化工学报 ›› 2023, Vol. 74 ›› Issue (1): 170-191.DOI: 10.11949/0438-1157.20221071
焦巡1( ), 童成1, 李存璞1,2(
), 童成1, 李存璞1,2( ), 魏子栋1,2(
), 魏子栋1,2( )
)
收稿日期:2022-08-01
修回日期:2022-12-12
出版日期:2023-01-05
发布日期:2023-03-20
通讯作者:
李存璞,魏子栋
作者简介:焦巡(1995—),女,博士研究生,20211801052@cqu.edu.cn
基金资助:
Xun JIAO1( ), Cheng TONG1, Cunpu LI1,2(
), Cheng TONG1, Cunpu LI1,2( ), Zidong WEI1,2(
), Zidong WEI1,2( )
)
Received:2022-08-01
Revised:2022-12-12
Online:2023-01-05
Published:2023-03-20
Contact:
Cunpu LI, Zidong WEI
摘要:
锂硫(Li-S)电池因其超高的理论能量密度(2600 Wh·kg-1)有望成为下一代高能量密度电池的候选者之一。然而,它存在硫利用率低、容量衰减快以及多硫化锂(LiPSs)发生“流失效应”等问题,这使得Li-S电池反应动力学缓慢,严重限制了其实际应用。物理限制、化学吸附等方法可以加速硫、LiPSs和Li2S之间的氧化还原反应,减少LiPSs的流失,加速动力学过程,使电池具有高能量密度和长循环稳定性。基于整体电化学反应过程,对近些年使用的材料如何促进动力学进程、阻止LiPSs的流失,以及相应策略的评价进行了综述,以指导提升电池动力学性能的合理设计和Li-S电池的实际应用。
中图分类号:
焦巡, 童成, 李存璞, 魏子栋. 锂硫电池的动力学调控策略[J]. 化工学报, 2023, 74(1): 170-191.
Xun JIAO, Cheng TONG, Cunpu LI, Zidong WEI. Kinetic regulation strategies in lithium-sulfur batteries[J]. CIESC Journal, 2023, 74(1): 170-191.
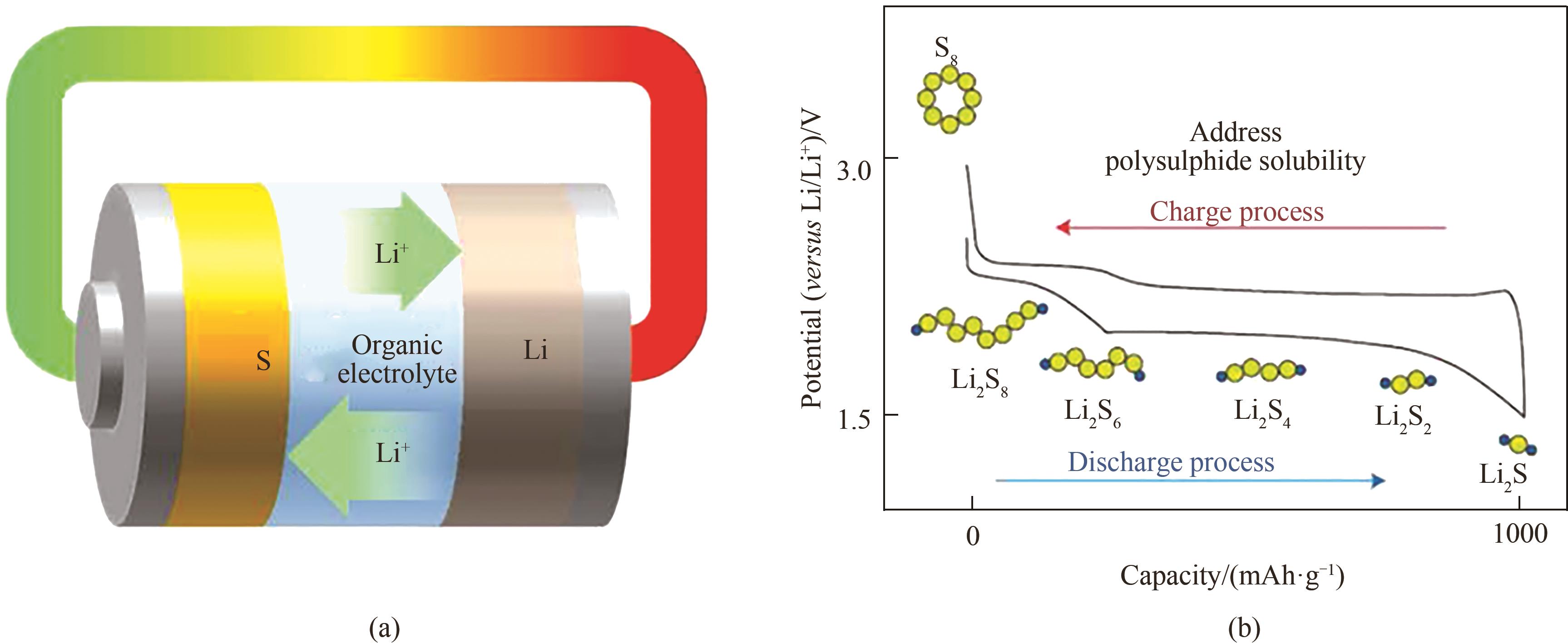
图1 (a)Li-S电池的结构示意图;(b)Li-S电池的一般电位分布[12]
Fig.1 (a) Schematic illustration of the construction of a Li-S battery cell; (b) General potential profile of a Li-S battery[12]
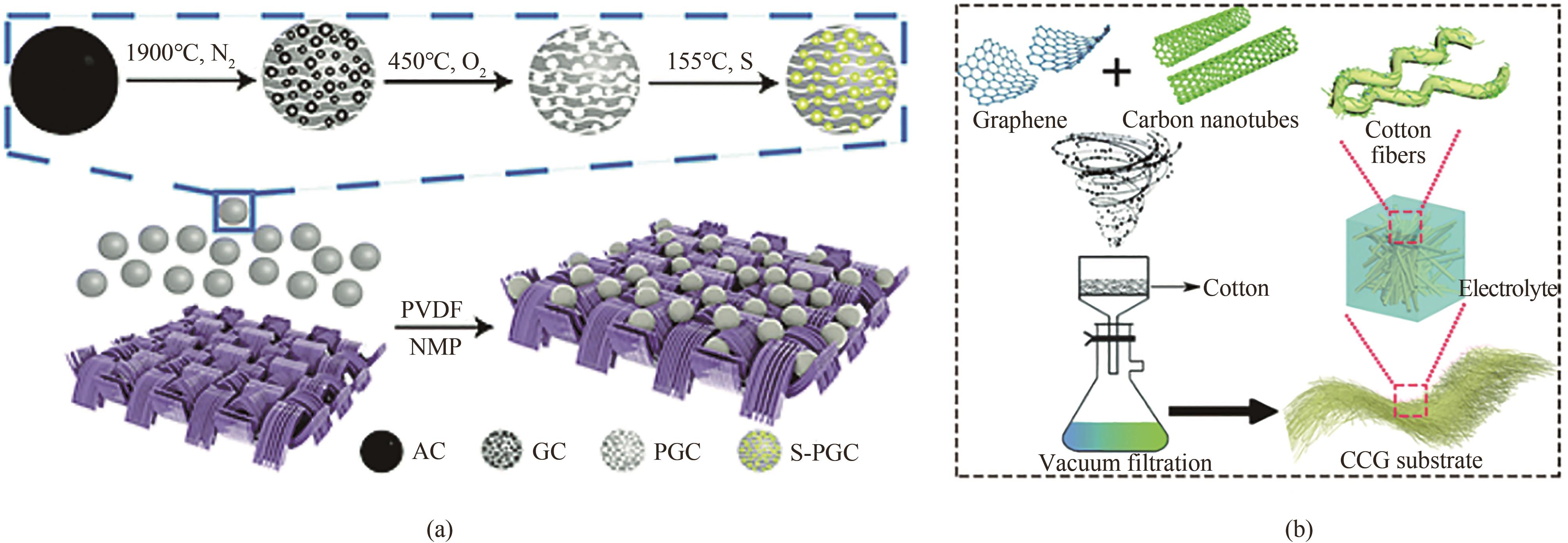
图2 (a)制造S-PGC-CFF正极的过程示意图和每个组件的微观结构[46];(b)CCG底物制备及其相应配置的示意图[48]
Fig.2 (a) Schematic illustration of the procedure for fabricating S-PGC-CFF cathode and the microstructures of each component[46]; (b) Schematic of the CCG substrate preparation and its corresponding configurations[48]
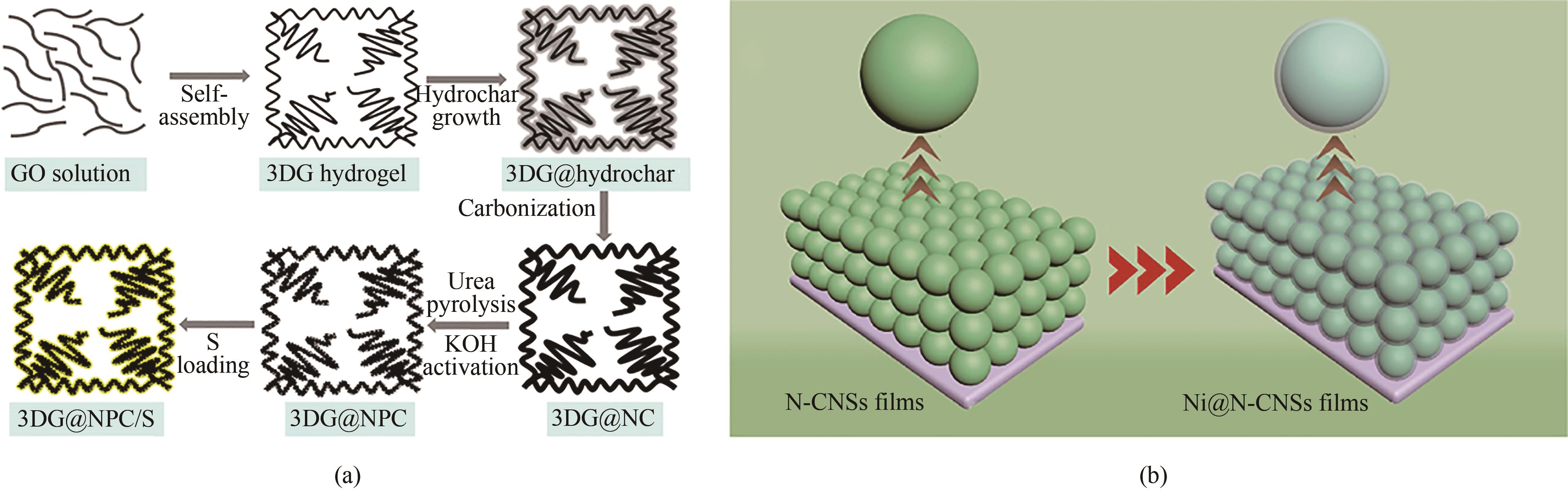
图3 (a)3DG@NPC和3DG@NPC/S的合成过程示意图[55];(b)Ni@N-CNSs薄膜的制造工艺示意图[56]
Fig.3 (a) Schematic synthesis process of 3DG@NPC and 3DG@NPC/S[55]; (b) Schematic fabrication process of Ni@N-CNSs films[56]
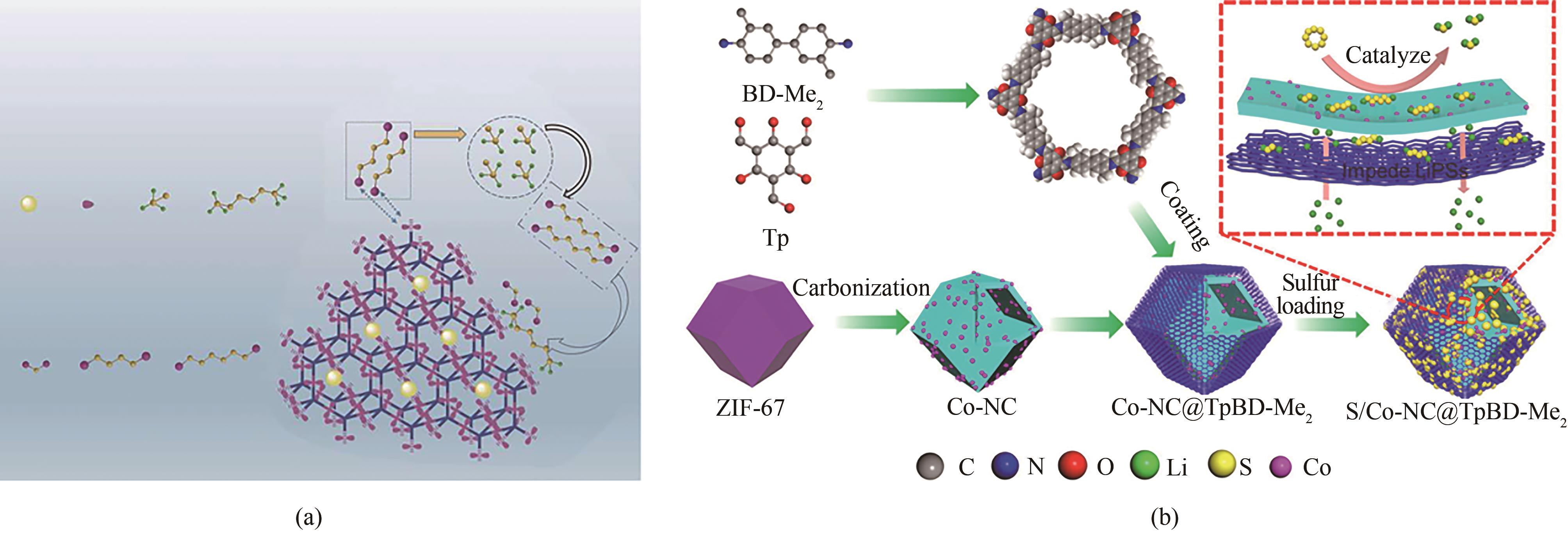
图4 (a)LiPSs与羟基之间相互作用的示意图[57];(b)Co-NC@TpBD-Me2正极的制备工艺及其作为硫正极的工作机理[58]
Fig.4 (a) Schematic illustration of the interactions between polysulfides and hydroxyl groups[57]; (b) Preparation procedure of Co-NC@TpBD-Me2 cathode and its working mechanism as sulfur cathode[58]
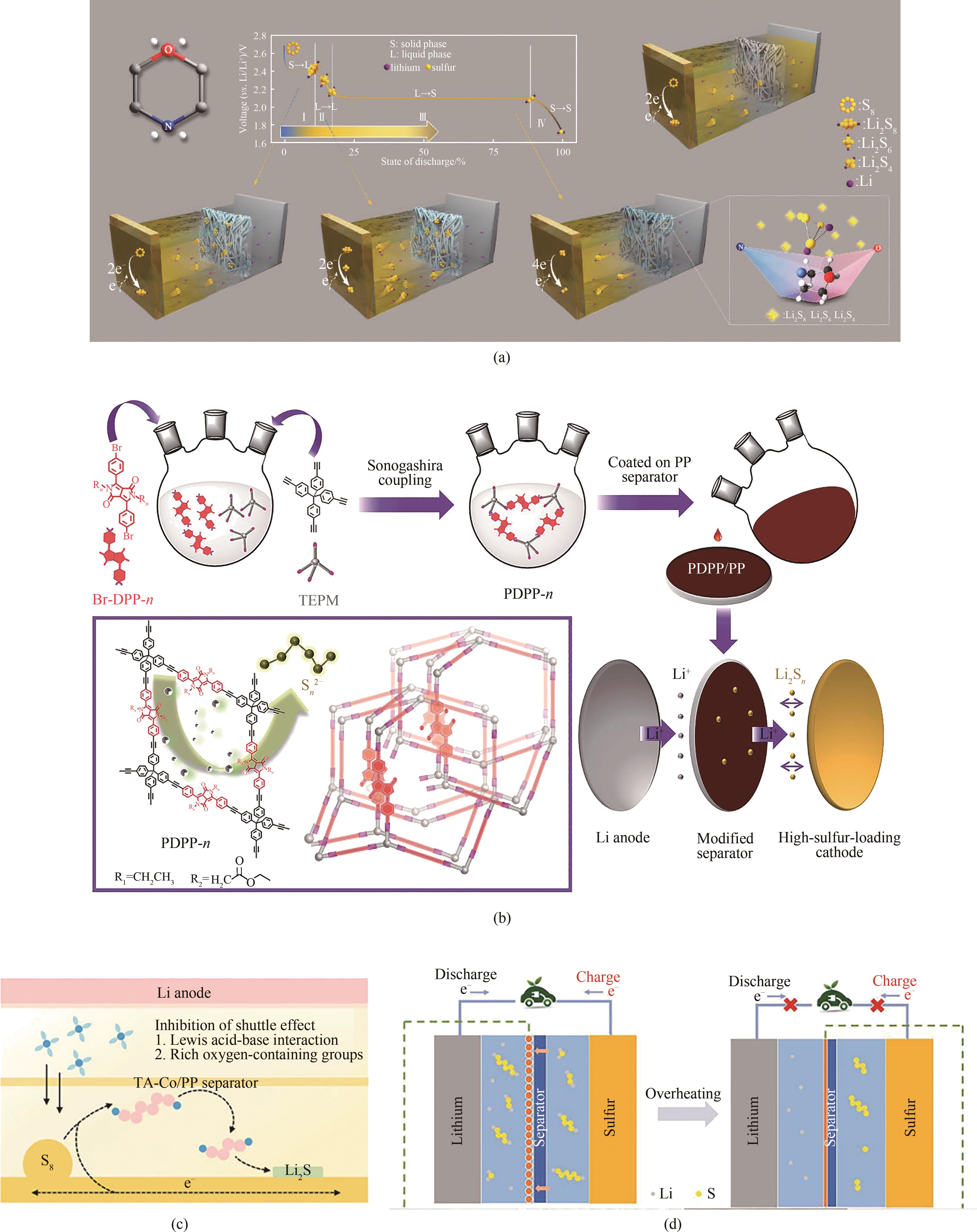
图5 (a)MP的化学结构以及PP-C-St-MP隔膜实现可逆的咬合效果[62];(b)PDPP改性隔膜的示意图以及其在Li-S电池中作用的效果图[63];(c)TA-Co隔膜的Li-S电池示意图[64];(d)具有EVA微球涂层隔膜的Li-S电池示意图[65]
Fig.5 (a) Chemical structure of MP and PP-C-St-MP diaphragm for reversible clinching effect[62]; (b) Schematic diagram of PDPP modified separator and its effect in Li-S battery[63]; (c) Schematic diagram of TA-Co separator in Li-S battery[64]; (d) Schematic illustration of the Li-S battery with EVA microspheres-coated separators[65]
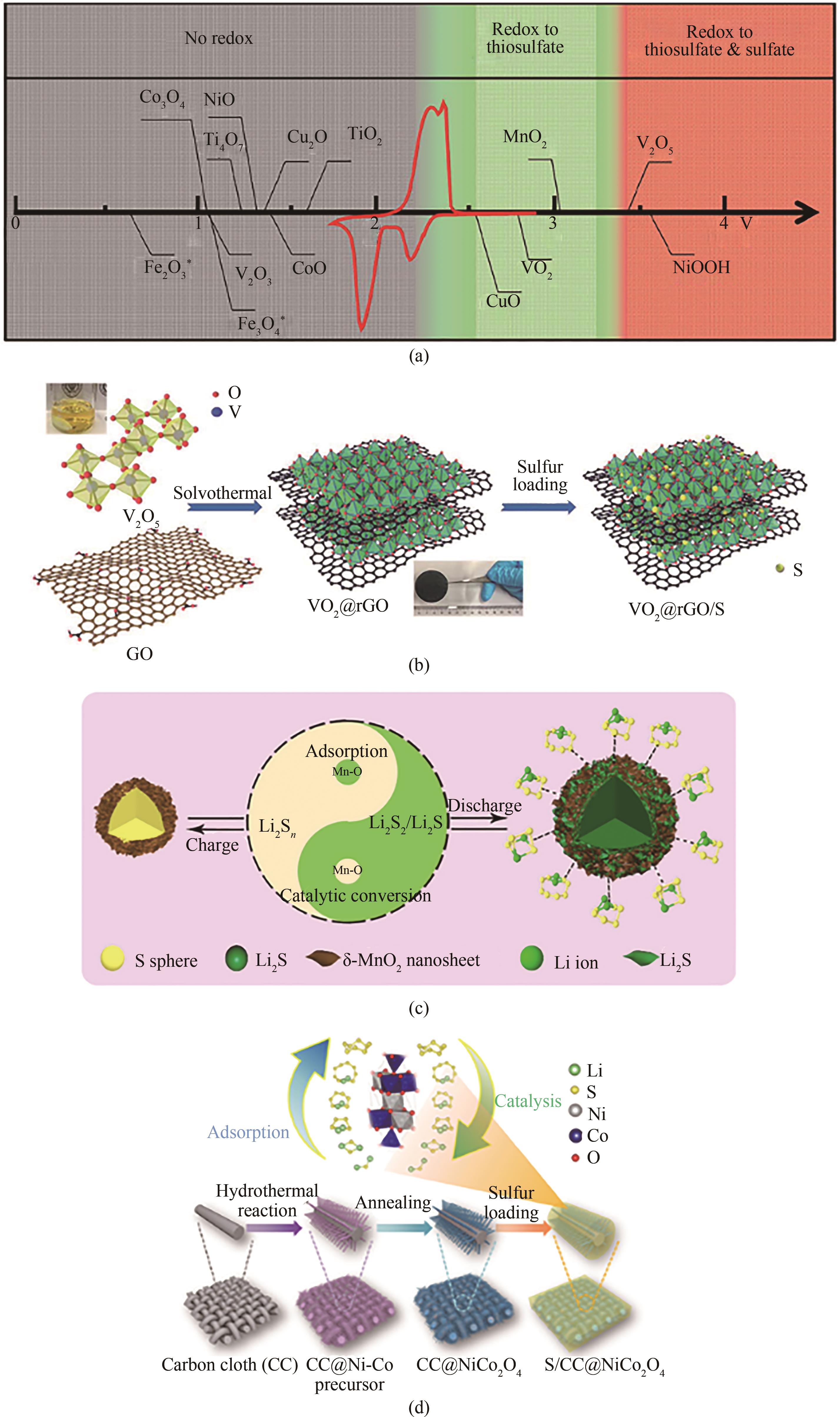
图6 (a)不同金属氧化物与LiPSs的化学反应性作为氧化还原电势与Li/Li+的函数,叠加了典型的Li-S循环伏安曲线(以红色显示)[69];(b)VO2@rGO/S产品的制造过程示意图[70];(c)核壳结构中超薄δ-MnO2纳米片的催化转化机理[72];(d)S/CC@NiCo2O4复合材料的合成步骤示意图,NiCo2O4纳米纤维阵列可以有效吸附和催化LiPSs的转化反应[73]
Fig.6 (a) Chemical reactivity of different metal oxides with LiPSs as a function of redox potential versus Li/Li+, superimposed with a typical Li-S cyclic voltammetry curve (shown in red)[69]; (b) Schematic of the fabrication processes of VO2@rGO/S product[70]; (c) Catalytic conversion mechanism of ultrathin δ-MnO2 nanosheets in the core-shell structure[72]; (d) Schematic of the synthesis steps for the S/CC@NiCo2O4 composite, the NiCo2O4 nanofiber arrays can effectively adsorb and catalyze the conversion reaction of LiPSs[73]
