化工学报 ›› 2020, Vol. 71 ›› Issue (12): 5361-5375.DOI: 10.11949/0438-1157.20200711
栾鹏仟1( ),周丹丹1,王晓天1,陈冉1,高士耆1,赵浩1,黄琛1,刘运亭1(
),周丹丹1,王晓天1,陈冉1,高士耆1,赵浩1,黄琛1,刘运亭1( ),高静1,姜艳军1,2(
),高静1,姜艳军1,2( )
)
收稿日期:2020-06-05
修回日期:2020-07-22
出版日期:2020-12-05
发布日期:2020-12-05
通讯作者:
刘运亭,姜艳军
作者简介:栾鹏仟(1995—),男,硕士研究生,基金资助:
LUAN Pengqian1( ),ZHOU Dandan1,WANG Xiaotian1,CHEN Ran1,GAO Shiqi1,ZHAO Hao1,HUANG Chen1,LIU Yunting1(
),ZHOU Dandan1,WANG Xiaotian1,CHEN Ran1,GAO Shiqi1,ZHAO Hao1,HUANG Chen1,LIU Yunting1( ),GAO Jing1,JIANG Yanjun1,2(
),GAO Jing1,JIANG Yanjun1,2( )
)
Received:2020-06-05
Revised:2020-07-22
Online:2020-12-05
Published:2020-12-05
Contact:
LIU Yunting,JIANG Yanjun
摘要:
化学-酶级联催化结合了化学催化的广泛反应性与生物催化的高选择性,是不对称合成高附加值手性化合物的有效途径。然而,化学催化剂和酶之间以及它们反应条件之间的不相容性极大地限制了这一领域的发展。因此设计可行的方法解决这些问题,实现两种催化范畴的兼容和优势互补,将使化学-酶级联催化反应得到更广泛的应用。综述了近年来克服化学催化与酶催化不相容性所采取的一些策略以及相关的研究进展,如时间分隔、空间分隔和集成催化剂等,并介绍了化学-酶级联催化在手性化合物动态动力学拆分及手性药物合成方面的应用,最后展望了该领域未来的局限性和发展趋势。
中图分类号:
栾鹏仟,周丹丹,王晓天,陈冉,高士耆,赵浩,黄琛,刘运亭,高静,姜艳军. 架起化学-酶催化之间的桥梁:构建策略及催化应用[J]. 化工学报, 2020, 71(12): 5361-5375.
LUAN Pengqian,ZHOU Dandan,WANG Xiaotian,CHEN Ran,GAO Shiqi,ZHAO Hao,HUANG Chen,LIU Yunting,GAO Jing,JIANG Yanjun. Bridging gap between chemo- and biocatalysis: strategies and applications[J]. CIESC Journal, 2020, 71(12): 5361-5375.
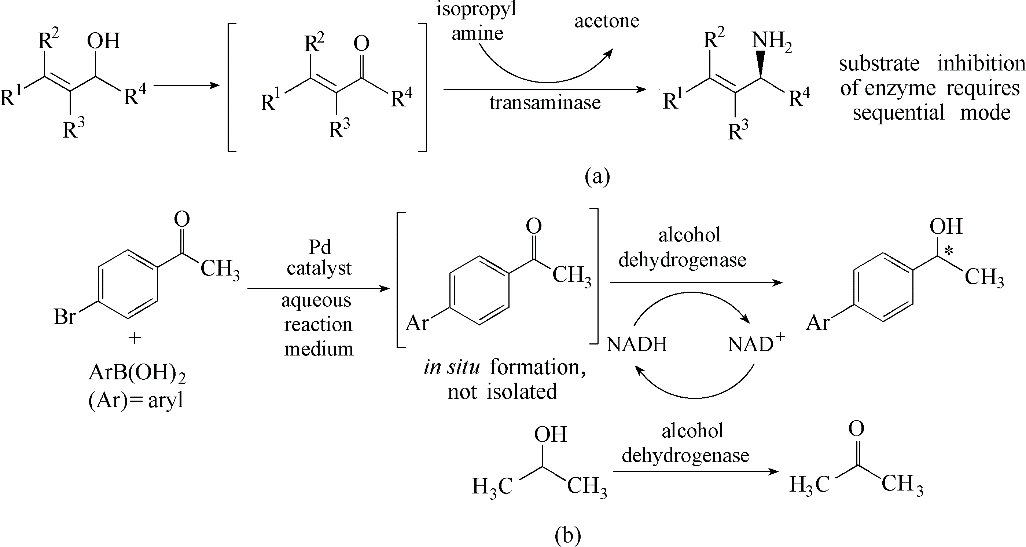
图1 级联金属Ru和ω-TA[20](a)及金属Pd和ADH[21](b)的一锅化学-酶催化转化
Fig.1 One-pot chemoenzymatic conversion through the combination of Ru catalyst and ω-transaminase[20](a), and Pd catalyst and alcohol dehydrogenase[21] (b)
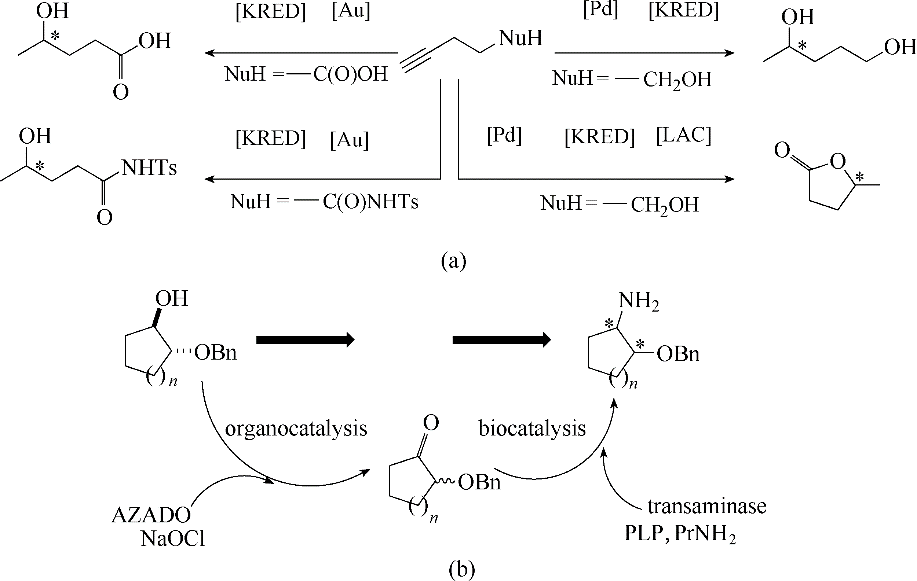
图2 水溶液中化学-酶催化不对称级联合成1,4-二醇、内酯、γ-羟基羰基化合物[24](a)及2-(苄氧基)环烷胺[25](b)
Fig.2 Chemoenzymatic for the asymmetric synthesis of 1,4-diols, lactones, γ-hydroxy-carbonyl compounds[24] (a) and (benzyloxy)cycloalkanamines[25] (b) in aqueous medium

图4 (a)PAD封装于PVA/PEG凝胶中用于一锅化学-酶催化反应[37];(b)TPGS-750-M在水相中形成的胶束[38]
Fig.4 Encapsulate PAD in PVA/PEG for one-pot chemoenzymatic cascade reaction[37](a);The designer surfactant TPGS-750-M forms micelles in water[38](b)

图6 利用PDMS套管分隔催化剂活性位点实现Wacker氧化和酶还原反应的级联反应[66]
Fig.6 Site‐isolation of catalysts using a PDMS thimble for the combination of a Wacker oxidation and an enzymatic reduction[66]
| Chemical catalysts | Biocatalysts | Products | Strategy | Ref. |
|---|---|---|---|---|
| Ni | KRED | orphenadrine | temporal separation | [ |
| Pd | PAL | biarylalanines | temporal separation | [ |
| oxaziridine oxidant | MsrA | sulfoxides | biphasic system | [ |
| Pd | ADH | biaryl alcohols | non-conventional media | [ |
| oxovanadium | lipase | optically active esters | space separation | [ |
| proline-derivative | ADH | 1,3-diol | space separation | [ |
| Au | glucosidase | 4-aminophenol | space separation | [ |
| Pd | CalB | benzyl hexanoate | space separation | [ |
| Pd | MAO-N-D5 | (R)-MTQ | space separation | [ |
| Pd | halogenase | indole heterocycle | space separation | [ |
表1 克服化学-酶催化不相容性的实例
