化工学报 ›› 2020, Vol. 71 ›› Issue (10): 4652-4662.DOI: 10.11949/0438-1157.20200653
刘洋洋1( ),孙超2,Malhi Haripal Singh1,位重洋1,张振洲2(
),孙超2,Malhi Haripal Singh1,位重洋1,张振洲2( ),涂维峰1,2(
),涂维峰1,2( )
)
收稿日期:2020-05-25
修回日期:2020-08-14
出版日期:2020-10-05
发布日期:2020-10-05
通讯作者:
张振洲,涂维峰
作者简介:刘洋洋(1995—),男,硕士研究生,基金资助:
Yangyang LIU1( ),Chao SUN2,Malhi Haripal Singh1,Chongyang WEI1,Zhenzhou ZHANG2(
),Chao SUN2,Malhi Haripal Singh1,Chongyang WEI1,Zhenzhou ZHANG2( ),Weifeng TU1,2(
),Weifeng TU1,2( )
)
Received:2020-05-25
Revised:2020-08-14
Online:2020-10-05
Published:2020-10-05
Contact:
Zhenzhou ZHANG,Weifeng TU
摘要:
铁基催化剂CO2加氢直接合成烯烃是实现CO2减排及CO2转化与利用的最佳途径之一。目前铁基催化剂的CO2加氢活性及反应过程中铁基催化剂结构强度仍然较低,成为CO2加氢制烯烃产业化生产的重要挑战。通过浸渍法制备一系列负载型铁基催化剂,研究载体材料性质对铁基催化剂结构及CO2加氢直接合成烯烃的影响特性。研究发现,载体可诱导铁基催化剂在CO2加氢反应过程中形成的铁物种,同时影响铁基催化剂表面碳物种的有序度,调变对CO2吸附及活化能力;研究结果表明ZrO2负载的Fe催化剂展现出最佳的CO2加氢合成烯烃催化性能,在温度320℃和反应压力2.0 MPa时,CO2转化率>30%,C2~C7烃类产物中烯烃选择性高达85%以上,烯烷比为8.2,且CO选择性较低为17.1%。
中图分类号:
刘洋洋, 孙超, Malhi Haripal Singh, 位重洋, 张振洲, 涂维峰. 载体对铁基催化剂结构及CO2加氢制烯烃反应性能的影响特性[J]. 化工学报, 2020, 71(10): 4652-4662.
Yangyang LIU, Chao SUN, Malhi Haripal Singh, Chongyang WEI, Zhenzhou ZHANG, Weifeng TU. Effects of identities of supports on Fe-based catalyst and their consequences on activities of CO2 hydrogenation to olefins[J]. CIESC Journal, 2020, 71(10): 4652-4662.
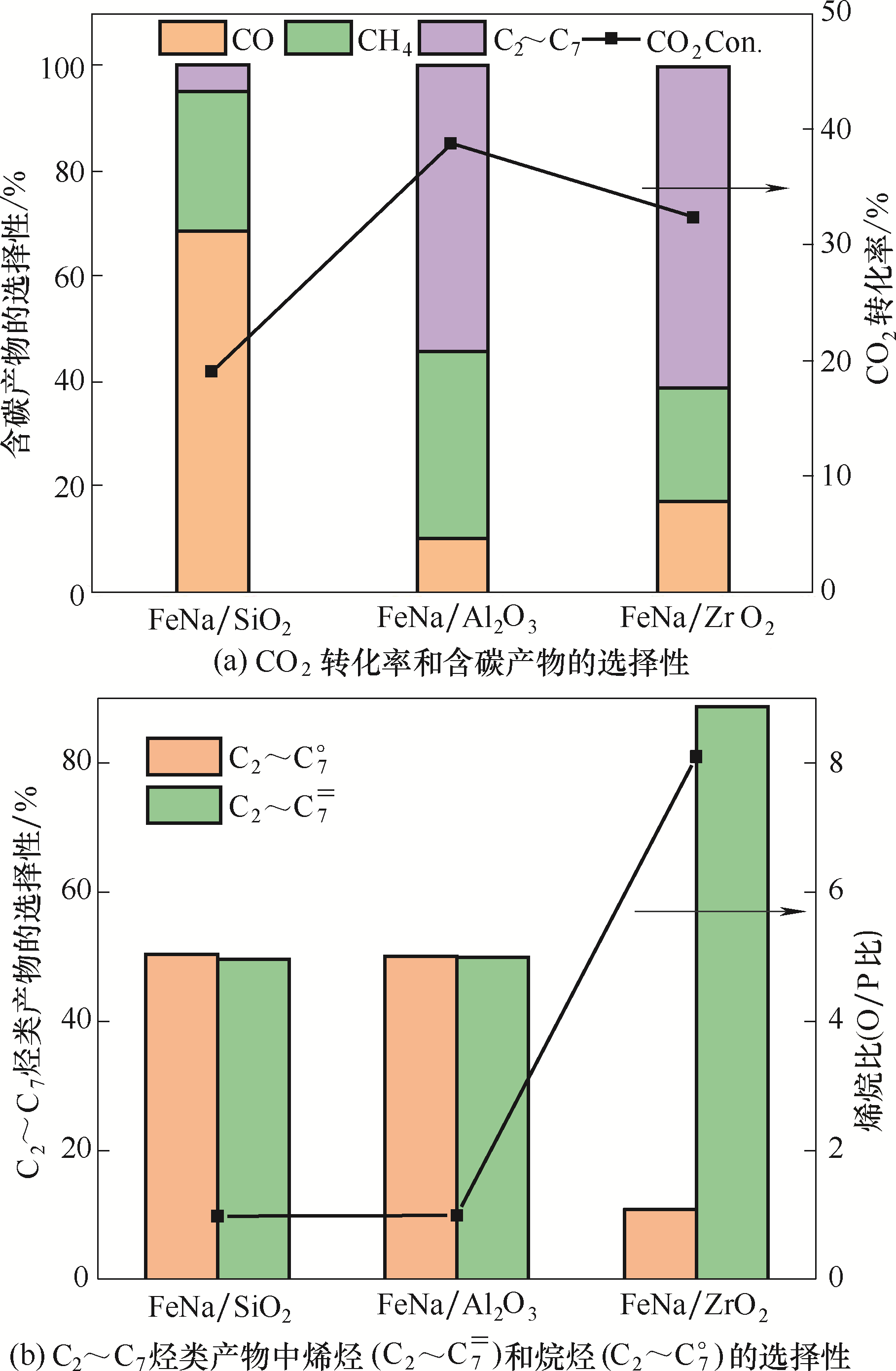
图1 不同载体材料负载的Fe基催化剂CO2加氢反应性能[反应条件:320℃、2.0 MPa、CO2/H2/Ar=1/3/3和空速9000 ml/(g·h)]
Fig.1 Catalytic performance of the Fe based catalysts supported on different supports for CO2 hydrogenation [reaction conditions:320℃, 2.0 MPa, CO2/H2/Ar =1/3/3 and GHSV=9000 ml/(g·h)]
| 催化剂 | CO2转化率 /% | 含碳产物的选择性/C% | C2~C7烷烃和烯烃的 选择性/C% | C2~C7烃类 O/P比 | C2~C7= 时空产率/(g/(kg·h)) | |||
|---|---|---|---|---|---|---|---|---|
| CO | CH4 | C2~C7 | C2~C7o | C2~C7= | ||||
| FeNa/SiO2 | 18.9 | 68.3 | 27.9 | 4.8 | 50.4 | 49.6 | 1.0 | 3.0 |
| FeNa/Al2O3 | 38.4 | 10.0 | 34.4 | 55.6 | 50.1 | 49.9 | 1.0 | 67.8 |
| FeNa/ZrO2 | 32.6 | 17.1 | 20.2 | 62.7 | 10.8 | 89.2 | 8.2 | 111.4 |
表1 负载型Fe基催化剂CO2加氢性能
Table 1 Performance of supported Fe catalysts for CO2 hydrogenation