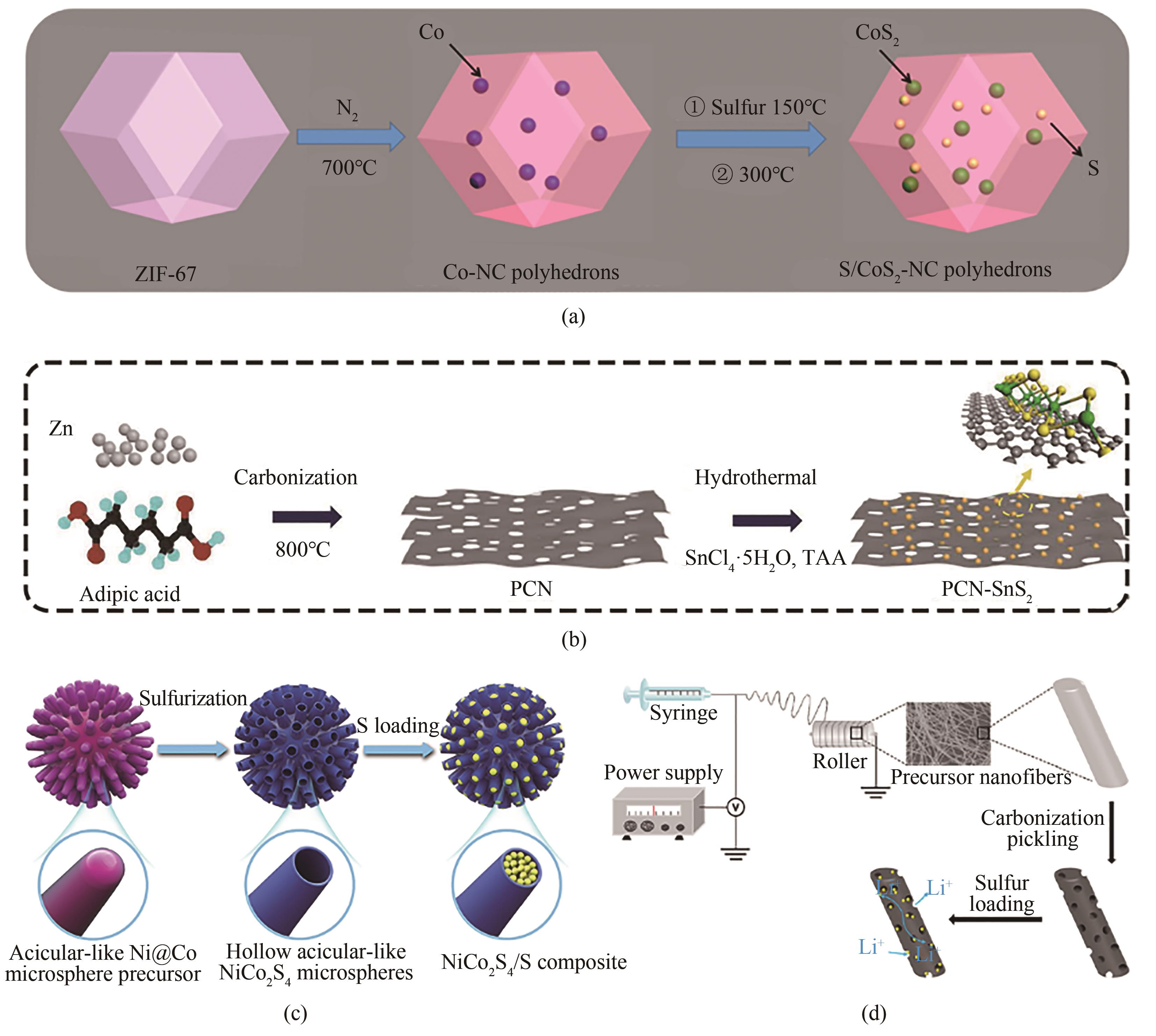
图7 (a)S/CoS2-NC复合材料的合成过程示意图[74];(b)PCN-SnS2复合材料的制备示意图[75];(c)NiCo2S4/S复合材料合成过程示意图[76];(d)MoS2@CNF和S@MoS2@CNF复合材料的制备[77]
Fig.7 (a) Schematic illustration of the synthesis process of S/CoS2-NC composites[74]; (b) Schematic illustration of the preparation of PCN-SnS2 composites[75]; (c) Schematic illustration of the synthetic processes of NiCo2S4/S composite[76]; (d) Preparation of MoS2@CNF and S@MoS2@CNF composite[77]
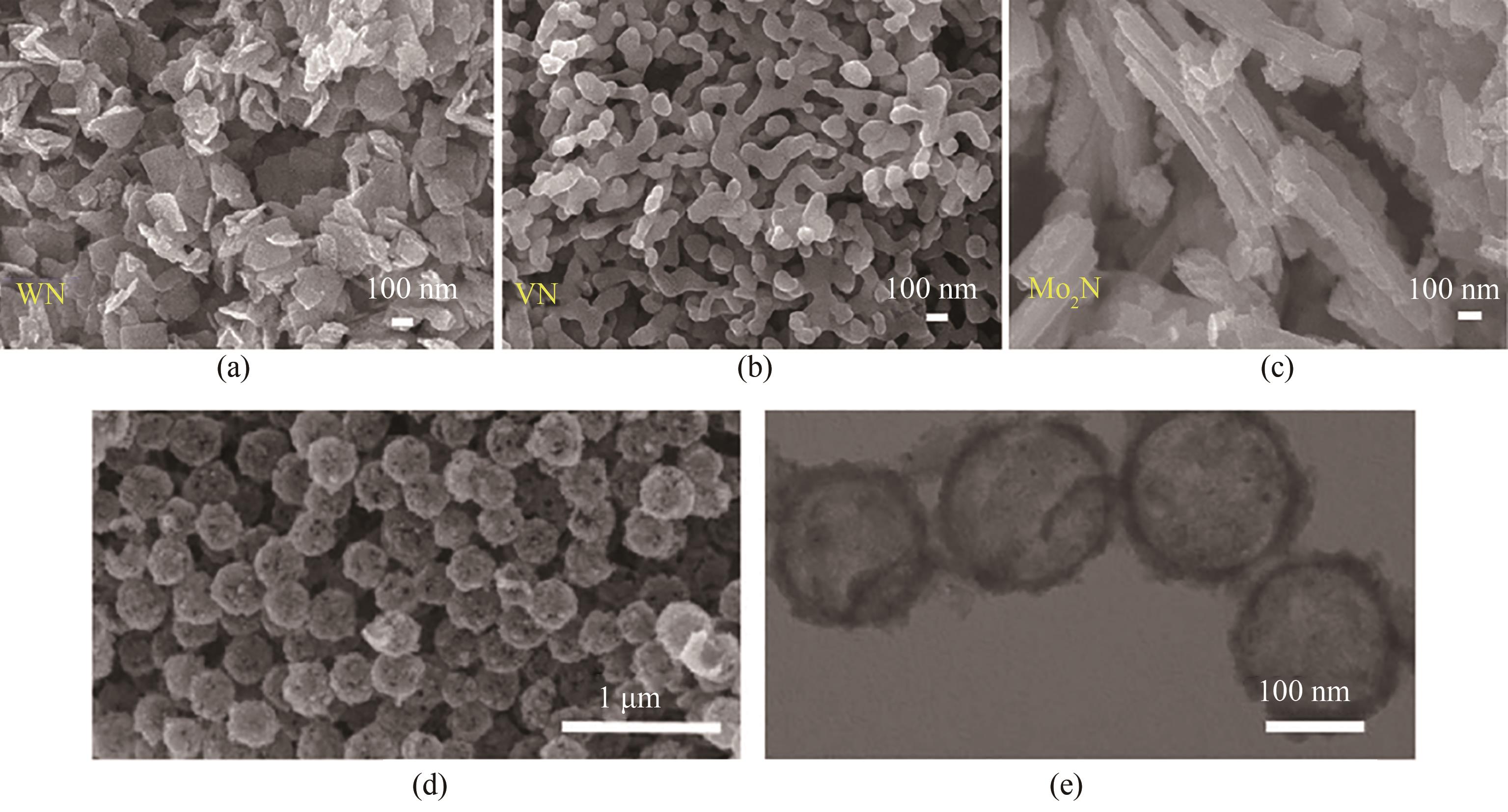
图8 (a)~(c)WN、VN和Mo2N的SEM图[78];(d)、(e)TiN中空纳米球的SEM图和TEM图[79]
Fig.8 (a)—(c) SEM images of WN, VN and Mo2N[78]; (d), (e) SEM image and TEM image of TiN hollow nanospheres[79]
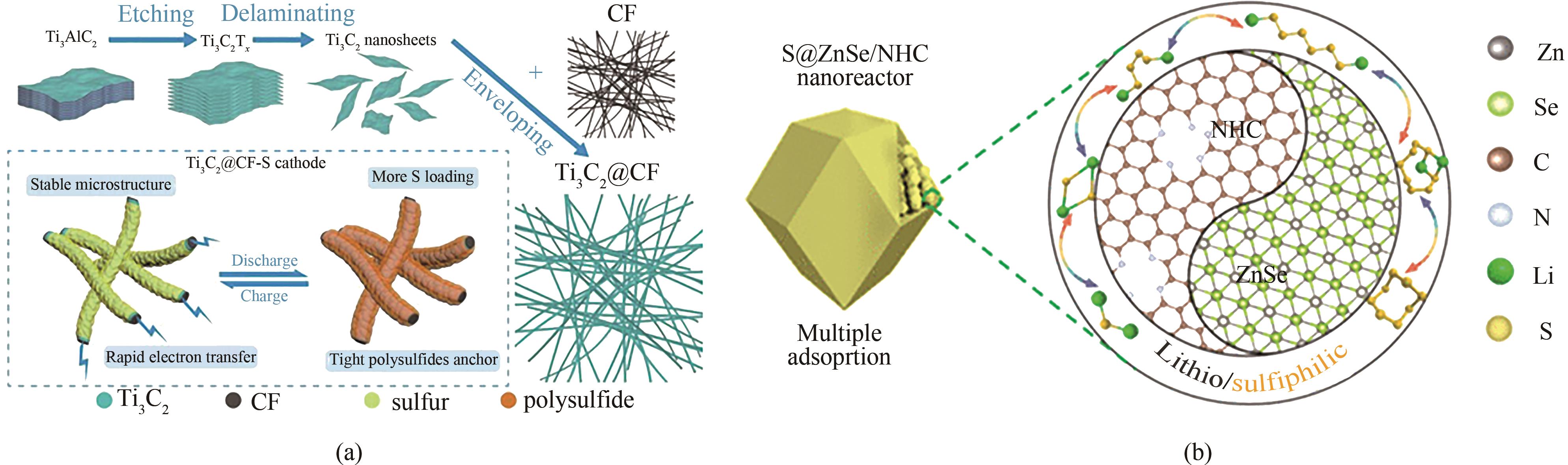
图9 (a)Ti3C2@CF制备示意图以及作为多功能正极材料改善Li-S电池性能的示意图[80];(b)S@ZnSe/NHC正极对Li-S电池的作用示意图[81]
Fig.9 (a) Schematic illustration of Ti3C2@CF preparation and as a multifunctional cathode material to ameliorate the performance of Li-S batteries[80]; (b) Schematic diagram of the effect of S@ZnSe/NHC cathode on Li-S batteries[81]

图10 (a)~(c)钙钛矿促进剂对工作中Li-S电池中LiPSs抑制和Li2S调节的作用及Ba0.5Sr0.5Co0.8Fe0.2O3-δ PrNP的晶体结构[82];(d)Li2S在常规导电表面(左)和具有均匀分布的成核位点的导电与催化纳米三相界面(右)上成核和生长的示意图[83];(e)LiPSs氧化还原反应和Li2S成核示意图[83]
Fig.10 (a)—(c) The role of perovskite promoter on LiPSs suppression and Li2S regulation in a working Li-S battery and the crystal structure of Ba0.5Sr0.5Co0.8Fe0.2O3-δ PrNP[82]; (d) Schematic illustration of the Li2S nucleation and growth on routine conductive surface (left) and on conductive and catalytic nanotriple-phase interface with uniformly distributed nucleation sites (right)[83]; (e) Schematic illustration of polysulfide redox reaction and Li2S nucleation[83]
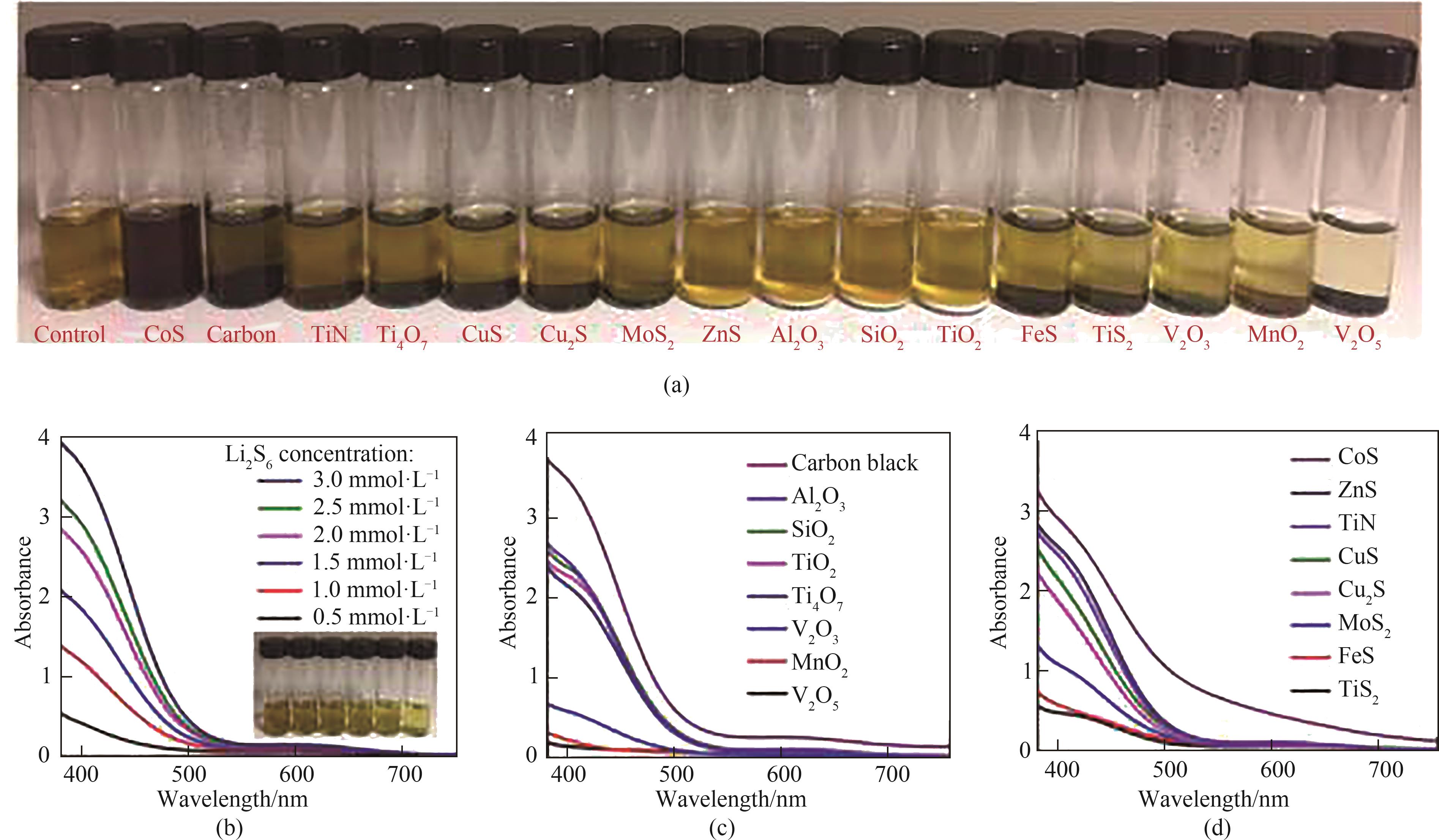
图11 Li2S6 LiPSs吸附试验:(a)候选材料的光学照片;(b)无候选材料的DOL/DME溶液中不同浓度Li2S6的UV-Vis数据;(c)、(d)候选材料添加到3.0 mmol·L-1 Li2S6中的UV-Vis数据[84]
Fig.11 Li2S6 polysulfide adsorption test: (a) Optical photos of candidate materials; (b) UV-Vis data of varying concentrations of Li2S6 in DOL/DME solution without candidate materials; (c), (d) UV-Vis data of candidate materials added in 3.0 mmol·L-1 Li2S6[84]

图13 MoO2(a)、α-MoC(b)和MoO2/α-MoC(c)在2.09 V时的无量纲瞬态曲线;MoO2、α-MoC和MoO2/α-MoC电极放电到最大电流[(d)~(f)]和Li2S沉积后[(g)~(i)]的SEM图像[89]
Fig.13 Dimensionless transient profiles of MoO2 (a), α-MoC (b) and MoO2/α-MoC (c) at 2.09 V; SEM images of MoO2, α-MoC and, MoO2/α-MoC electrodes after discharged to maximum current [(d)—(f)] and after Li2S deposition [(g)—(i)][89]

图14 (a)液相LiPSs被Co9S8强烈吸附,因此不能转移到MoS2上以实现快速转化;(b)硫空位可以协调组分的化学吸附以均匀吸附LiPSs;(c)~(e)CMG-L、CMG-M和CMG-H在1.6~2.8 V电压范围内不同扫描速率下的CV曲线;(f)CMG-L、CMG-M和CMG-H在不同扫描速度下的NTR值;(g)CMG-L、CMG-M和CMG-H在0.5 C下的长循环性能图[91]
