化工学报 ›› 2020, Vol. 71 ›› Issue (10): 4642-4651.DOI: 10.11949/0438-1157.20200609
黄守莹( ),熊雄,贺培,王建豪,李媖,刘宏开,吕静,马新宾(
),熊雄,贺培,王建豪,李媖,刘宏开,吕静,马新宾( )
)
收稿日期:2020-05-18
修回日期:2020-07-15
出版日期:2020-10-05
发布日期:2020-10-05
通讯作者:
马新宾
作者简介:黄守莹(1985—),女,博士,副教授,基金资助:
Shouying HUANG( ),Xiong XIONG,Pei HE,Jianhao WANG,Ying LI,Hongkai LIU,Jing LYU,Xinbin MA(
),Xiong XIONG,Pei HE,Jianhao WANG,Ying LI,Hongkai LIU,Jing LYU,Xinbin MA( )
)
Received:2020-05-18
Revised:2020-07-15
Online:2020-10-05
Published:2020-10-05
Contact:
Xinbin MA
摘要:
二甲醚(DME)羰基化制乙酸甲酯(MA),MA加氢合成乙醇工艺是一种新颖、绿色且经济的乙醇合成路线,催化剂成型对实现该工艺工业化具有重要意义。本论文选用拟薄水铝石及硅溶胶为黏结剂,对丝光沸石分子筛(MOR)进行挤条成型,制备了一系列不同黏结剂种类与含量的成型MOR催化剂。通过强度测试以及Weibull函数统计分析,探究了黏结剂对成型MOR的强度以及强度可靠性的影响;X射线衍射、N2物理吸附、NH3程序升温脱附、吡啶吸附原位红外等表征结合活性评价,探究了黏结剂对MOR的物理结构、酸性以及催化性能的作用。结果表明:黏结剂的引入不会影响MOR的晶体结构,且以拟薄水铝石为黏结剂时,催化剂力学性能及DME羰基化催化性能最佳。通过建立MA时空收率、TOF与微孔比表面积之间的定量关系,确定黏结剂不影响MOR内单位活性位点催化能力,产物时空收率与催化剂微孔比表面积呈线性正相关。
中图分类号:
黄守莹, 熊雄, 贺培, 王建豪, 李媖, 刘宏开, 吕静, 马新宾. 二甲醚羰基化丝光沸石成型催化剂黏结剂的研究[J]. 化工学报, 2020, 71(10): 4642-4651.
Shouying HUANG, Xiong XIONG, Pei HE, Jianhao WANG, Ying LI, Hongkai LIU, Jing LYU, Xinbin MA. Study on binder of extruded mordenite catalyst for dimethyl ether carbonylation[J]. CIESC Journal, 2020, 71(10): 4642-4651.
| MOR质量/g | 黏结剂种类 | 黏结剂质量/g | 去离子水质量/g | 硝酸质量/g | 命名 |
|---|---|---|---|---|---|
| 10 | 无 | 0 | 0 | 0 | MOR-P |
| 9 | 拟薄水铝石 | 1 | 6 | 0.125 | 10NI |
| 8 | 拟薄水铝石 | 2 | 6 | 0.125 | 20NI |
| 9 | 硅溶胶 | 1 | 6 | 0.125 | 10Si |
| 8 | 硅溶胶 | 2 | 6 | 0.125 | 20Si |
| 8 | 拟薄水铝石+硅溶胶 | 2 | 6 | 0.125 | 10NI10Si |
表1 催化剂配方及命名
Table 1 Formula and nomenclature for catalyst
| MOR质量/g | 黏结剂种类 | 黏结剂质量/g | 去离子水质量/g | 硝酸质量/g | 命名 |
|---|---|---|---|---|---|
| 10 | 无 | 0 | 0 | 0 | MOR-P |
| 9 | 拟薄水铝石 | 1 | 6 | 0.125 | 10NI |
| 8 | 拟薄水铝石 | 2 | 6 | 0.125 | 20NI |
| 9 | 硅溶胶 | 1 | 6 | 0.125 | 10Si |
| 8 | 硅溶胶 | 2 | 6 | 0.125 | 20Si |
| 8 | 拟薄水铝石+硅溶胶 | 2 | 6 | 0.125 | 10NI10Si |
| 样品 | F/(N/cm) | F0/(N/cm) | m | F10% |
|---|---|---|---|---|
| 10NI | 83 | 93 | 2.80 | 35 |
| 20NI | 177 | 197 | 3.21 | 103 |
| 10Si | 47 | 52 | 2.84 | 22 |
| 20Si | 105 | 117 | 3.28 | 47 |
