化工学报 ›› 2019, Vol. 70 ›› Issue (8): 3188-3195.DOI: 10.11949/j.issn.0438-1157.20190067
收稿日期:2019-01-21
修回日期:2019-03-02
出版日期:2019-08-05
发布日期:2019-08-05
通讯作者:
张海
作者简介:王康(1993—),男,硕士研究生,<email>18612526286@163.com</email>
基金资助:
Kang WANG( ),Yang ZHANG,Lilin HU,Hai ZHANG(
),Yang ZHANG,Lilin HU,Hai ZHANG( ),Junfu LYU,Guangxi YUE
),Junfu LYU,Guangxi YUE
Received:2019-01-21
Revised:2019-03-02
Online:2019-08-05
Published:2019-08-05
Contact:
Hai ZHANG
摘要:
针对水煤浆循环流化床锅炉水蒸气浓度高的特点,在较宽的水蒸气浓度内采用固定床反应器对石灰石脱硫过程的煅烧和硫化阶段分别开展机理研究,并利用低温氮吸附法、真密度计和扫描电子显微镜分析煅烧产物和硫化产物的孔结构特征、表观形貌。研究表明水蒸气促进煅烧产物的烧结,减小了煅烧产物的孔隙率和比表面积,增大了大孔份额,使产物氧化钙失去活性;水蒸气对硫化反应的作用呈非单调性,随着水蒸气浓度提高,同一时刻的钙转化率表现为先促进后抑制,在水蒸气浓度10%左右时钙转化率最高。其原因可以归结为水蒸气一方面促进硫化产物固态离子扩散,另一方面促进未反应氧化钙烧结成块。
中图分类号:
王康, 张扬, 胡丽琳, 张海, 吕俊复, 岳光溪. 高分压水蒸气对石灰石脱硫过程的影响作用[J]. 化工学报, 2019, 70(8): 3188-3195.
Kang WANG, Yang ZHANG, Lilin HU, Hai ZHANG, Junfu LYU, Guangxi YUE. Effects of high steam partial pressure on desulfurization process of limestone[J]. CIESC Journal, 2019, 70(8): 3188-3195.
| No. | CaO | MgO | SiO2 | Al2O3 | Fe2O3 | 其他 |
|---|---|---|---|---|---|---|
| 1 | 91.70 | 4.21 | 1.60 | 1.01 | 0.64 | 0.84 |
| 2 | 0.07 | 0.05 | 99.61 | 0.10 | 0.01 | 0.16 |
表1 石灰石和石英砂成分
Table 1 Compound of limestone and quartz sand/%
| No. | CaO | MgO | SiO2 | Al2O3 | Fe2O3 | 其他 |
|---|---|---|---|---|---|---|
| 1 | 91.70 | 4.21 | 1.60 | 1.01 | 0.64 | 0.84 |
| 2 | 0.07 | 0.05 | 99.61 | 0.10 | 0.01 | 0.16 |
| 煅烧气氛 | 比表 面积/(m2/g) | 孔体积/ (cm3/g) | 真密度/ (kg/m3) | 颗粒密度/ (kg/m3) | 孔隙率 |
|---|---|---|---|---|---|
| 无水 | 29.67 | 0.236 | 3176 | 1816 | 0.428 |
| 含水20% | 12.46 | 0.151 | 3204 | 2159 | 0.326 |
| 变化率/% | -58.1 | -36.0 | +0.9 | +18.9 | -23.8 |
表2 煅烧产物颗粒性质
Table 2 Properties of calcination product
| 煅烧气氛 | 比表 面积/(m2/g) | 孔体积/ (cm3/g) | 真密度/ (kg/m3) | 颗粒密度/ (kg/m3) | 孔隙率 |
|---|---|---|---|---|---|
| 无水 | 29.67 | 0.236 | 3176 | 1816 | 0.428 |
| 含水20% | 12.46 | 0.151 | 3204 | 2159 | 0.326 |
| 变化率/% | -58.1 | -36.0 | +0.9 | +18.9 | -23.8 |

图6 不同H2O(g)浓度下的煅烧产物硫化过程的钙转化率随时间的变化
Fig.6 Temporal variations of calcium conversion during sulfation at water free condition for calcined products at different H2O(g) concentrations
| H2O(g)/% | 颗粒密度/(kg/m3) |
|---|---|
| 0 | 2420 |
| 10 | 2790 |
| 20 | 2881 |
| 30 | 3070 |
表3 硫化产物颗粒密度
Table 3 Particle density of sulfation product
| H2O(g)/% | 颗粒密度/(kg/m3) |
|---|---|
| 0 | 2420 |
| 10 | 2790 |
| 20 | 2881 |
| 30 | 3070 |
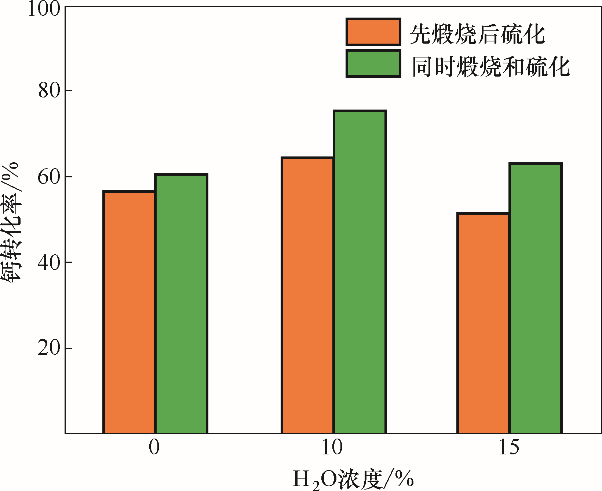
图10 不同H2O(g)浓度下煅烧和硫化独立进行和耦合进行的石灰石钙转化率
Fig. 10 Effect of H2O(g) concentration on calcium conversion between decoupled and coupled calcination and sulfation processes
