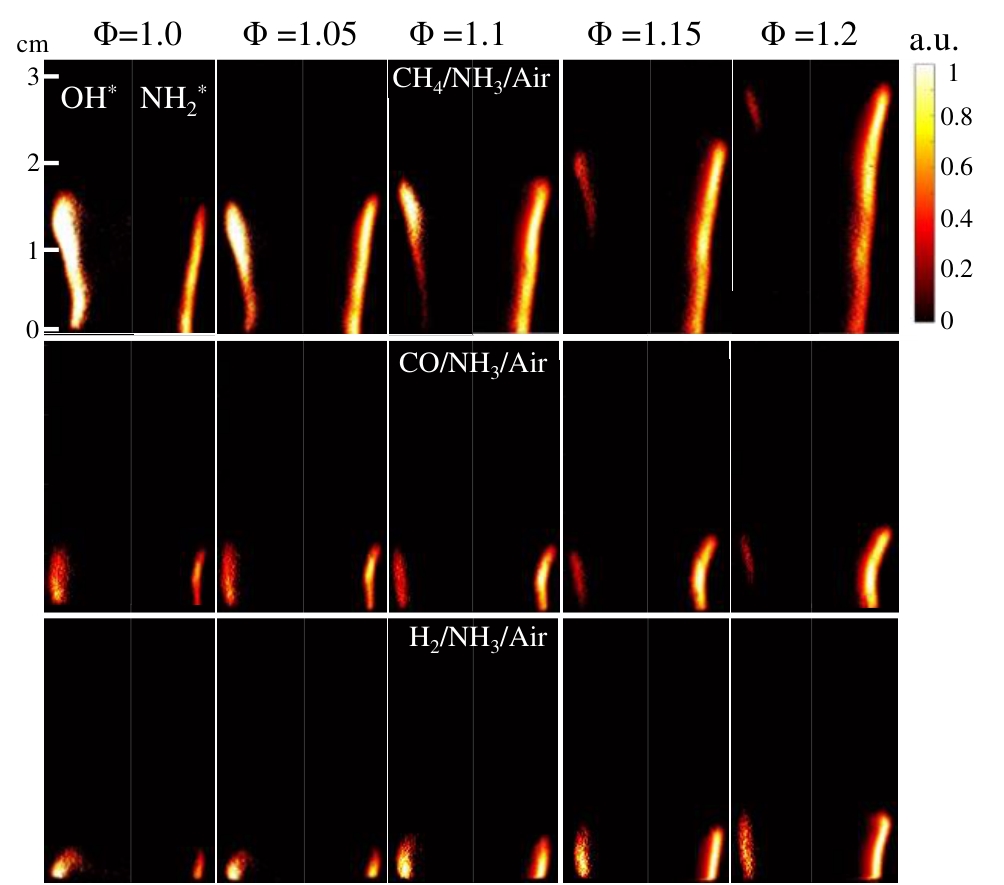
图8 不同掺混燃料燃烧过程OH*(左)和NH2*(右)化学自发光信号的Abel变换与时均图像,ENH3=0.8注:blending combustion under typical operating conditions. ENH3=0.8
Fig.8 Abel-transformed and time-averaged images of OH* (left) and NH2* (right) chemiluminescence signals in various fuel
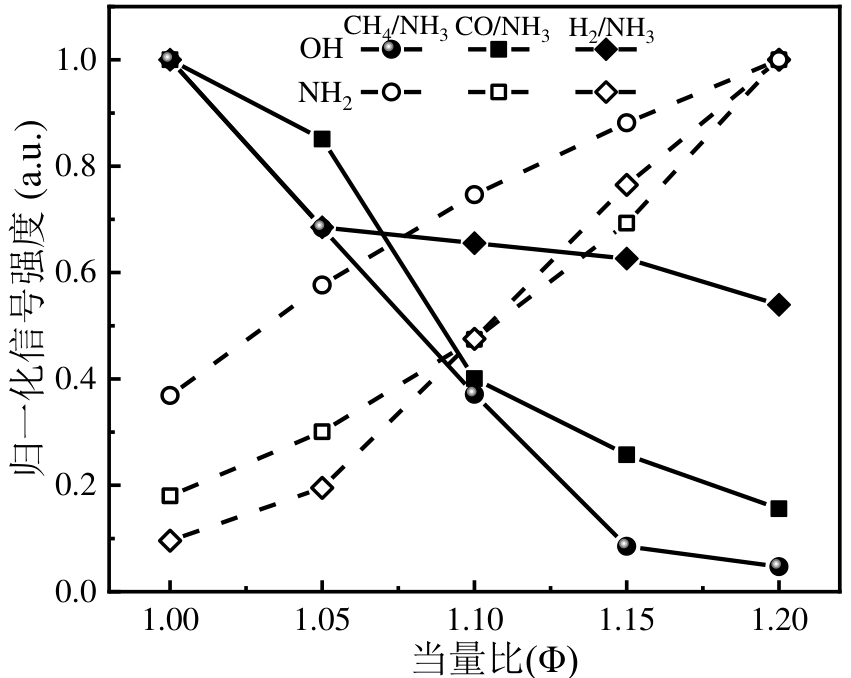
图9 不同掺混燃料OH*和NH2*归一化信号强度随当量比变化特性注:different fuel blended
Fig.9 Variations of the normalized plane-integrated time-averaged OH*and NH2* chemiluminescence signals intensities with
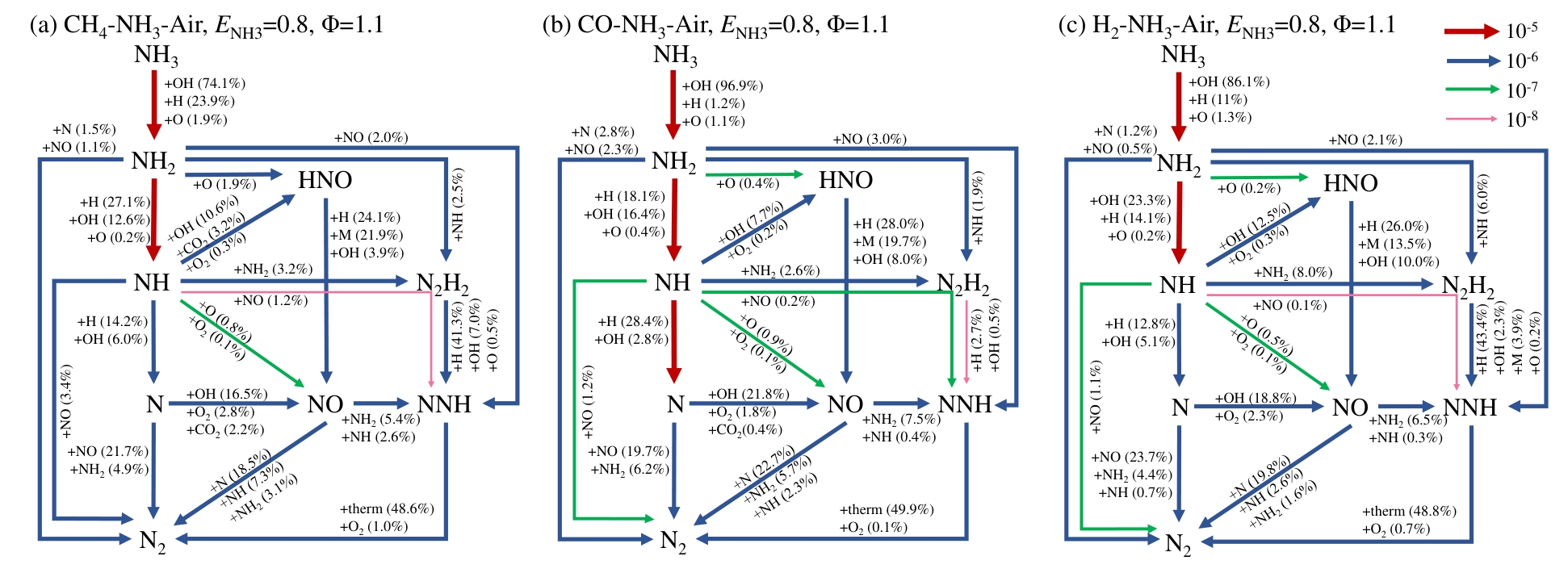
图11 CH4/CO/H2掺氨燃料燃烧主火焰区域NO反应路径(箭头宽度反映特定反应路径的相对重要性)注:arrows reflects the relative importance of a specific reaction pathway)
Fig.11 Reaction pathways of NO in the main flame region during combustion of CH4/CO/H2–NH3 blended fuels (the width of
| [1] | 黄震, 谢晓敏. 碳中和愿景下的能源变革[J]. 中国科学院院刊, 2021, 36(9): 1010-1018. |
| Huang Z, XIE X M. Energy Revolution under Vision of Carbon Neutrality[J]. Bulletin of Chinese Academy of Sciences, 2021, 36(9): 1010-1018. | |
| [2] | Abdalla A M, Hossain S, Nisfindy O B, et al. Hydrogen production, storage, transportation and key challenges with applications: a review[J]. Energy Conversion and Management, 2018, 165: 602-627. |
| [3] | Giddey S, Badwal S P S, Munnings C, et al. Ammonia as a renewable energy transportation media[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(11): 10231-10239. |
| [4] | Valera-Medina A, Xiao H, Owen-Jones M, et al. Ammonia for power[J]. Progress in Energy and Combustion Science, 2018, 69: 63-102. |
| [5] | Kobayashi H, Hayakawa A, Somarathne K D K A, et al. Science and technology of ammonia combustion[J]. Proceedings of the Combustion Institute, 2019, 37(1): 109-133. |
| [6] | Han X L, Wang Z H, Costa M, et al. Experimental and kinetic modeling study of laminar burning velocities of NH3/air, NH3/H2/air, NH3/CO/air and NH3/CH4/air premixed flames[J]. Combustion and Flame, 2019, 206: 214-226. |