Fig.14 (a) Liquid-phase LiPSs are strongly adsorbed by Co9S8 and therefore cannot be transferred to MoS2 to accomplish fast conversion; (b) Sulfur vacancies can harmonize the chemisorption of components to uniformly adsorb LiPSs; (c)—(e) CV curves within the voltage range of 1.6—2.8 V at different sweep rates of CMG-L, CMG-M and CMG-H; (f) The NTR values of CMG-L, CMG-M and CMG-H at different sweep rates; (g) Long cycle performances of CMG-L, CMG-M and CMG-H at 0.5 C[91]
| 1 | Armand M, Tarascon J M. Building better batteries[J]. Nature, 2008, 451(7179): 652-657. |
| 2 | Palacín M R, de Guibert A. Why do batteries fail?[J]. Science, 2016, 351(6273): 1253292. |
| 3 | Olabi A G, Onumaegbu C, Wilberforce T, et al. Critical review of energy storage systems[J]. Energy, 2021, 214: 118987. |
| 4 | Manthiram A. An outlook on lithium ion battery technology[J]. ACS Central Science, 2017, 3(10): 1063-1069. |
| 5 | Yoshino A. The birth of the lithium-ion battery[J]. Angewandte Chemie International Edition, 2012, 51(24): 5798-5800. |
| 6 | Manthiram A, Fu Y, Su Y S. Challenges and prospects of lithium-sulfur batteries[J]. Accounts of Chemical Research, 2013, 46(5): 1125-1134. |
| 7 | Manthiram A, Chung S H, Zu C. Lithium-sulfur batteries: progress and prospects[J]. Advanced Materials, 2015, 27(12): 1980-2006. |
| 8 | Kim J, Lee D J, Jung H G, et al. An advanced lithium-sulfur battery[J]. Advanced Functional Materials, 2013, 23(8): 1076-1080. |
| 9 | Li T, Bai X, Gulzar U, et al. A comprehensive understanding of lithium-sulfur battery technology[J]. Advanced Functional Materials, 2019, 29(32): 1901730. |
| 10 | Evers S, Nazar L F. New approaches for high energy density lithium-sulfur battery cathodes[J]. Accounts of Chemical Research, 2013, 46(5): 1135-1143. |
| 11 | Li G R, Wang S, Zhang Y N, et al. Revisiting the role of polysulfides in lithium-sulfur batteries[J]. Advanced Materials, 2018, 30(22): 1705590. |
| 12 | Kim S J, Kim K, Park J, et al. Role and potential of metal sulfide catalysts in lithium-sulfur battery applications[J]. ChemCatChem, 2019, 11(10): 2373-2387. |
| 13 | Hu Y, Chen W, Lei T Y, et al. Strategies toward high-loading lithium-sulfur battery[J]. Advanced Energy Materials, 2020, 10(17): 2000082. |
| 14 | Nazar L F, Cuisinier M, Pang Q. Lithium-sulfur batteries[J]. MRS Bulletin, 2014, 39(5): 436-442. |
| 15 | Fang R P, Zhao S Y, Sun Z H, et al. More reliable lithium-sulfur batteries: status, solutions and prospects[J]. Advanced Materials, 2017, 29(48): 1606823. |
| 16 | Seh Z W, Sun Y, Zhang Q, et al. Designing high-energy lithium-sulfur batteries[J]. Chemical Society Reviews, 2016, 45(20): 5605-5634. |
| 17 | Kolosnitsyn V S, Karaseva E V. Lithium-sulfur batteries: problems and solutions[J]. Russian Journal of Electrochemistry, 2008, 44(5): 506-509. |
| 18 | Yang X F, Li X, Adair K, et al. Structural design of lithium-sulfur batteries: from fundamental research to practical application[J]. Electrochemical Energy Reviews, 2018, 1(3): 239-293. |
| 19 | Bhargav A, He J, Gupta A, et al. Lithium-sulfur batteries: attaining the critical metrics[J]. Joule, 2020, 4(2): 285-291. |
| 20 | Yan J, Liu X, Li B. Capacity fade analysis of sulfur cathodes in lithium-sulfur batteries[J]. Advanced Science, 2016, 3(12): 1600101. |