| 1 | 岳光溪, 吕俊复, 徐鹏, 等 .循环流化床燃烧发展现状及前景分析[J].中国电力, 2016, 49(1): 1-13. |
| Yue G X , Lyu J F , Xu P , et al . The up-to-date development and future of circulating fluidized bed combustion technology[J]. Electric Power, 2016, 49(1): 1-13. | |
| 2 | 宋畅, 吕俊复, 杨海瑞, 等 . 超临界及超超临界循环流化床锅炉技术研究与应用[J]. 中国电机工程学报, 2018, 38(2): 338-347. |
| Song C , Lyu J F , Yang H R , et al . Research and application of supercritical and ultra-supercritical circulating fluidized bed boiler technology[J]. Proceedings of the CSEE, 2018, 38(2): 338-347. | |
| 3 | 段伦博, 陈晓平, 梁财, 等 . 以煤焦混合物为燃料的循环流化床锅炉SO2 排放特性[J]. 化工学报, 2008, 59(3): 728-734. |
| Duan L B , Chen X P , Liang C , et al . SO2 emission characteristics of circulating fluidized bed boiler co-firing coal and petroleum coke[J]. Journal of Chemical Industry and Engineering(China), 2008, 59(3): 728-734. | |
| 4 | 朱廷钰, 叶猛, 荆鹏飞, 等 . 1.5MW循环流化床锅炉内煤与垃圾混烧的烟气特性[J]. 化工学报, 2010, 61(9): 2468-2473. |
| Zhu T Y , Ye M , Jing P F , et al . Properties of flue gas from mixed incineration of municipal solid waste and coal in a 1.5MW circulating fluidized bed boiler[J]. CIESC Journal, 2010, 61(9): 2468-2473. | |
| 5 | 姜秀民, 马玉峰, 崔志刚, 等 . 水煤浆流化悬浮高效洁净燃烧技术研究与应用[J]. 化学工程, 2006, 34(1): 62-65. |
| Jiang X M , Ma Y F , Cui Z G , et al . Study and application of fluidization-suspension high efficiency cleaning combustion technology of coal-water slurry[J]. Chemical Engineering, 2006, 34(1): 62-65. | |
| 6 | Anthony E J , Granatstein D L . Sulfation phenomena in fluidized bed combustion systems[J]. Progress in Energy and Combustion Science, 2001, 27(2): 215-236. |
| 7 | Ar I , Dogu G . Calcination kinetics of high purity limestones[J]. Chemical Engineering Journal, 2001, 83(2): 131-137. |
| 8 | Khinast J , Krammer G F , Brunner C , et al . Decomposition of limestone: the influence of CO2 and particle size on the reaction rate[J]. Chemical Engineering Science, 1996, 51(4): 623-634. |
| 9 | 陈亮, 王子铭, 王春波 . 流化床锅炉内石灰石同时煅烧/硫化反应中煅烧动力学特性[J]. 化工学报, 2017, 68(12): 4615-4624. |
| Chen L , Wang Z M , Wang C B . Limestone calcination kinetics in simultaneous calcination and sulfation under CFB conditions[J]. CIESC Journal, 2017, 68(12): 4615-4624. | |
| 10 | 杨海波, 武增华, 邱新平 . 固硫反应机理研究的新进展[J]. 燃料化学学报, 2003, 31(1): 92-96. |
| Yang H B , Wu Z H , Qiu X P . Development of mechanism of CaO sulfation reaction[J]. Journal of Fuel Chemistry and Technology, 2003, 31(1): 92-96. | |
| 11 | Hu G L , Dam-Johansen K , Wedel S , et al . Review of the direct sulfation reaction of limestone [J]. Progress in Energy & Combustion Science, 2006, 32(4): 386-407. |
| 12 | 贾传凯 . 水煤浆水分对锅炉燃烧的影响[J]. 工业锅炉, 2008, (6): 5-7. |