| 10NI10Si | 142 | 158 | 3.99 | 80 |
表2 成型MOR催化剂的强度数据统计
Table 2 Statistics of strength data for the extruded MOR
| 样品 | F/(N/cm) | F0/(N/cm) | m | F10% |
|---|---|---|---|---|
| 10NI | 83 | 93 | 2.80 | 35 |
| 20NI | 177 | 197 | 3.21 | 103 |
| 10Si | 47 | 52 | 2.84 | 22 |
| 20Si | 105 | 117 | 3.28 | 47 |
| 10NI10Si | 142 | 158 | 3.99 | 80 |
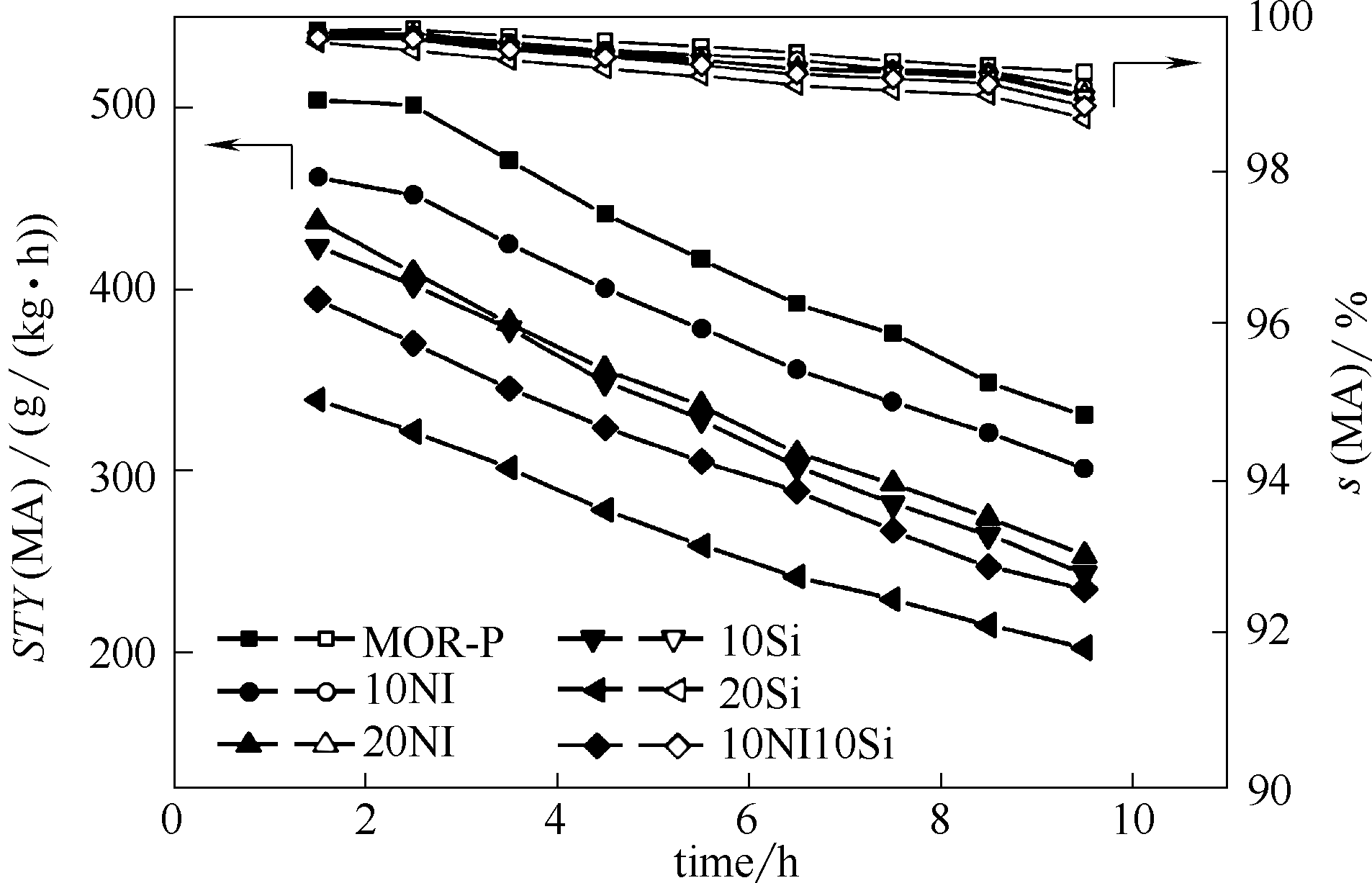
图3 不同成型催化剂及未成型分子筛DME羰基化催化性能(反应条件:温度200℃,总压1.5 MPa, 混合气比例DME∶CO∶Ar=1∶47∶2,空速6000 h-1)
Fig. 3 Catalytic performance of parent and extruded catalyst in DME carbonylation (Reaction conditions: T=200℃, p=1.5 MPa, DME∶CO∶Ar=1∶47∶2, GHSV=6000 h-1)
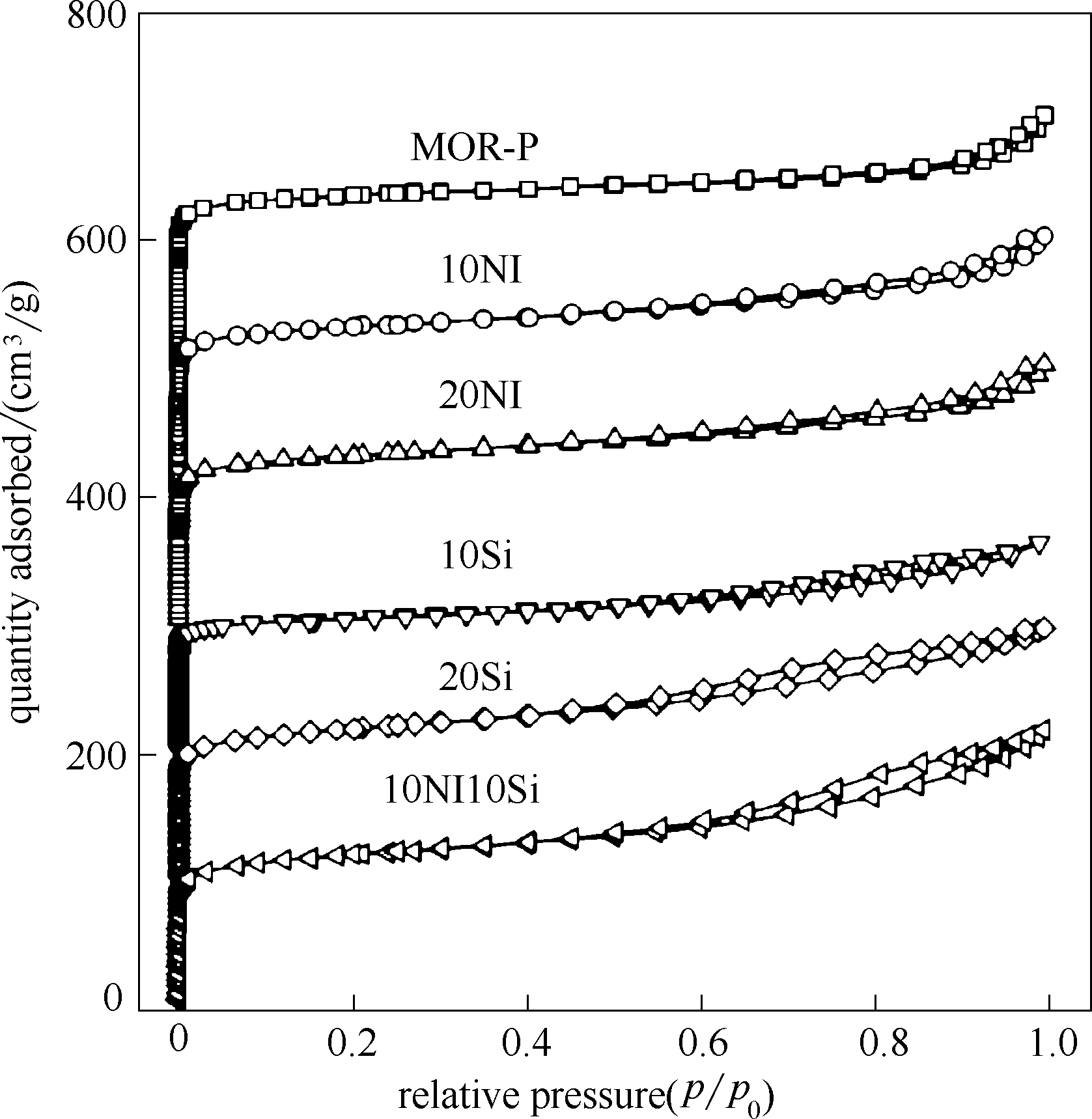
图5 成型催化剂与未成型MOR的N2吸脱附等温线(除10NI10Si样品,其余吸脱附等温线依次向上平移100 cm3/g)
Fig.5 Nitrogen adsorption-desorption isotherms of the parent and extruded MOR (except for 10NI10Si, the isotherms for other samples are shifted 100 cm3/g upward respect to each other)
| 样品 | SBET/ (m2/g) | Smicro/ (m2/g) | Smeso/ (m2/g) | Vmicro/ (cm3/g) | Vmeso/(cm3/g) | Dmeso/nm |
|---|---|---|---|---|---|---|
| MOR-P | 462 | 431 | 31 | 0.159 | 0.109 | 8.1 |
| 10NI | 449 | 399 | 50 | 0.145 | 0.126 | 5.6 |
| 20NI | 449 | 385 | 74 | 0.141 | 0.136 | 5.7 |
| 10Si | 435 | 365 | 70 | 0.132 | 0.132 | 4.2 |
| 20Si | 398 | 299 | 99 | 0.109 | 0.154 | 4.4 |
| 10NI10Si | 435 | 338 | 97 | 0.116 | 0.159 | 5.0 |
| NI① | 283 | — | 283 | — | 0.296 | 6.0 |
| Si② | 331 | — | 331 | — | 0.411 | 4.1 |
表3 成型催化剂与未成型MOR催化剂的物理结构性质
Table 3 Textural properties of parent and extruded MOR
| 样品 | SBET/ (m2/g) | Smicro/ (m2/g) | Smeso/ (m2/g) | Vmicro/ (cm3/g) | Vmeso/(cm3/g) | Dmeso/nm |
|---|---|---|---|---|---|---|
| MOR-P | 462 | 431 | 31 | 0.159 | 0.109 | 8.1 |
| 10NI | 449 | 399 | 50 | 0.145 | 0.126 | 5.6 |
| 20NI | 449 | 385 | 74 | 0.141 | 0.136 | 5.7 |
| 10Si | 435 | 365 | 70 | 0.132 | 0.132 | 4.2 |
| 20Si | 398 | 299 | 99 | 0.109 | 0.154 | 4.4 |
| 10NI10Si | 435 | 338 | 97 | 0.116 | 0.159 | 5.0 |
| NI① | 283 | — | 283 | — | 0.296 | 6.0 |
| Si② | 331 | — | 331 | — | 0.411 | 4.1 |
| 样品 | 弱酸峰/℃ | 酸含量/(μmol/g) | 中强酸峰/℃ | 酸含量/(μmol/g) | 强酸峰/℃ | 酸含量/(μmol/g) |