| 21 | 李存璞, 王建川, 魏子栋. 电化学反应器隔膜材料的分子设计与介尺度策略[J]. 化工学报, 2020, 71(10): 4490-4501. |
| Li C P, Wang J C, Wei Z D. Mesoscopic strategies and molecular design of diaphragm for electrochemical reactors[J]. CIESC Journal, 2020, 71(10): 4490-4501. | |
| 22 | Chen Y, Wang T Y, Tian H J, et al. Advances in lithium-sulfur batteries: from academic research to commercial viability[J]. Advanced Materials, 2021, 33(29): 2003666. |
| 23 | Zheng C, Niu S, Lv W, et al. Propelling polysulfides transformation for high-rate and long-life lithium-sulfur batteries[J]. Nano Energy, 2017, 33: 306-312. |
| 24 | Li G, Chen Z, Lu J. Lithium-sulfur batteries for commercial applications[J]. Chem, 2018, 4(1): 3-7. |
| 25 | Peng H J, Huang J Q, Cheng X B, et al. Review on high-loading and high-energy lithium-sulfur batteries[J]. Advanced Energy Materials, 2017, 7(24): 1700260. |
| 26 | Zhao M, Li B Q, Chen X, et al. Redox comediation with organopolysulfides in working lithium-sulfur batteries[J]. Chem, 2020, 6(12): 3297-3311. |
| 27 | Liang X, Hart C, Pang Q, et al. A highly efficient polysulfide mediator for lithium-sulfur batteries[J]. Nature communications, 2015, 6(1): 1-8. |
| 28 | Huang L, Li J J, Liu B, et al. Electrode design for lithium-sulfur batteries: problems and solutions[J]. Advanced Functional Materials, 2020, 30(22): 1910375. |
| 29 | 高希雅, 邓子华, 李存璞, 等. 锂硫电池中的催化应用[J]. 化工进展, 2021, 40(9): 5073-5087. |
| Gao X Y, Deng Z H, Li C P, et al. Catalytic application in lithium-sulfur batteries[J]. Chemical Industry and Engineering Progress, 2021, 40(9): 5073-5087. | |
| 30 | Liu D H, Zhang C, Zhou G M, et al. Catalytic effects in lithium-sulfur batteries: promoted sulfur transformation and reduced shuttle effect[J]. Advanced Science, 2018, 5(1): 1700270. |
| 31 | Peres-Benito J F. Iron (Ⅲ)-hydrogen peroxide reaction: kinetic evidence of a hydroxyl-mediated chain mechanism[J]. The Journal of Physical Chemistry A, 2004, 108(22): 4853-4858. |
| 32 | Manthiram A, Fu Y, Chung S H, et al. Rechargeable lithium-sulfur batteries[J]. Chemical Reviews, 2014, 114(23): 11751-11787. |
| 33 | Huang S, Wang Z, Lim Y V, et al. Recent advances in heterostructure engineering for lithium-sulfur batteries[J]. Advanced Energy Materials, 2021, 11(10): 2003689. |
| 34 | Dong Q, Shen R P, Li C P, et al. Construction of soft base tongs on separator to grasp polysulfides from shuttling in lithium-sulfur batteries[J]. Small, 2018, 14(52): 1804277. |
| 35 | Wang H L, Yang Y, Liang Y Y, et al. Graphene-wrapped sulfur particles as a rechargeable lithium-sulfur battery cathode material with high capacity and cycling stability[J]. Nano Letters, 2011, 11(7): 2644-2647. |
| 36 | Xu G Y, Ding B, Pan J, et al. High performance lithium-sulfur batteries: advances and challenges[J]. Journal of Materials Chemistry A, 2014, 2(32): 12662-12676. |
| 37 | Wang P, Xi B J, Huang M, et al. Emerging catalysts to promote kinetics of lithium-sulfur batteries[J]. Advanced Energy Materials, 2021, 11(7): 2002893. |