| [7] | Ji L J, Wang J H, Hu G Y, et al. Experimental study on structure and blow-off characteristics of NH3/CH4 co-firing flames in a swirl combustor[J]. Fuel, 2022, 314: 123027. |
| [8] | Khateeb A A, Guiberti T F, Zhu X R, et al. Stability limits and exhaust NO performances of ammonia-methane-air swirl flames[J]. Experimental Thermal and Fluid Science, 2020, 114: 110058. |
| [9] | Okafor E C, K D A Somarathne, Ratthanan R, et al. Control of NO x and other emissions in micro gas turbine combustors fuelled with mixtures of methane and ammonia[J]. Combustion and Flame, 2020, 211: 406-416. |
| [10] | 杨浩杰, 刘春雨, 李雪娇, 等. 低旋流配置下氨-甲烷-空气预混旋流火焰稳定性和排放特性[J]. 化工学报, 2025, 76(6): 3029-3040. |
| Yang H J, Liu C Y, Li X J, et al. Study of stability limits and emission characteristics in premixed ammonia-methane-air swirling flames in low swirl configurations[J]. CIESC Journal, 2025, 76(6): 3029-3040. | |
| [11] | 彭维康, 赵晓尧, 李子妍, 等. 管状火焰燃烧器中高氧气浓度丙烷燃烧特性[J]. 工程热物理学报, 2018, 39(1): 218-223. |
| Peng W K, Zhao X Y, Li Z Y, et al. Characteristics of Propane Combustion in a Tubular Flame Burner Under High Oxygen Mole Fraction[J]. Journal of Engineering Thermophsics, 2018, 39(1): 218-223. | |
| [12] | 朱志强, 初庆钊, 石保禄, 等. 基于急速混合管状火焰的甲烷富氧燃烧特性[J]. 燃烧科学与技术, 2017, 23(3): 835-845. |
| Zhu Z Q, Chu Q Z, Shi B L, et al. Oxy-Fuel Combustion of Methane in a Rapidly Mixed Tubular Flame Burner[J]. Journal of Combustion Science and Technology, 2017, 23(3): 242-248. | |
| [13] | 张泽雨, 王平, 戴凯论, 等. 轴向双级氨/甲烷湍流预混火焰燃烧特性及NO生成[J]. 化工学报, 2025, 76(2): 1085-1092. |
| Zhang Z Y, Wang P, Dai K L, et al. Combustion characteristics and NO production of axially staged premixed NH3/CH4 turbulent swirling flames[J]. CIESC Journal, 2025, 76(2): 835-845. | |
| [14] | Okafor E C, Naito Y, Colson S, et al. Measurement and modelling of the laminar burning velocity of methane-ammonia-air flames at high pressures using a reduced reaction mechanism[J]. Combustion and Flame, 2019, 204: 162-175. |
| [15] | 毛晨林, 王平, Shrotriya P, 等. 含氨燃料预混火焰的层流火焰速度及NO排放特性[J]. 化工学报, 2021, 72(10): 5330-5343. |