Table 1 Examples of overcoming chemoenzymatic incompatibility
| Chemical catalysts | Biocatalysts | Products | Strategy | Ref. |
|---|---|---|---|---|
| Ni | KRED | orphenadrine | temporal separation | [ |
| Pd | PAL | biarylalanines | temporal separation | [ |
| oxaziridine oxidant | MsrA | sulfoxides | biphasic system | [ |
| Pd | ADH | biaryl alcohols | non-conventional media | [ |
| oxovanadium | lipase | optically active esters | space separation | [ |
| proline-derivative | ADH | 1,3-diol | space separation | [ |
| Au | glucosidase | 4-aminophenol | space separation | [ |
| Pd | CalB | benzyl hexanoate | space separation | [ |
| Pd | MAO-N-D5 | (R)-MTQ | space separation | [ |
| Pd | halogenase | indole heterocycle | space separation | [ |

图7 PdPt@PDA[56],V-MPD与CalB[48]和4-甲基吗啉与CalB[78]动态动力学拆分的产物谱
Fig.7 Scope of DKR systems involving PdPt@PDA[56], V-MPD and CalB[48], and 4-methylmorpholine and CalB[78]
| Chiral building blocks | Pharmaceuticals | Indications | Ref. |
|---|---|---|---|
 | Orphenadrine (邻甲苯海明) | antihistamines, muscle relaxants | [ |
 | Odanacatib (奥达那卡蒂) | cathepsin C inhibitor | [ |
 | Adrenergic (去肾上腺素) | anti-asthma drug | [ |
 | Amphetamine (安非他命) | narcolepsy | [ |
 | Propranolol (普萘洛尔) | arrhythmia | [ |
 | Sertraline (舍曲林) | anti-depressant drug | [ |
 | Cinacalcet (西那卡塞) | hypercalcaemia associated to parathyroid carcinoma | [ |
 | Lipitor (立普妥) | cholesterol-low-ering drug | [ |
 | Sitagliptin (西他列汀) | anti-diabetic drug | [ |
 | Dantrolene (丹曲林) | muscle relaxant | [ |
表2 化学-酶催化制备的手性药物与中间体
Table 2 Chemoenzymatic catalyzed preparation of chiral drugs and intermediates
| Chiral building blocks | Pharmaceuticals | Indications | Ref. |
|---|---|---|---|
 | Orphenadrine (邻甲苯海明) | antihistamines, muscle relaxants | [ |
 | Odanacatib (奥达那卡蒂) | cathepsin C inhibitor | [ |
 | Adrenergic (去肾上腺素) | anti-asthma drug | [ |
 | Amphetamine (安非他命) | narcolepsy | [ |
 | Propranolol (普萘洛尔) | arrhythmia | [ |
 | Sertraline (舍曲林) | anti-depressant drug | [ |
 | Cinacalcet (西那卡塞) | hypercalcaemia associated to parathyroid carcinoma | [ |
 | Lipitor (立普妥) | cholesterol-low-ering drug | [ |
 | Sitagliptin (西他列汀) | anti-diabetic drug | [ |
 | Dantrolene (丹曲林) | muscle relaxant | [ |
| 1 | Hu M Y, He Q, Fan S J, et al. Ligands with 1,10-phenanthroline scaffold for highly regioselective iron-catalyzed alkene hydrosilylation[J]. Nature Communications, 2018, 9(1): 221. |
| 2 | Li M L, Yu J H, Li Y H, et al. Highly enantioselective carbene insertion into N—H bonds of aliphatic amines[J]. Science, 2019, 366(6468): 990-994. |
| 3 | Li Y P, Li Z Q, Zhou B Y, et al. Chiral spiro phosphoric acid-catalyzed Friedel-Crafts conjugate addition/enantioselective protonation reactions[J]. ACS Catalysis, 2019, 9(7): 6522-6529. |
| 4 | Lohse M S, Bein T. Covalent organic frameworks: structures, synthesis, and applications[J]. Advanced Functional Materials, 2018, 28(33): 1705553. |