| 催化剂 | CO2转化率 /% | 含碳产物的选择性/C% | C2~C7烷烃和烯烃的 选择性/C% | C2~C7烃类 O/P比 | C2~C7= 时空产率/(g/(kg·h)) | |||
|---|---|---|---|---|---|---|---|---|
| CO | CH4 | C2~C7 | C2~C7o | C2~C7= | ||||
| FeNa/SiO2 | 18.9 | 68.3 | 27.9 | 4.8 | 50.4 | 49.6 | 1.0 | 3.0 |
| FeNa/Al2O3 | 38.4 | 10.0 | 34.4 | 55.6 | 50.1 | 49.9 | 1.0 | 67.8 |
| FeNa/ZrO2 | 32.6 | 17.1 | 20.2 | 62.7 | 10.8 | 89.2 | 8.2 | 111.4 |
| 催化剂 | BET / (m2/g)① | 总孔容v/ (cm3/g)② | 平均孔径d /nm② | Fe2O3尺寸/nm③ |
|---|---|---|---|---|
| SiO2 | 311.66 | 1.09 | 10.96 | — |
| Al2O3 | 150.44 | 0.57 | 11.98 | — |
| ZrO2 | 4.39 | 0.04 | 25.87 | — |
| FeNa/SiO2 | 167.43 | 0.51 | 9.30 | 16.7 |
| FeNa/Al2O3 | 140.31 | 0.38 | 8.30 | 15.9 |
| FeNa/ZrO2 | 14.20 | 0.08 | 18.82 | 19.0 |
表2 载体和负载型Fe催化剂的物理化学性质
Table 2 Physicochemical properties of supports and supported Fe catalysts
| 催化剂 | BET / (m2/g)① | 总孔容v/ (cm3/g)② | 平均孔径d /nm② | Fe2O3尺寸/nm③ |
|---|---|---|---|---|
| SiO2 | 311.66 | 1.09 | 10.96 | — |
| Al2O3 | 150.44 | 0.57 | 11.98 | — |
| ZrO2 | 4.39 | 0.04 | 25.87 | — |
| FeNa/SiO2 | 167.43 | 0.51 | 9.30 | 16.7 |
| FeNa/Al2O3 | 140.31 | 0.38 | 8.30 | 15.9 |
| FeNa/ZrO2 | 14.20 | 0.08 | 18.82 | 19.0 |

图3 不同载体负载Fe基催化剂的XRD谱图[反应条件:320℃、2 MPa、CO2/H2/Ar=1/3/3和空速9000 ml/(g·h)]
Fig.3 XRD patterns of Fe-based catalyst on different supports [reaction conditions:320℃, 2 MPa, CO2/H2/Ar =1/3/3 and GHSV=9000 ml/(g·h)]
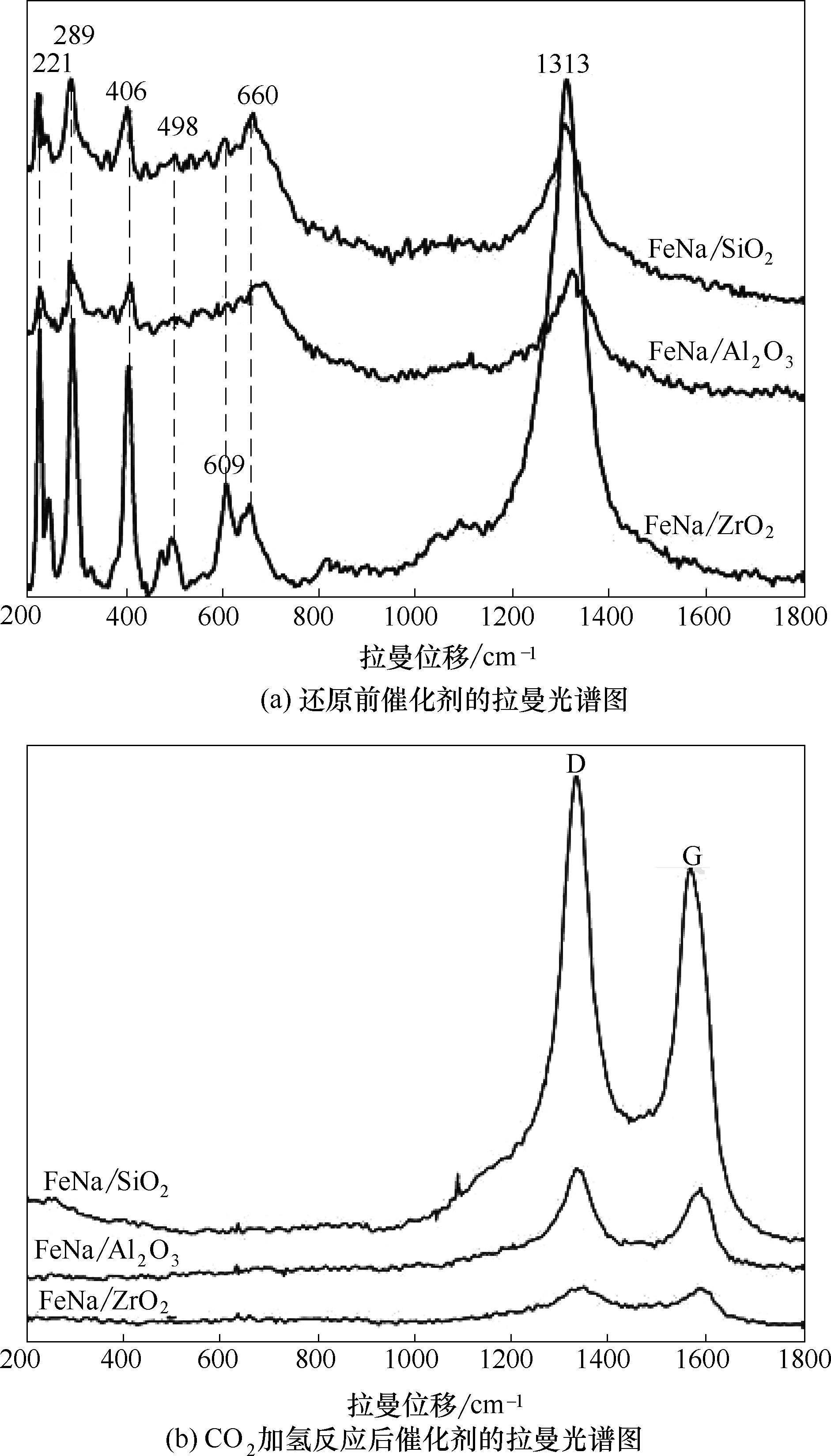
图4 不同载体负载的Fe基催化剂的拉曼光谱图[反应条件:320℃、2 MPa、CO2/H2/Ar=1/3/3和空速9000 ml/(g·h)]
Fig.4 Raman spectra of Fe-based catalyst on different supports [reaction conditions:320℃, 2 MPa, CO2/H2/Ar =1/3/3 and GHSV=9000 ml/(g·h)]

图5 H2预处理的载体和催化剂在50℃、Ar流中CO2吸附稳定后的红外光谱图
Fig.5 FTIR spectra of H2 pretreated supports and catalysts after CO2 adsorption and stabilization in Ar flow at 50℃

图6 载体对铁物种演化的影响及由此调节CO2加氢制烯烃的示意图
Fig.6 Schematic diagram of the effect of supports on the evolution of iron species and consequent tuning the hydrogenation of CO2 to olefins
| 1 | 白雪楠, 白昕, 尤慧君. 中国碳交易市场发展现状及问题分析[J]. 中外企业家, 2020, 14: 100. |
| Bai X N, Bai X, You H J. Development status and problems of carbon trading market in China [J]. Chinese and Foreign Entrepreneurs, 2020, 14: 100. | |