| Mao C L, Wang P, Shrotriya P, et al. Laminar flame speed and NO emission characteristics of premixed flames with different ammonia-containing fuels[J]. CIESC Journal, 2021, 72(10): 5330-5343. | |
| [16] | Mashruk S, Xiao H, Valera-Medina A. Rich-Quench-Lean model comparison for the clean use of humidified ammonia/hydrogen combustion systems[J]. International Journal of Hydrogen Energy, 2021, 46(5): 4472-4484. |
| [17] | Okafor E C, Somarathne K D K A, Hayakawa A, et al. Towards the development of an efficient low-NO x ammonia combustor for a micro gas turbine[J]. Proceedings of the Combustion Institute, 2019, 37(4): 4597-4606. |
| [18] | Yang Y P, Huang Q, Si T, et al. Expanding low NO emission range of NH3/CH4 flames via fuel nitrogen/hydrocarbon separation in two-stage tangential swirling burner[J]. Fuel, 2025, 385: 134029. |
| [19] | Zhu X R, Du J G, Yu Z, et al. NO x emission and control in ammonia combustion: state-of-the-art review and future perspectives[J]. Energy & Fuels, 2024, 38(1): 43-60. |
| [20] | Yang Y P, Huang Q, Sun J G, et al. Reducing NO x emission of swirl-stabilized ammonia/methane tubular flames through a fuel-oxidizer mixing strategy[J]. Energy & Fuels, 2022, 36(4): 2277-2287. |
| [21] | Li B, Shi B L, Zhao X Y, et al. Oxy-fuel combustion of methane in a swirl tubular flame burner under various oxygen contents: Operation limits and combustion instability[J]. Experimental Thermal and Fluid Science, 2018, 90: 115-124. |
| [22] | Zhang Y Y, Shimokuri D, Mukae Y, et al. Flow field in swirl-type tubular flame burner[J]. JSME International Journal Series B, 2005, 48(4): 830-838. |
| [23] | Okafor E C, Naito Y, Colson S, et al. Experimental and numerical study of the laminar burning velocity of CH4–NH3–air premixed flames[J]. Combustion and Flame, 2018, 187: 185-198. |
| [24] | Tu Y J, Zhang H Y, Guiberti T F, et al. Experimental and numerical study of combustion and emission characteristics of NH3/CH4/air premixed swirling flames with air-staging in a model combustor[J]. Applied Energy, 2024, 367: 123370. |