| 5 | Jiao L, Wang Y, Jiang H L, et al. Metal-organic frameworks as platforms for catalytic applications[J]. Advanced Materials, 2018, 30(37): 1703663. |
| 6 | Wang Y, Kong B, Zhao D Y, et al. Strategies for developing transition metal phosphides as heterogeneous electrocatalysts for water splitting[J]. Nano Today, 2017, 15(2017): 26-55. |
| 7 | Wan F, Zhang L, Dai X, et al. Aqueous rechargeable zinc/sodium vanadate batteries with enhanced performance from simultaneous insertion of dual carriers[J]. Nature Communications, 2018, 9(1): 1656. |
| 8 | Zhang Y, Wan F, Huang S, et al. A chemically self-charging aqueous zinc-ion battery[J]. Nature Communications, 2020, 11(1): 2199. |
| 9 | Lu Y, Hou X, Miao L, et al. Cyclohexanehexone with ultrahigh capacity as cathode materials for lithium-ion batteries[J]. Angewandte Chemie-International Edition, 2019, 58(21): 7020-7024. |
| 10 | Sheldon R A, Pereira P C. Biocatalysis engineering: the big picture[J]. Chemical Society Reviews, 2017, 46(10): 2678-2691. |
| 11 | Clague M J, Urbe S, Komander D. Breaking the chains: deubiquitylating enzyme specificity begets function[J]. Nature Reviews Molecular Cell Biology, 2019, 20(6): 338-352. |
| 12 | Clomburg J M, Crumbley A M, Gonzalez R. Industrial biomanufacturing: the future of chemical production[J]. Science, 2017, 355(6320): aag0804. |
| 13 | Sheldon R A, Woodley J M. Role of biocatalysis in sustainable chemistry[J]. Chemical Reviews, 2018, 118(2): 801-838. |
| 14 | Larsson A L E, Persson B A, Bäckvall J E. Enzymatic resolution of alcohols coupled with ruthenium-catalyzed racemization of the substrate alcohol[J]. Angewandte Chemie-International Edition, 1997, 36(11): 1211-1212. |
| 15 | Wang A Q, Zhang T. One-pot conversion of cellulose to ethylene glycol with multifunctional tungsten-based catalysts[J]. Accounts of Chemical Research, 2012, 46(7): 1377-1386. |
| 16 | Corma A, Navas J, Sabater M J. Advances in one-pot synthesis through borrowing hydrogen catalysis[J]. Chemical Reviews, 2018, 118(4): 1410-1459. |
| 17 | Zhang W, Lee J H, Younes S H H, et al. Photobiocatalytic synthesis of chiral secondary fatty alcohols from renewable unsaturated fatty acids[J]. Nature Communications, 2020, 11(1): 2258. |
| 18 | Latham J, Henry J M, Sharif H H, et al. Integrated catalysis opens new arylation pathways via regiodivergent enzymatic C—H activation[J]. Nature Communications, 2016, 7: 11873. |
| 19 | Sheng X, Himo F. Theoretical study of enzyme promiscuity: mechanisms of hydration and carboxylation activities of phenolic acid decarboxylase[J]. ACS Catalysis, 2017, 7(3): 1733-1741. |
| 20 | Rios-Lombardia N, Vidal C, Cocina M, et al. Chemoenzymatic one-pot synthesis in an aqueous medium: combination of metal-catalysed allylic alcohol isomerisation-asymmetric bioamination[J]. Chemical Communications, 2015, 51(54): 10937-10940. |