|---|---|---|---|---|---|---|
| MOR-P | 209.7 | 255 | 331.1 | 262 | 535.1 | 1110 |
| 10NI | 213.4 | 233 | 380.4 | 288 | 537.3 | 1007 |
| 20NI | 214.0 | 234 | 378.2 | 317 | 538.1 | 950 |
| 10Si | 211.5 | 217 | 340.2 | 215 | 530.2 | 903 |
| 20Si | 212.0 | 180 | 341.2 | 176 | 531.2 | 739 |
| 10NI10Si | 213.0 | 247 | 350.6 | 227 | 533.4 | 864 |
表4 NH3-TPD测试及分峰结果
Table 4 The de-convolution results of NH3-TPD profiles
| 样品 | 弱酸峰/℃ | 酸含量/(μmol/g) | 中强酸峰/℃ | 酸含量/(μmol/g) | 强酸峰/℃ | 酸含量/(μmol/g) |
|---|---|---|---|---|---|---|
| MOR-P | 209.7 | 255 | 331.1 | 262 | 535.1 | 1110 |
| 10NI | 213.4 | 233 | 380.4 | 288 | 537.3 | 1007 |
| 20NI | 214.0 | 234 | 378.2 | 317 | 538.1 | 950 |
| 10Si | 211.5 | 217 | 340.2 | 215 | 530.2 | 903 |
| 20Si | 212.0 | 180 | 341.2 | 176 | 531.2 | 739 |
| 10NI10Si | 213.0 | 247 | 350.6 | 227 | 533.4 | 864 |
| 样品 | Btotal/(μmol/g) | B12-MR/(μmol/g) | L12-MR/(μmol/g) | B8-MR/(μmol/g) | B8-MR/B12-MR |
|---|---|---|---|---|---|
| MOR-P | 1110 | 351 | 47 | 759 | 2.2 |
| 10NI | 1007 | 307 | 73 | 700 | 2.3 |
| 20NI | 950 | 292 | 91 | 658 | 2.3 |
| 10Si | 903 | 294 | 45 | 609 | 2.1 |
| 20Si | 739 | 241 | 41 | 498 | 2.1 |
| 10NI10Si | 864 | 279 | 63 | 585 | 2.1 |
表5 成型催化剂及未成型MOR酸量
Table 5 The amounts of acid for parent and extruded MOR
| 样品 | Btotal/(μmol/g) | B12-MR/(μmol/g) | L12-MR/(μmol/g) | B8-MR/(μmol/g) | B8-MR/B12-MR |
|---|---|---|---|---|---|
| MOR-P | 1110 | 351 | 47 | 759 | 2.2 |
| 10NI | 1007 | 307 | 73 | 700 | 2.3 |
| 20NI | 950 | 292 | 91 | 658 | 2.3 |
| 10Si | 903 | 294 | 45 | 609 | 2.1 |
| 20Si | 739 | 241 | 41 | 498 | 2.1 |
| 10NI10Si | 864 | 279 | 63 | 585 | 2.1 |
| 样品 | Smicro/ (m2/g) | B8-MR/ (μmol/g) | STY(MA)/ (g/(kg·h)) | TOF/h-1 |
|---|---|---|---|---|
| MOR-P | 431 | 759 | 504 | 9.1 |
| 10NI | 399 | 700 | 469 | 9.0 |
| 20NI | 385 | 658 | 437 | 9.0 |
| 10Si | 365 | 609 | 414 | 9.1 |
| 20Si | 299 | 498 | 340 | 9.1 |
| 10NI10Si | 338 | 585 | 395 | 9.1 |
表6 成型催化剂及未成型分子筛微孔比表面、8元环Br?nsted酸量及催化性能汇总
Table 6 The specific surface area (Smicro), B8-MR and catalytic performance of the parent and extruded MOR