| [25] | Smith G P, Golden D M, Frenklach M, et al. GRI-mech 3.0[EB/OL]. 1999. |
| [26] | Tian Z Y, Li Y Y, Zhang L D, et al. An experimental and kinetic modeling study of premixed NH3/CH4/O2/Ar flames at low pressure[J]. Combustion and Flame, 2009, 156(7): 1413-1426. |
| [27] | Valera-Medina A, Amer-Hatem F, Azad A K, et al. Review on ammonia as a potential fuel: from synthesis to economics[J]. Energy & Fuels, 2021, 35(9): 6964-7029. |
| [28] | Carrera S A, Villarreal J S, Acosta P I, et al. Designing an efficient and recoverable magnetic nanocatalyst based on Ca, Fe and pectin for biodiesel production[J]. Fuel, 2022, 310: 122456. |
| [29] | Zhu W C, Zhang M K, Zhang X R, et al. A comprehensive kinetic modeling study on NH3/H2, NH3/CO and NH3/CH4 blended fuels[J]. International Journal of Hydrogen Energy, 2024, 85: 228-241. |
| [30] | Zhou S K, Cui B C, Yang W J, et al. An experimental and kinetic modeling study on NH3/air, NH3/H2/air, NH3/CO/air, and NH3/CH4/air premixed laminar flames at elevated temperature[J]. Combustion and Flame, 2023, 248: 112536. |
| [31] | Zhang X Y, Yalamanchi K K, Mani Sarathy S. Combustion chemistry of ammonia/C1 fuels: a comprehensive kinetic modeling study[J]. Fuel, 2023, 341: 127676. |
| [32] | Shrestha K P, Lhuillier C, Barbosa A A, et al. An experimental and modeling study of ammonia with enriched oxygen content and ammonia/hydrogen laminar flame speed at elevated pressure and temperature[J]. Proceedings of the Combustion Institute, 2021, 38(2): 2163-2174. |
| [33] | Zhang X Y, Moosakutty S P, Rajan R P, et al. Combustion chemistry of ammonia/hydrogen mixtures: Jet-stirred reactor measurements and comprehensive kinetic modeling[J]. Combustion and Flame, 2021, 234: 111653. |
| [34] | Stagni A, Cavallotti C, Arunthanayothin S, et al. An experimental, theoretical and kinetic-modeling study of the gas-phase oxidation of ammonia[J]. Reaction Chemistry & Engineering, 2020, 5(4): 696-711. |