| 2 | 巩金龙. CO2化学转化研究进展概述[J]. 化工学报, 2017, 68(4): 1282-1285. |
| Gong J L. A brief overview on recent progress on chemical conversion of CO2[J]. CIESC Journal, 2017, 68(4): 1282-1285. | |
| 3 | 靳治良, 钱玲, 吕功煊. 二氧化碳化学—现状及展望[J]. 化学进展, 2010, 22(6): 1102-1115. |
| Jin Z L, Qian L, Lyu G X. CO2 Chemistry—actuality and expectation[J]. Progress in Chemistry, 2010, 22(6): 1102-1115. | |
| 4 | Wang S, Xi C. Recent advances in nucleophile-triggered CO2-incorporated cyclization leading to heterocycles[J]. Chem. Soc. Rev., 2019, 48(1): 382-404. |
| 5 | 成康, 张庆红, 康金灿, 等. 二氧化碳直接制备高值化学品中的接力催化方法[J]. 中国科学: 化学, 2020, 50(7): 743-755. |
| Cheng K, Zhang Q H, Kang J C, et al. Relay catalysis in the direct conversion of carbon dioxide to high-value chemicals[J]. Sci. Sin. Chim., 2020, 50(7): 743-755. | |
| 6 | 陈倩倩, 顾宇, 唐志永, 等. 以二氧化碳规模化利用技术为核心的碳减排方案[J]. 中国科学院院刊, 2019, 34(4): 478-487. |
| Chen Q Q, Gu Y, Tang Z Y, et al. Carbon dioxide sizable utilization technology based carbon reduction solutions[J]. Bulletin of the Chinese Academy of Sciences, 2019, 34(4): 478-487. | |
| 7 | 金涌, 周禹成, 胡山鹰. 低碳理念指导的煤化工产业发展探讨[J]. 化工学报, 2012, 63(1): 3-8. |
| Jin Y, Zhou Y C, Hu S Y. Discussion on development of coal chemical industry using low-carbon concept[J]. CIESC Journal, 2012, 63(1): 3-8. | |
| 8 | Yang H Y, Zhang C, Gao P, et al. A review of the catalytic hydrogenation of carbon dioxide into value-added hydrocarbons[J]. Catalysis Science & Technology, 2017, 7(20): 4580-4598. |
| 9 | 高新华, 王康洲, 张建利, 等. CO/CO2催化加氢双功能催化剂新进展[J]. 石油学报(石油加工), 2019, 35(6): 1228-1238. |
| Gao X H, Wang K Z, Zhang J L, et al. Recent advances in bifunctional catalysts for hydrogenation of CO/CO2[J]. Acta Petrolei Sinica(Petroleum Processing Section), 2019, 35(6): 1228-1238. | |
| 10 | 高鹏, 崔勖, 钟良枢, 等. CO/CO2加氢高选择性合成化学品和液体燃料[J]. 化工进展, 2019, 38(1): 183-195. |
| Gao P, Cui X, Zhang L S, et al. CO/CO2 hydrogenation to chemicals and liquid fuels with high selectivity[J]. Chemical Industry and Engineering Progress, 2019, 38(1): 183-195. | |
| 11 | Jiao F, Li J J, Pan X L, et al. Selective conversion of syngas to light olefins[J]. Science, 2016, 351: 1065-1068. |