| 21 | Burda E, Hummel W, Gröger H. Modular chemoenzymatic one-pot syntheses in aqueous media: combination of a palladium-catalyzed cross-coupling with an asymmetric biotransformation[J]. Angewandte Chemie-International Edition, 2008, 47(49): 9551-9554. |
| 22 | Dander J E, Giroud M, Racine S, et al. Chemoenzymatic conversion of amides to enantioenriched alcohols in aqueous medium[J]. Communications Chemistry, 2019, 2(1): 1-9. |
| 23 | Ahmed S T, Parmeggiani F, Weise N J, et al. Chemoenzymatic synthesis of optically pure L- and D-biarylalanines through biocatalytic asymmetric amination and palladium-catalyzed arylation[J]. ACS Catalysis, 2015, 5(9): 5410-5413. |
| 24 | Rodríguez-Álvarez M J, Ríos-Lombardía N, Schumacher S, et al. Combination of metal-catalyzed cycloisomerizations and biocatalysis in aqueous media: asymmetric construction of chiral alcohols, lactones, and γ-hydroxy-carbonyl compounds[J]. ACS Catalysis, 2017, 7(11): 7753-7759. |
| 25 | Liardo E, Ríos-Lombardía N, Morís F, et al. Hybrid organo- and biocatalytic process for the asymmetric transformation of alcohols into amines in aqueous medium[J]. ACS Catalysis, 2017, 7(7): 4768-4774. |
| 26 | Litman Z C, Wang Y, Zhao H, et al. Cooperative asymmetric reactions combining photocatalysis and enzymatic catalysis[J]. Nature, 2018, 560(7718): 355-359. |
| 27 | Kędziora K, Díaz-Rodríguez A, Lavandera I, et al. Laccase/tempo-mediated system for the thermodynamically disfavored oxidation of 2,2-dihalo-1-phenylethanol derivatives[J]. Green Chemistry, 2014, 16(5): 2448. |
| 28 | Scalacci N, Black G W, Mattedi G, et al. Unveiling the biocatalytic aromatizing activity of monoamine oxidases MAO-N and 6-HDNO: development of chemoenzymatic cascades for the synthesis of pyrroles[J]. ACS Catalysis, 2017, 7(2): 1295-1300. |
| 29 | Risi C, Zhao F, Castagnolo D. Chemo-enzymatic metathesis/aromatization cascades for the synthesis of furans: disclosing the aromatizing activity of laccase/tempo in oxygen-containing heterocycles[J]. ACS Catalysis, 2019, 9(8): 7264-7269. |
| 30 | Denard C A, Bartlett M J, Wang Y, et al. Development of a one-pot tandem reaction combining ruthenium-catalyzed alkene metathesis and enantioselective enzymatic oxidation to produce aryl epoxides[J]. ACS Catalysis, 2015, 5(6): 3817-3822. |
| 31 | Denard C A, Huang H, Bartlett M J, et al. Cooperative tandem catalysis by an organometallic complex and a metalloenzyme[J]. Angewandte Chemie-International Edition, 2014, 53(2): 465-469. |
| 32 | De Winter K, Desmet T, Devlamynck T, et al. Biphasic catalysis with disaccharide phosphorylases: chemoenzymatic synthesis of α-D-glucosides using sucrose phosphorylase[J]. Organic Process Research & Development, 2014, 18(6): 781-787. |
| 33 | Bentley R. Role of sulfur chirality in the chemical processes of biology[J]. Chemical Society Reviews, 2005, 34(7): 609-624. |