| Jia C K . Effect of moisture content in coal water mixture on boiler combustion[J]. Industrial Boiler, 2008, (6): 5-7. | |
| 13 | Wang C B , Zhang Y , Jia L F , et al . Effect of water vapor on the pore structure and sulfation of CaO[J]. Fuel, 2014, 130(15): 60-65. |
| 14 | 陈亮, 赵帆, 闫广精, 等 . H2O和SO2对CFB内石灰石同时煅烧/硫化反应中煅烧动力学的协同作用[J]. 化工学报, 2018, 69(9): 3859-3868. |
| Chen L , Zhao F , Yan G J , et al . Synergetic effect of H2O and SO2 on calcination kinetics of limestone during simultaneous calcination/sulfation reaction in CFB boilers[J]. CIESC Journal, 2018, 69(9): 3859-3868. | |
| 15 | Wang H , Guo S , Liu D Y , et al . A dynamic study on the impacts of water vapor and impurities on limestone calcination and CaO sulfurization processes in a micro fluidized bed reactor analyzer[J]. Energy Fuels, 2016, 30(6): 4625-4634. |
| 16 | Borgwardt R H . Calcium oxide sintering in atmospheres containing water and carbon dioxide[J]. Industrial & Engineering Chemistry Research, 1989, 28(4): 493-500. |
| 17 | 刘妮, 程乐鸣, 骆仲泱, 等 . 钙基吸收剂微观结构特性及其反应性能[J]. 化工学报, 2004, 55(4): 635-639. |
| Liu N , Cheng L M , Luo Z Y , et al . Microstructure and sulfation characteristics of calcium-based sorbents[J]. Journal of Chemical Industry and Engineering(China), 2004, 55(4): 635-639. | |
| 18 | Dennis J S . The desulphurisation of flue gases using calcareous materials[D]. Cambridge, UK: Selwyn College, 1985. |
| 19 | Suyadal Y , Erol M , Oğuz H . Deactivation model for dry desulphurization of simulated flue gas with calcined limestone in a fluidized-bed reactor[J]. Fuel, 2005, 84(12/13): 1705-1712. |
| 20 | 王世昌, 徐旭常, 姚强 . 水蒸气对CaO颗粒脱硫反应催化作用的实验研究[J]. 中国电机工程学报, 2004, 24(9): 256-260. |
| Wang S C , Xu X C , Yao Q . Experimental study on the catalysis effect of steam in the dry flue gas desulfurization reaction by CaO particles[J]. Proceedings of the CSEE, 2004, 24(9): 256-260. | |
| 21 | Stewart M C , Manovic V , Anthony E J , et al . Enhancement of indirect sulphation of limestone by steam addition[J]. Environmental Science & Technology, 2010, 44(22): 8781-8786. |
| 22 | Wang C B , Jia L F , Tan Y W , et al . The effect of water on the sulphation of limestone[J]. Fuel, 2010, 89(9): 2628-2632. |
| 23 | Wang C B , Jia L F , Tan Y W , et al . Influence of water vapor on the direct sulfation of limestone under simulated oxy-fuel fluidized-bed combustion (FBC) conditions[J]. Energy Fuels, 2011, 25(2): 617-623. |
| 24 | 姜中孝, 段伦博, 陈晓平, 等 . 空气燃烧与O2/CO2燃烧气氛下水蒸气对石灰石煅烧/硫化特性的影响[J]. 中国电机工程学报, 2013, 26: 14-20. |
| Jiang Z X , Duan L B , Chen X P , et al . Effect of water vapor on indirect sulfation during air and O2/CO2 combustion[J] Proceedings of the CSEE, 2013, 26: 14-20. | |