| 12 |
Wei C Y, Tu W F, Jia L Y, et al. The evolutions of carbon and iron species modified by Na and their tuning effect on the hydrogenation of CO2 to olefins[J]. Applied Surface Science, 2020, DOI: 10.1016/j.apsusc.2020.146622.
DOI |
| 13 | 张玉龙, 邵光印, 张征湃, 等. 活化气氛对CO2加氢制取低碳烯烃Fe-K催化剂构-效关系[J]. 化工学报, 2018, 69(2): 690-698. |
| Zhang Y L, Shao G Y, Zhang Z P, et al. Activation atmospheres on structure-performance relationship of K-promoted Fe catalysts for lower olefin synthesis from CO2 hydrogenation[J]. CIESC Journal, 2018, 69(2): 690-698. | |
| 14 | Liang B L, Sun T, Ma J G, et al. Mn decorated Na/Fe catalysts for CO2 hydrogenation to light olefins[J]. Catal. Sci. Technol., 2019, 9 (2): 456-464. |
| 15 | Zhang Z Z, Wei C Y, Jia L Y, et al. Insights into the regulation of FeNa catalysts modified by Mn promoter and their tuning effect on the hydrogenation of CO2 to light olefins[J]. Journal of Catalysis, 2020, 390: 12-22. |
| 16 | Liu J H, Zhang A F, Jiang X, et al. Overcoating the surface of Fe-based catalyst with ZnO and nitrogen-doped carbon toward high selectivity of light olefins in CO2 hydrogenation[J]. Ind. Eng. Chem. Res, 2019, 58 (10): 4017-4023. |
| 17 | Cheng K, Virginie M, Ordomsky V V, et al. Pore size effects in high-temperature Fischer–Tropsch synthesis over supported iron catalysts[J]. Journal of Catalysis, 2015, 328: 139-150. |
| 18 | Numpilai T, Chanlek N, Poo-Arporn Y, et al. Pore size effects on physicochemical properties of Fe-Co/K-Al2O3 catalysts and their catalytic activity in CO2 hydrogenation to light olefins[J]. Applied Surface Science, 2019, 483: 581-592. |
| 19 | 张俊, 张征湃, 苏俊杰, 等. 载体碱性对Fe基催化剂费-托合成反应的影响[J]. 化工学报, 2016, 67(2): 549-556. |
| Zhang J, Zhang Z P, Su J J, et al. Effect of support basicity on iron based catalysts for Fischer-Tropsch synthesis[J]. CIESC Journal, 2016, 67(2): 549-556. | |
| 20 | Wang J J, You Z Y, Zhang Q H, et al. Synthesis of lower olefins by hydrogenation of carbon dioxide over supported iron catalysts[J]. Catalysis Today, 2013, 215: 186-193. |