| 34 | Sipos G, Drinkel E E, Dorta R. The emergence of sulfoxides as efficient ligands in transition metal catalysis[J]. Chemical Society Reviews, 2015, 44(11): 3834-3860. |
| 35 | Nosek V, Míšek J. Chemoenzymatic deracemization of chiral sulfoxides[J]. Angewandte Chemie-International Edition, 2018, 57(31): 9849-9852. |
| 36 | Pesci L, Baydar M, Glueck S, et al. Development and scaling-up of the fragrance compound 4-ethylguaiacol synthesis via a two-step chemo-enzymatic reaction sequence[J]. Organic Process Research & Development, 2016, 21(1): 85-93. |
| 37 | Baraibar Á G, Reichert D, Mügge C, et al. Ein-topf-reaktionskaskaden durch kombination einer eingekapselten decarboxylase mit metathese zur synthese biobasierter antioxidantien[J]. Angewandte Chemie-International Edition, 2016, 128(47): 15043-15047. |
| 38 | Cortes-Clerget M, Akporji N, Zhou J, et al. Bridging the gap between transition metal- and bio-catalysis via aqueous micellar catalysis[J]. Nature Communications, 2019, 10(1): 2169. |
| 39 | Kourist R, González-Sabín J. Non‐conventional media as strategy to overcome the solvent dilemma in chemoenzymatic tandem catalysis[J]. ChemCatChem, 2020, 12(7): 1903-1912. |
| 40 | Gauchot V, Kroutil W, Schmitzer A R. Highly recyclable chemo-/biocatalyzed cascade reactions with ionic liquids: one-pot synthesis of chiral biaryl alcohols[J]. Chemistry, 2010, 16(23): 6748-6751. |
| 41 | Paris J, Telzerow A, Ríos-Lombardía N, et al. Enantioselective one-pot synthesis of biaryl-substituted amines by combining palladium and enzyme catalysis in deep eutectic solvents[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(5): 5486-5493. |
| 42 | Lan D, Wang X, Zhou P, et al. Deep eutectic solvents as performance additives in biphasic reactions[J]. RSC Advances, 2017, 7(64): 40367-40370. |
| 43 | Cicco L, Ríos-Lombardía N, Rodríguez-Álvarez M J, et al. Programming cascade reactions interfacing biocatalysis with transition-metal catalysis in deep eutectic solvents as biorenewable reaction media[J]. Green Chemistry, 2018, 20(15): 3468-3475. |
| 44 | Paris J, Ríos‐Lombardía N, Morís F, et al. Novel insights into the combination of metal‐ and biocatalysis: cascade one‐pot synthesis of enantiomerically pure biaryl alcohols in deep eutectic solvents[J]. ChemCatChem, 2018, 10(19): 4417-4423. |
| 45 | Zhou L, Zhuang Z, Zhao H, et al. Intricate hollow structures: controlled synthesis and applications in energy storage and conversion[J]. Advanced Materials, 2017, 29(20): 1602914. |
| 46 | Liu X, Zhang F, Jing X, et al. Complex silica composite nanomaterials templated with DNA origami[J]. Nature, 2018, 559(7715): 593-598. |
| 47 | Himiyama T, Waki M, Maegawa Y, et al. Cooperative catalysis of an alcohol dehydrogenase and rhodium-modified periodic mesoporous organosilica[J]. Angewandte Chemie-International Edition, 2019, 58(27): 9150-9154. |