| 38 | Lin Z, Liu Z C, Fu W J, et al. Lithium polysulfidophosphates: a family of lithium-conducting sulfur-rich compounds for lithium-sulfur batteries[J]. Angewandte Chemie International Edition, 2013, 52(29): 7460-7463. |
| 39 | Zhang X, Xie H, Kim C S, et al. Advances in lithium-sulfur batteries[J]. Materials Science and Engineering R: Reports, 2017, 121: 1-29. |
| 40 | Hou T Z, Xu W T, Chen X, et al. Lithium bond chemistry in lithium-sulfur batteries[J]. Angewandte Chemie International Edition, 2017, 129(28): 8290-8294. |
| 41 | Yin Y X, Xin S, Guo Y G, et al. Lithium-sulfur batteries: electrochemistry, materials, and prospects[J]. Angewandte Chemie International Edition, 2013, 52(50): 13186-13200. |
| 42 | Fan X J, Sun W W, Meng F C, et al. Advanced chemical strategies for lithium-sulfur batteries: a review[J]. Green Energy & Environment, 2018, 3(1): 2-19. |
| 43 | Cao J, Chen C, Zhao Q, et al. A flexible nanostructured paper of a reduced graphene oxide-sulfur composite for high-performance lithium-sulfur batteries with unconventional configurations[J]. Advanced Materials, 2016, 28(43): 9629-9636. |
| 44 | Zhou G, Paek E, Hwang G S, et al. Long-life Li/polysulphide batteries with high sulphur loading enabled by lightweight three-dimensional nitrogen/sulphur-codoped graphene sponge[J]. Nature Communications, 2015, 6(1): 1-11. |
| 45 | Li Z, Zhang J T, Chen Y M, et al. Pie-like electrode design for high-energy density lithium-sulfur batteries[J]. Nature Communications, 2015, 6(1): 1-8. |
| 46 | Chen H, Hong H F, Zhang X, et al. Integration of porous graphitic carbon and carbon fiber framework for ultrahigh sulfur-loading lithium-sulfur battery[J]. Dalton Transactions, 2022, 51(8): 3357-3365. |
| 47 | Cao D X, Jiao Y C, Cai Q F, et al. Stable lithium-sulfur full cells enabled by dual functional and interconnected mesocarbon arrays[J]. Journal of Materials Chemistry A, 2019, 7(7): 3289-3297. |
| 48 | Wu Y, Wang C, Yang Z W, et al. Designing conductive networks of hybrid carbon enables stable and long-lifespan cotton-fiber-based lithium-sulfur batteries[J]. RSC Advances, 2021, 11(55): 34955-34962. |
| 49 | Shen Z H, Jin X, Tian J M, et al. Cation-doped ZnS catalysts for polysulfide conversion in lithium-sulfur batteries[J]. Nature Catalysis, 2022, 5(6): 555-563. |
| 50 | Yang S, Xiao R, Hu T Z, et al. Ni2P electrocatalysts decorated hollow carbon spheres as bi-functional mediator against shuttle effect and Li dendrite for Li-S batteries[J]. Nano Energy, 2021, 90: 106584. |
| 51 | Xiao R, Yu T, Yang S, et al. Electronic structure adjustment of lithium sulfide by a single-atom copper catalyst toward high-rate lithium-sulfur batteries[J]. Energy Storage Materials, 2022, 51: 890-899. |
| 52 | Yan B, Li Y, Gao L, et al. Confining ZnS/SnS2 ultrathin heterostructured nanosheets in hollow N-doped carbon nanocubes as novel sulfur host for advanced Li-S batteries[J]. Small, 2022, 18(24): e2107727. |
| 53 | Yan L, Zhang Z X, Yu F, et al. Rational design of NiCo2S4@MoS2 ball-in-ball heterostructure nanospheres for advanced lithium-sulfur batteries[J]. Electrochimica Acta, 2021, 383: 138268. |