| 样品 | Smicro/ (m2/g) | B8-MR/ (μmol/g) | STY(MA)/ (g/(kg·h)) | TOF/h-1 |
|---|---|---|---|---|
| MOR-P | 431 | 759 | 504 | 9.1 |
| 10NI | 399 | 700 | 469 | 9.0 |
| 20NI | 385 | 658 | 437 | 9.0 |
| 10Si | 365 | 609 | 414 | 9.1 |
| 20Si | 299 | 498 | 340 | 9.1 |
| 10NI10Si | 338 | 585 | 395 | 9.1 |
| 1 | Goldemberg J. Ethanol for a sustainable energy future[J]. Science, 2007, 315(5813): 808-810. |
| 2 | Li Y, Huang S Y, Cheng Z Z, et al. Promoting the activity of Ce-incorporated MOR in dimethyl ether carbonylation through tailoring the distribution of Brønsted acids[J]. Applied Catalysis B: Environmental, 2019, 256: 117777-117788. |
| 3 | 李振宇, 黄格省, 杨延翔, 等. 燃料乙醇生产技术路线分析及产业发展建议[J]. 现代化工, 2011, 31(8): 1-5. |
| Li Z Y, Huang G S, Yang Y X, et al. Technical route analysis of fuel ethanol production and industrial development suggestions[J]. Modern Chemical Industry, 2011, 31(8): 1-5. | |
| 4 | Zeng C Y, Hu Q L. 2018 petroleum & chemical industry development report[J]. Chinese Journal of Chemical Engineering, 2019, 27: 2606-2614. |
| 5 | 黄守莹, 王悦, 吕静, 等. 合成气经二甲醚/乙酸甲酯制无水乙醇的研究进展[J]. 化工学报, 2016, 67(1): 240-247. |
| Huang S Y, Wang Y, Lyu J, et al. Advances in indirect synthesis of ethanol from syngas via dimethyl ether/methyl acetate[J]. CIESC Journal, 2016, 67(1): 240-247. | |
| 6 | Yang G H, Meng F Z, Tsubaki N, et al. A new method of ethanol synthesis from dimethyl ether and syngas in a sequential dual bed reactor with the modified zeolite and Cu/ZnO catalysts[J]. Catalysis Today, 2011, 164(1): 425-428. |
| 7 | Cheung P, Bhan A, Sunley G J, et al. Site requirements and elementary steps in dimethyl ether carbonylation catalyzed by acidic zeolites[J]. Journal of Catalysis, 2007, 245(1): 110-123. |
| 8 | Bhan A, Iglesia E. A link between reactivity and local structure in acid catalysis on zeolites[J]. Accounts of Chemical Research, 2008, 41(4): 559-567. |
| 9 | Bhan A, Allian A D, Sunley G J, et al. Specificity of sites within eight-membered ring zeolite channels for carbonylation of methyls to acetyls[J]. Journal of the American Chemical Society, 2007, 129(16): 4919-4924. |