| 21 | Gu H, Ding J, Zhong Q, et al. Promotion of surface oxygen vacancies on the light olefins synthesis from catalytic CO2 hydrogenation over Fe–K/ZrO2 catalysts[J]. International Journal of Hydrogen Energy, 2019, 44(23): 11808-11816. |
| 22 | Lu J Z, Yang L J, Xu B L, et al. Promotion effects of nitrogen doping into carbon nanotubes on supported iron Fischer–Tropsch catalysts for lower olefins[J]. ACS Catalysis, 2014, 4(2): 613-621. |
| 23 | Su X J, Zhang J L, Fan S B, et al. Effect of preparation of Fe-Zr-K catalyst on the product distribution of CO2 hydrogenation[J]. RSC Advances, 2015, 5(98): 80196-80202. |
| 24 | Torres Galvis H M, Bitter J H, Khare C B, et al. Supported iron nanoparticles as catalysts for sustainable production of lower olefins[J]. Science, 2012, 335: 835-838. |
| 25 | Park J Y, Lee Y J, Khanna P K, et al. Alumina-supported iron oxide nanoparticles as Fischer-Tropsch catalysts: effect of particle size of iron oxide[J]. Journal of Molecular Catalysis A: Chemical, 2010, 323(1/2): 84-90. |
| 26 | Lee Y H, Lee D W, Kim H, et al. Fe–Zn catalysts for the production of high-calorie synthetic natural gas[J]. Fuel, 2015, 159: 259-268. |
| 27 | Jin Y, Datye A K. Phase transformations in iron Fischer–Tropsch catalysts during temperature-programmed reduction[J]. Journal of Catalysis, 2000, 196(1): 8-17. |
| 28 | Li S Z, Li A W, Krishnamoorthy S, et al. Effects of Zn, Cu, and K promoters on the structure and on the reduction, carburization, and catalytic behavior of iron-based Fischer–Tropsch synthesis catalysts[J]. Catalysis Letters, 2001, 77(4): 197-205. |
| 29 | Zhang C H, Wan H J, Yang Y, et al. Study on the iron–silica interaction of a co-precipitated Fe/SiO2 Fischer–Tropsch synthesis catalyst[J]. Catalysis Communications, 2006, 7(9): 733-738. |
| 30 | Yuen S, Kubsh J E, Dumesic J A, et al. Metal oxide-support interactions in silica-supported iron oxide catalysts probed by nitric oxide adsorption[J]. The Journal of Physical Chemistry, 1982, 86(15): 3022-3032. |
| 31 | Xie T Z, Wang J Y, Ding F S, et al. CO2 hydrogenation to hydrocarbons over alumina-supported iron catalyst: effect of support pore size[J]. Journal of CO2 Utilization, 2017, 19: 202-208. |