| 54 | Han P, Chung S H, Manthiram A. Pyrrolic-type nitrogen-doped hierarchical macro/mesoporous carbon as a bifunctional host for high-performance thick cathodes for lithium-sulfur batteries[J]. Small, 2019, 15(16): 1900690. |
| 55 | Cheng D D, Zhao Y L, An T, et al. 3D interconnected crumpled porous carbon sheets modified with high-level nitrogen doping for high performance lithium sulfur batteries[J]. Carbon, 2019, 154: 58-66. |
| 56 | Liu J, Wei A X, Pan G X, et al. Atomic layer deposition-assisted construction of binder-free Ni@N-doped carbon nanospheres films as advanced host for sulfur cathode[J]. Nano-Micro Letters, 2019, 11(1): 1-14. |
| 57 | Li Z, Zhou H Y, Zhao F L, et al. Three-dimensional covalent organic frameworks as host materials for lithium-sulfur batteries[J]. Chinese Journal of Polymer Science, 2020, 38(5): 550-557. |
| 58 | Du B W, Luo Y H, Yang Y Q, et al. COFs-confined multifunctional sulfur-host design towards high-performance lithium-sulfur batteries[J]. Chemical Engineering Journal, 2022, 442: 135823. |
| 59 | Zhu F L, Tao Y L, Bao H F, et al. Ferroelectric metal-organic framework as a host material for sulfur to alleviate the shuttle effect of lithium-sulfur battery[J]. Chemistry-A European Journal, 2020, 26(61): 13779-13782. |
| 60 | Fan X L, Liu Y Q, Tan J Y, et al. An ultrathin and highly efficient interlayer for lithium-sulfur batteries with high sulfur loading and lean electrolyte[J]. Journal of Materials Chemistry A, 2022, 10(14): 7653-7659. |
| 61 | Xiao R, Yang S, Yu T, et al. A janus separator for inhibiting shuttle effect and lithium dendrite in lithium-sulfur batteries[J]. Batteries & Supercaps, 2022, 5(4): e202100389. |
| 62 | Dong Q, Wang T, Gan R Y, et al. Balancing the seesaw: investigation of a separator to grasp polysulfides with diatomic chemisorption[J]. ACS Applied Materials & Interfaces, 2020, 12(18): 20596-20604. |
| 63 | Xu J, Bi S M, Tang W Q, et al. Duplex trapping and charge transfer with polysulfides by a diketopyrrolopyrrole-based organic framework for high-performance lithium-sulfur batteries[J]. Journal of Materials Chemistry A, 2019, 7(30): 18100-18108. |
| 64 | Yang L W, Wang Y, Li Q, et al. Inhibition of the shuttle effect of lithium-sulfur batteries via a tannic acid-metal one-step in situ chemical film-forming modified separator[J]. Nanoscale, 2021, 13(9): 5058-5068. |
| 65 | Wei Z Z, Zhang N X, Feng T, et al. A copolymer microspheres-coated separator to enhance thermal stability of lithium-sulfur batteries[J]. Chemical Engineering Journal, 2022, 430: 132678. |
| 66 | Wang P, Zhang Z A, Hong B, et al. Multifunctional porous VN nanowires interlayer as polysulfides barrier for high performance lithium sulfur batteries[J]. Journal of Electroanalytical Chemistry, 2019, 832: 475-479. |
| 67 | Song N, Xi B J, Wang P, et al. Immobilizing VN ultrafine nanocrystals on N-doped carbon nanosheets enable multiple effects for high-rate lithium-sulfur batteries[J]. Nano Research, 2022, 15(2): 1424-1432. |
| 68 | Xu J, Yang L K, Cao S F, et al. Sandwiched cathodes assembled from CoS2-modified carbon clothes for high-performance lithium-sulfur batteries[J]. Advanced Science, 2021, 8(16): 2101019. |