| 10 | Boronat M, Sánchez C M, Law D, et al. Enzyme-like specificity in zeolites: a unique site position in mordenite for selective carbonylation of methanol and dimethyl ether with CO[J]. Journal of the American Chemical Society, 2008, 130(48): 16316-16323. |
| 11 | Li B J, Wang C, Han B, et al. Insight into dimethyl ether carbonylation reaction over mordenite zeolite from in-situ solid-state NMR spectroscopy[J]. The Journal of Physical Chemistry C, 2013, 117(11): 5840-5847. |
| 12 | Reule A A, Sawada J A, Semagina N. Effect of selective 4-membered ring dealumination on mordenite-catalyzed dimethyl ether carbonylation[J]. Journal of Catalysis, 2017, 349: 98-109. |
| 13 | Wang X S, Li R J, Yu C C, et al. Influence of acid site distribution on dimethyl ether carbonylation over mordenite[J]. Industrial & Engineering Chemistry Research, 2019, 58(39): 18065-18072. |
| 14 | Wang M X, Huang S Y, Lv J, et al. Modifying the acidity of H-MOR and its catalytic carbonylation of dimethyl ether[J]. Chinese Journal of Catalysis, 2016, 37(9): 1530-1537. |
| 15 | Li Y, Yu M, Cai K, et al. Template-induced Al distribution in MOR and enhanced activity in dimethyl ether carbonylation[J]. Physical Chemistry Chemical Physics, 2020, 22: 11374-11381. |
| 16 | Liu J L, Xue H F, Huang X M, et al. Stability enhanacement of H-mordenite in dimethyl ether carbonylation to methyl acetate by pre-adsorption of pyridine[J]. Chinese Journal of Catalysis, 2010, 31(7): 729-738. |
| 17 | Ma M, Huang X M, Zhang E S, et al. Synthesis of mordenite nanosheets with shortened channel lengths and enhanced catalytic activity[J]. Journal of Materials Chemistry A, 2017, 5: 8887-8891. |
| 18 | Wang X S, Li R J, Yu C C, et al. Enhancing the dimethyl ether carbonylation performance over mordenite catalysts by simple alkaline treatment[J]. Fuel, 2019, 239: 794-893. |
| 19 | de Jong K P. Synthesis of Solid Catalyst[M]. Winheim: Wiley-VCH Verlag GmbH & Co., 2009. |