| [35] | 段正巧, 龚岩, 郭庆华, 等. 氨/甲烷扩散火焰中OH*、NH2 *和NH*光谱辐射特性研究[J]. 化工学报, 2023, 74(11): 4710-4719. |
| Duan Z Q, Gong Y, Guo Q H, et al. Spectral radiation characterization of OH*, NH2 * and NH* in ammonia/methane diffusion flame[J]. CIESC Journal, 2023, 74(11): 4710-4719. | |
| [36] | Vijrumbana Y, Shankar Singh A, Jik Lee B, et al. Chemical kinetic analysis to study the potential of fuel staging in reducing the emissions from NH3/CH4-air combustion at different pressures[J]. Fuel, 2023, 339: 127404. |
| [1] | 徐佳琪, 张文君, 余燕萍, 苏宝根, 任其龙, 杨启炜. 热等离子体重整炼厂气制合成气过程数值模拟与实验研究[J]. 化工学报, 2025, 76(9): 4462-4473. |
| [2] | 赵清萍, 张敏, 赵辉, 王刚, 邱永福. 乙烯氢甲酯化合成丙酸甲酯的氢键作用机制及反应动力学研究[J]. 化工学报, 2025, 76(6): 2701-2713. |
| [3] | 麦棹铭, 武颖韬, 王维, 穆海宝, 黄佐华, 汤成龙. 正十二烷-甲烷双燃料非线性着火特性及稀释气体效应研究[J]. 化工学报, 2025, 76(6): 3115-3124. |
| [4] | 杨猛, 丁晓倩, 余涛, 刘畅, 汤成龙, 黄佐华. 甲烷/氧化亚氮绿色推进剂自着火特性实验及动力学[J]. 化工学报, 2025, 76(3): 1221-1229. |
| [5] | 郭珊, 田雨, 徐永滨, 王朋, 刘治明. 废旧电池再资源化制备高性能中熵合金催化剂及其性能研究[J]. 化工学报, 2025, 76(1): 231-240. |
| [6] | 赵焕娟, 包颖昕, 于康, 刘婧, 钱新明. 多元组分爆轰不稳定性定量实验研究[J]. 化工学报, 2024, 75(S1): 339-348. |
| [7] | 王学云, 郁肖兵, 彭万旺, 沈岩松. 熔渣气化炉喷嘴燃烧区行为的数值模拟研究[J]. 化工学报, 2024, 75(2): 659-674. |
| [8] | 张兆想, 蔡茂坤, 任志英, 贾晓红, 郭飞. 温度及其波动对橡胶密封硫化过程影响的仿真分析[J]. 化工学报, 2024, 75(2): 715-726. |
| [9] | 卓红英, 赵忠正, 沈铮, 杨小峰, 黄延强. 正-仲氢催化转化研究进展[J]. 化工学报, 2024, 75(11): 3883-3895. |
| [10] | 程成, 段钟弟, 孙浩然, 胡海涛, 薛鸿祥. 表面微结构对析晶沉积特性影响的格子Boltzmann模拟[J]. 化工学报, 2023, 74(S1): 74-86. |
| [11] | 汪林正, 陆俞冰, 张睿智, 罗永浩. 基于分子动力学模拟的VOCs热氧化特性分析[J]. 化工学报, 2023, 74(8): 3242-3255. |
| [12] | 张蒙蒙, 颜冬, 沈永峰, 李文翠. 电解液类型对双离子电池阴阳离子储存行为的影响[J]. 化工学报, 2023, 74(7): 3116-3126. |
| [13] | 禹进, 余彬彬, 蒋新生. 一种基于虚拟组分的燃烧调控化学作用量化及分析方法研究[J]. 化工学报, 2023, 74(3): 1303-1312. |
| [14] | 刘世君, 郑安庆, 陈晓丽, 付娟, 苏秋成. 纤维素增强环氧树脂复合材料热解特性研究[J]. 化工学报, 2023, 74(12): 4968-4978. |
| [15] | 刘宗鹏, 胡少剑, 张宇宁, 马玲, 李磊, 武本成, 朱建华. 复合型多元醇酯合成反应的热力学分析及动力学研究[J]. 化工学报, 2023, 74(11): 4475-4486. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号