| 32 | Wu J H, Wang L C, Yang X, et al. Support effect of the Fe/BN catalyst on Fischer–Tropsch performances: role of the surface B-O defect[J]. Industrial & Engineering Chemistry Research, 2018, 57(8): 2805-2810. |
| 33 | Zhang Y L, Cao C X, Zhang C, et al. The study of structure-performance relationship of iron catalyst during a full life cycle for CO2 hydrogenation[J]. Journal of Catalysis, 2019, 378: 51-62. |
| 34 | Pham T H, Qi Y Y, Yang J, et al. Insights into Hägg iron-carbide-catalyzed Fischer–Tropsch synthesis: suppression of CH4 formation and enhancement of C–C coupling on χ-Fe5C2 (510)[J]. ACS Catalysis, 2015, 5(4): 2203-2208. |
| 35 | Moodley D J, van de loosdrecht J, Saib A M, et al. Carbon deposition as a deactivation mechanism of cobalt-based Fischer–Tropsch synthesis catalysts under realistic conditions[J]. Applied Catalysis A: General, 2009, 354(1/2): 102-110. |
| 36 | Zhang Y L, Fu D D, Liu X L, et al. Operando spectroscopic study of dynamic structure of iron oxide catalysts during CO2 hydrogenation[J]. ChemCatChem, 2018, 10(6): 1272-1276. |
| 37 | Zhang Z P, Zhang J, Wang X, et al. Promotional effects of multiwalled carbon nanotubes on iron catalysts for Fischer-Tropsch to olefins[J]. Journal of Catalysis, 2018, 365: 71-85. |
| 38 | Thomsen C, Reich S. Double resonant Raman scattering in graphite[J]. Physical Review Letters, 2000, 85(24): 5214-5217. |
| 39 | Tuinstra F, Koenig J L. Raman spectrum of graphite[J]. The Journal of Chemical Physics, 1970, 53(3): 1126-1130. |
| 40 | Gruver V, Young R, Engman J, et al. The role of accumulated carbon in deactivating cobalt catalysts during FT synthesis in a slurry-bubble-column reactor[J]. American Chemical Society, Division of Petroleum Chemistry, Preprints, 2005, 50: 164-166. |
| 41 | Solis-Garcia A, Louvier-Hernandez J F, Almendarez-Camarillo A, et al. Participation of surface bicarbonate, formate and methoxy species in the carbon dioxide methanation catalyzed by ZrO2-supported Ni[J]. Applied Catalysis B: Environmental, 2017, 218: 611-620. |