| 20 | Li L Y, Wang Q Y, Liu H C, et al. Preparation of spherical mordenite zeolite assemblies with excellent catalytic performance for dimethyl ether carbonylation[J]. ACS Applied Materials & Interfaces, 2018, 10(38): 32239-32246. |
| 21 | Jasra R A, Tyagi B, Badheka Y M, et al. Effect of clay binder on sorption and catalytic properties of zeolite pellets[J]. Industrial & Engineering Chemistry Research, 2003, 42(14): 3263-3272. |
| 22 | Lucas A D, Valverde J L, Sanchez P, et al. Influence of the binder on the n-octane hydroisomerization over palladium-containing zeolite catalysts[J]. Industrial & Engineering Chemistry Research, 2004, 43(26): 8217-8225. |
| 23 | Shen X L, Zhou Y M, Kong J, et al. Influence of pseudo-boehmite binder modified dealuminated mordenite on Friedel-Crafts alkylation[J]. Journal of Porous Materials, 2015, 22: 179-185. |
| 24 | Li Y, Sun Q, Huang S Y, et al. Dimethyl ether carbonylation over pyridine-modified MOR: enhanced stability influenced by acidity[J]. Catalysis Today, 2018, 311: 81-88. |
| 25 | Cheng Z Z, Huang S Y, Li Y, et al. Deactivation kinetics for the carbonylation of dimethyl ether to methyl acetate on H-MOR[J]. Industrial & Engineering Chemistry Research, 2017, 56(46): 13618-13627. |
| 26 | Li Y D, Wu D F, Zhang J P, et al. Measurement and statistics of single pellet mechanical strength of differently shaped catalysts[J]. Powder Technology, 2000, 113(1/2): 176-184. |
| 27 | Deng S W, Wang Y B, Zhuang G L, et al. Micromechanical simulation of the pore size effect on the structural stability of brittle porous materials with bicontinuous morphology[J]. Physical Chemistry Chemical Physics, 2019, 21: 12895-12904. |
| 28 | Liu G Z, Guo J H, Meng F X, et al. Effects of colloidal silica binder on catalytic activity and adhesion of HZSM-5 coatings for structured reactors[J]. Chinese Journal of Chemical Engineering, 2014, 22(8): 875-881. |
| 29 | Whiting G T, Chung S H, Stosic D, et al. Multiscale mechanistic insights of shaped catalyst body formulations and their impact on catalytic properties[J]. ACS Catalysis, 2019, 9(6): 4792-4803. |