| 69 | Liang X, Kwok C Y, Lodi-Marzano F, et al. Tuning transition metal oxide-sulfur interactions for long life lithium sulfur batteries: the "Goldilocks" principle[J]. Advanced Energy Materials, 2016, 6(6): 1501636. |
| 70 | Li S, Cen Y, Xiang Q, et al. Vanadium dioxide-reduced graphene oxide binary host as an efficient polysulfide plague for high-performance lithium-sulfur batteries[J]. Journal of Materials Chemistry A, 2019, 7(4): 1658-1668. |
| 71 | Patil S B, Kim H J, Lim H K, et al. Exfoliated 2D lepidocrocite titanium oxide nanosheets for high sulfur content cathodes with highly stable Li-S battery performance[J]. ACS Energy Letters, 2018, 3(2): 412-419. |
| 72 | Li Q, Ma Z P, Li J J, et al. Core-shell-structured sulfur cathode: ultrathin δ-MnO2 nanosheets as the catalytic conversion shell for lithium polysulfides in high sulfur content lithium-sulfur batteries[J]. ACS Applied Materials & Interfaces, 2020, 12(31): 35049-35057. |
| 73 | Chen S J, Zhang J X, Wang Z Y, et al. Electrocatalytic NiCo2O4 nanofiber arrays on carbon cloth for flexible and high-loading lithium-sulfur batteries[J]. Nano Letters, 2021, 21(12): 5285-5292. |
| 74 | Zhou J, Lin N, long Cai W, et al. Synthesis of S/CoS2 nanoparticles-embedded N-doped carbon polyhedrons from polyhedrons ZIF-67 and their properties in lithium-sulfur batteries[J]. Electrochimica Acta, 2016, 218: 243-251. |
| 75 | Zhou N, Dong W D, Zhang Y J, et al. Embedding tin disulfide nanoparticles in two-dimensional porous carbon nanosheet interlayers for fast-charging lithium-sulfur batteries[J]. Science China Materials, 2021, 64(11): 2697-2709. |
| 76 | Li S, Xu P, Aslam M K, et al. Propelling polysulfide conversion for high-loading lithium-sulfur batteries through highly sulfiphilic NiCo2S4 nanotubes[J]. Energy Storage Materials, 2020, 27: 51-60. |
| 77 | Wang H X, Wei D, Zheng J C, et al. Electrospinning MoS2-decorated porous carbon nanofibers for high-performance lithium-sulfur batteries[J]. ACS Applied Energy Materials, 2020, 3(12): 11893-11899. |
| 78 | Mosavati N, Salley S O, Ng K Y S. Characterization and electrochemical activities of nanostructured transition metal nitrides as cathode materials for lithium sulfur batteries[J]. Journal of Power Sources, 2017, 340: 210-216. |
| 79 | Li C C, Shi J J, Zhu L, et al. Titanium nitride hollow nanospheres with strong lithium polysulfide chemisorption as sulfur hosts for advanced lithium-sulfur batteries[J]. Nano Research, 2018, 11(8): 4302-4312. |
| 80 | Gan R Y, Yang N, Dong Q, et al. Enveloping ultrathin Ti3C2 nanosheets on carbon fibers: a high-density sulfur loaded lithium-sulfur battery cathode with remarkable cycling stability[J]. Journal of Materials Chemistry A, 2020, 8(15): 7253-7260. |
| 81 | Yang D W, Zhang C Q, Biendicho J J, et al. ZnSe/N-doped carbon nanoreactor with multiple adsorption sites for stable lithium-sulfur batteries[J]. ACS Nano, 2020, 14(11): 15492-15504. |