| 42 | Takano H, Kirihata Y, Izumiya K, et al. Highly active Ni/Y-doped ZrO2 catalysts for CO2 methanation[J]. Applied Surface Science, 2016, 388: 653-663. |
| 43 | Szamyi J, Kwak J H. Dissecting the steps of CO2 reduction(1). The interaction of CO and CO2 with γ-Al2O3: an in situ FTIR study[J]. Phys Chem Chem Phys, 2014, 16(29): 15117-15125. |
| 44 | Proano L, Tello E, Arellano-Trevino M A, et al. In-situ DRIFTS study of two-step CO2 capture and catalytic methanation over Ru, "Na2O"/Al2O3 dual functional material[J]. Applied Surface Science, 2019, 479: 25-30. |
| 45 | Szanyi J, Kwak J H. Dissecting the steps of CO2 reduction(2). The interaction of CO and CO2 with Pd/γ-Al2O3: an in situ FTIR study[J]. Phys. Chem. Chem. Phys., 2014, 16(29): 15126-15138. |
| 46 | Guo J Z, Hou Z Y, Gao J, et al. DRIFTS study on adsorption and activation of CH4 and CO2 over Ni/SiO2 catalyst with various Ni particle sizes[J]. Chinese Journal of Catalysis, 2007, 28(1): 22-26. |
| 47 | Chang K, Ordomsky V V, Legras B, et al. Sodium-promoted iron catalysts prepared on different supports for high temperature Fischer-Tropsch synthesis[J]. Applied Catalysis A: General, 2015, 502: 204-214. |
| [1] | 杨欣, 王文, 徐凯, 马凡华. 高压氢气加注过程中温度特征仿真分析[J]. 化工学报, 2023, 74(S1): 280-286. |
| [2] | 吴曦, 区祖迪, 张鑫杰, 徐士鸣, 朱晓静. HFO-1243zf爆燃特性实验研究[J]. 化工学报, 2023, 74(S1): 346-352. |
| [3] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [4] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [5] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [6] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [7] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [8] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [9] | 曹跃, 余冲, 李智, 杨明磊. 工业数据驱动的加氢裂化装置多工况切换过渡状态检测[J]. 化工学报, 2023, 74(9): 3841-3854. |
| [10] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [11] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [12] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [13] | 洪瑞, 袁宝强, 杜文静. 垂直上升管内超临界二氧化碳传热恶化机理分析[J]. 化工学报, 2023, 74(8): 3309-3319. |
| [14] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [15] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号