| 25 | Duan L B , Jiang Z X , Chen X P , et al . Investigation on water vapor effect on direct sulfation during wet-recycle oxy-coal combustion[J]. Applied Energy, 2013, 108: 121-127. |
| 26 | 郭帅 . 流化床锅炉含水蒸气条件下石灰石脱硫反应机理研究[D]. 哈尔滨: 哈尔滨工业大学, 2016. |
| Guo S . Studies on influence mechanism of water vapor on limestone desulfurization in fluidized bed boiler[D]. Harbin: Harbin Institute of Technology, 2016. | |
| 27 | Wang H , Guo S , Liu D Y , et al . Understanding the impacts of water vapor on CaO sulfurization in a laboratory-scale fluidized bed[J]. Energy Fuels, 2016, 30(9): 7108-7117. |
| 28 | German R M . Sintering Theory and Practice[M]. New York: Wiley, 1996. |
| 29 | Hsia C , St Pierre G R , Raghunathan K , et al .Diffusion through CaSO4 formed during the reaction of CaO with SO2 and O2 [J]. AIChE Journal, 1993, 39(4): 698-700. |
| 30 | Hsia C , St Pierre G R , Fan L S . Isotope study on diffusion in CaSO4 formed during sorbent-flue-gas reaction[J].AIChE Journal, 1995, 41(10): 2337-2340. |
| 31 | Wang C B , Chen L . The effect of steam on simultaneous calcination and sulfation of limestone in CFBB[J]. Fuel, 2016, 175: 164-171. |
| [1] | 曾如宾, 沈中杰, 梁钦锋, 许建良, 代正华, 刘海峰. 基于分子动力学模拟的Fe2O3纳米颗粒烧结机制研究[J]. 化工学报, 2023, 74(8): 3353-3365. |
| [2] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [3] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [4] | 朱风, 陈凯琳, 黄小凤, 鲍银珠, 李文斌, 刘嘉鑫, 吴玮强, 高王伟. KOH改性电石渣脱除羰基硫的性能研究[J]. 化工学报, 2023, 74(6): 2668-2679. |
| [5] | 王承泽, 顾凯丽, 张晋华, 石建轩, 刘艺娓, 李锦祥. 硫化协同老化零价铁增效去除水中Cr(Ⅵ)的作用机制[J]. 化工学报, 2023, 74(5): 2197-2206. |
| [6] | 葛泽峰, 吴雨青, 曾名迅, 查振婷, 马宇娜, 侯增辉, 张会岩. 灰化学成分对生物质气化特性的影响规律[J]. 化工学报, 2023, 74(5): 2136-2146. |
| [7] | 白天昊, 王晓雯, 杨梦滋, 段新伟, 米杰, 武蒙蒙. 类水滑石衍生锌基氧化物高温煤气脱硫过程中COS释放行为及其抑制研究[J]. 化工学报, 2023, 74(4): 1772-1780. |
| [8] | 吴选军, 王超, 曹子健, 蔡卫权. 数据与物理信息混合驱动的固定床吸附穿透深度学习模型[J]. 化工学报, 2023, 74(3): 1145-1160. |
| [9] | 焦巡, 童成, 李存璞, 魏子栋. 锂硫电池的动力学调控策略[J]. 化工学报, 2023, 74(1): 170-191. |
| [10] | 鞠小兵, 李雪纯, 孙芳. 二硫代水杨酸衍生物对光固化材料性能的影响[J]. 化工学报, 2022, 73(9): 4187-4193. |
| [11] | 彭琳, 牛明鑫, 白羽, 孙克宁. 中空硫球-MoS2/rGO材料的制备及其在锂硫电池中的应用[J]. 化工学报, 2022, 73(8): 3688-3698. |
| [12] | 李亚芾, 付亮亮, 白浩隆, 白丁荣, 许光文. 菱镁矿浮选尾矿直接合成同时制备镁橄榄石和镁砂研究[J]. 化工学报, 2022, 73(8): 3679-3687. |
| [13] | 郭志强, 燕可洲, 张吉元, 柳丹丹, 高阳艳, 郭彦霞. 煤矸石/粉煤灰对赤泥钠化还原焙烧反应的影响机制[J]. 化工学报, 2022, 73(5): 2194-2205. |
| [14] | 白文轩, 陈锦湘, 刘芬, 张静淙, 谷志平, 熊成铭, 施王军, 余江. 非水相金属基离子液体湿法氧化脱硫工艺:发展与展望[J]. 化工学报, 2022, 73(5): 1847-1862. |
| [15] | 许世佩, 王超, 李庆远, 张炳康, 许世伟, 张雪琴, 王诗颖, 丛梦晓. 氧化钙对油基钻屑热脱附产物影响的研究[J]. 化工学报, 2022, 73(4): 1724-1731. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号