| 82 | Kong L, Chen X, Li B Q, et al. A bifunctional perovskite promoter for polysulfide regulation toward stable lithium-sulfur batteries[J]. Advanced Materials, 2018, 30(2): 1705219. |
| 83 | Yuan H, Peng H J, Li B Q, et al. Conductive and catalytic triple-phase interfaces enabling uniform nucleation in high-rate lithium-sulfur batteries[J]. Advanced Energy Materials, 2019, 9(1): 1802768. |
| 84 | Wu D S, Shi F, Zhou G, et al. Quantitative investigation of polysulfide adsorption capability of candidate materials for Li-S batteries[J]. Energy Storage Materials, 2018, 13: 241-246. |
| 85 | Li J B, Qu Y R, Chen C Y, et al. Theoretical investigation on lithium polysulfide adsorption and conversion for high-performance Li-S batteries[J]. Nanoscale, 2021, 13(1): 15-35. |
| 86 | Zhu Y J, Wang C S. Galvanostatic intermittent titration technique for phase-transformation electrodes[J]. The Journal of Physical Chemistry C, 2010, 114(6): 2830-2841. |
| 87 | Dees D W, Kawauchi S, Abraham D P, et al. Analysis of the galvanostatic intermittent titration technique (GITT) as applied to a lithium-ion porous electrode[J]. Journal of Power Sources, 2009, 189(1): 263-268. |
| 88 | Yang B, Jiang H R, Zhou Y C, et al. Critical role of anion donicity in Li2S deposition and sulfur utilization in Li-S batteries[J]. ACS Applied Materials & Interfaces, 2019, 11(29): 25940-25948. |
| 89 | Cai D Q, Yang J L, Liu T, et al. Interfaces-dominated Li2S nucleation behavior enabled by heterostructure catalyst for fast kinetics Li-S batteries[J]. Nano Energy, 2021, 89: 106452. |
| 90 | Yang J L, Cai D Q, Hao X G, et al. Rich heterointerfaces enabling rapid polysulfides conversion and regulated Li2S deposition for high-performance lithium-sulfur batteries[J]. ACS Nano, 2021, 15(7): 11491-11500. |
| 91 | Xu R, Tang H A, Zhou Y Y, et al. Enhanced catalysis of LiS3· radical-to-polysulfide interconversion via increased sulfur vacancies in lithium-sulfur batteries[J]. Chemical Science, 2022, 13(21): 6224-6232. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 金正浩, 封立杰, 李舒宏. 氨水溶液交叉型再吸收式热泵的能量及 分析[J]. 化工学报, 2023, 74(S1): 53-63. 分析[J]. 化工学报, 2023, 74(S1): 53-63. |
| [3] | 程成, 段钟弟, 孙浩然, 胡海涛, 薛鸿祥. 表面微结构对析晶沉积特性影响的格子Boltzmann模拟[J]. 化工学报, 2023, 74(S1): 74-86. |
| [4] | 肖明堃, 杨光, 黄永华, 吴静怡. 浸没孔液氧气泡动力学数值研究[J]. 化工学报, 2023, 74(S1): 87-95. |
| [5] | 毕丽森, 刘斌, 胡恒祥, 曾涛, 李卓睿, 宋健飞, 吴翰铭. 粗糙界面上纳米液滴蒸发模式的分子动力学研究[J]. 化工学报, 2023, 74(S1): 172-178. |
| [6] | 于宏鑫, 邵双全. 水结晶过程的分子动力学模拟分析[J]. 化工学报, 2023, 74(S1): 250-258. |
| [7] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [8] | 吴雷, 刘姣, 李长聪, 周军, 叶干, 刘田田, 朱瑞玉, 张秋利, 宋永辉. 低阶粉煤催化微波热解制备含碳纳米管的高附加值改性兰炭末[J]. 化工学报, 2023, 74(9): 3956-3967. |
| [9] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [10] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [11] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [12] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [13] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [14] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| [15] | 杨越, 张丹, 郑巨淦, 涂茂萍, 杨庆忠. NaCl水溶液喷射闪蒸-掺混蒸发的实验研究[J]. 化工学报, 2023, 74(8): 3279-3291. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号