| 30 | Liu Y H, Zhao N, Xian H, et al. Facilely synthesized H-mordenite nanosheet assembly for carbonylation of dimethyl ether[J]. Applied Materials & Interfaces, 2015, 7(16): 8398-8403. |
| 31 | Osman A I, Dahrieh J K, Rooney D W, et al. Surface hydrophobicity and acidity effect on alumina catalyst in catalytic methanol dehydration reaction[J]. Journal of Chemical Technology & Biotechnology, 2017, 92(12): 2952-2962. |
| 32 | Zhou W W, Zhang Y N, Tao X J, et al. Effects of gallium addition to mesoporous alumina by impregnation on dibenzothiophene hydrodesulfurization performances of the corresponding NiMo supported catalysts[J]. Fuel, 2018, 228: 152-163. |
| 33 | Emeis C A. Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalyst[J]. Journal of Catalyst, 1993, 141: 347-354. |
| 34 | Garderen N V, Clemens F J, Aneziris C G, et al. Improved γ-alumina support based pseudo-boehmite shaped by micro-extrusion process for oxygen carrier support application[J]. Ceramics International, 2012, 38(7): 5481-5492. |
| [1] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [2] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [3] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [4] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [5] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [6] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [7] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [8] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| [9] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| [10] | 涂玉明, 邵高燕, 陈健杰, 刘凤, 田世超, 周智勇, 任钟旗. 钙基催化剂的设计合成及应用研究进展[J]. 化工学报, 2023, 74(7): 2717-2734. |
| [11] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [12] | 张希庆, 王琰婷, 徐彦红, 常淑玲, 孙婷婷, 薛定, 张立红. Mg量影响的纳米片负载Pt-In催化异丁烷脱氢性能[J]. 化工学报, 2023, 74(6): 2427-2435. |
| [13] | 张谭, 刘光, 李晋平, 孙予罕. Ru基氮还原电催化剂性能调控策略[J]. 化工学报, 2023, 74(6): 2264-2280. |
| [14] | 韩奎奎, 谭湘龙, 李金芝, 杨婷, 张春, 张永汾, 刘洪全, 于中伟, 顾学红. 四通道中空纤维MFI分子筛膜用于二甲苯异构体分离[J]. 化工学报, 2023, 74(6): 2468-2476. |
| [15] | 朱风, 陈凯琳, 黄小凤, 鲍银珠, 李文斌, 刘嘉鑫, 吴玮强, 高王伟. KOH改性电石渣脱除羰基硫的性能研究[J]. 化工学报, 2023, 74(6): 2668-2679. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号