| 48 | Egi M, Sugiyama K, Saneto M, et al. A mesoporous-silica-immobilized oxovanadium cocatalyst for the lipase-catalyzed dynamic kinetic resolution of racemic alcohols[J]. Angewandte Chemie-International Edition, 2013, 52(13): 3654-3658. |
| 49 | Köhler V, Wilson Y M, Dürrenberger M, et al. Synthetic cascades are enabled by combining biocatalysts with artificial metalloenzymes[J]. Nature Chemistry, 2013, 5(2): 93-99. |
| 50 | Okamoto Y, Köhler V, Paul C E, et al. Efficient in situ regeneration of NADH mimics by an artificial metalloenzyme[J]. ACS Catalysis, 2016, 6(6): 3553-3557. |
| 51 | Okamoto Y, Köhler V, Ward T R. An NAD(P)H-dependent artificial transfer hydrogenase for multienzymatic cascades[J]. Journal of the American Chemical Society, 2016, 138(18): 5781-5784. |
| 52 | Du Y J, Gao J, Zhou L Y, et al. MOF-based nanotubes to hollow nanospheres through protein-induced soft-templating pathways[J]. Advanced Science, 2019, 6(6): 1801684. |
| 53 | Yuan S, Feng L, Wang K, et al. Stable metal-organic frameworks: design, synthesis, and applications[J]. Advanced Materials, 2018, 30(37): 1704303. |
| 54 | Wang M, Wang X, Feng B, et al. Combining Pd nanoparticles on mofs with cross-linked enzyme aggregates of lipase as powerful chemoenzymatic platform for one-pot dynamic kinetic resolution of amines[J]. Journal of Catalysis, 2019, 378: 153-163. |
| 55 | Heidlindemann M, Rulli G, Berkessel A, et al. Combination of asymmetric organo- and biocatalytic reactions in organic media using immobilized catalysts in different compartments[J]. ACS Catalysis, 2014, 4(4): 1099-1103. |
| 56 | Gao S Q, Wang Z H, Ma L, et al. Mesoporous core-shell nanostructures bridging metal and biocatalyst for highly efficient cascade reactions[J]. ACS Catalysis, 2019, 10(2): 1375-1380. |
| 57 | Ganai A K, Shinde P, Dhar B B, et al. Development of a multifunctional catalyst for a “relay” reaction[J]. RSC Advances, 2013, 3(7): 2186. |
| 58 | Zhang N, Hübner R, Wang Y, et al. Surface-functionalized mesoporous nanoparticles as heterogeneous supports to transfer bifunctional catalysts into organic solvents for tandem catalysis[J]. ACS Applied Nano Materials, 2018, 1(11): 6378-6386. |
| 59 | Wang Y, Zhang N, Zhang E, et al. Heterogeneous metal-organic-framework-based biohybrid catalysts for cascade reactions in organic solvent[J]. Chemistry - A European Journal, 2019, 25(7): 1716-1721. |
| 60 | Zhang X M, Jing L Y, Chang F F, et al. Positional immobilization of Pd nanoparticles and enzymes in hierarchical yolk-shell@shell nanoreactors for tandem catalysis[J]. Chemical Communications, 2017, 53(55): 7780-7783. |
| 61 | Foulkes J M, Malone K J, Coker V S, et al. Engineering a biometallic whole cell catalyst for enantioselective deracemization reactions[J]. ACS Catalysis, 2011, 1(11): 1589-1594. |
| 62 | Wu S, Zhou Y, Gerngross D, et al. Chemo-enzymatic cascades to produce cycloalkenes from bio-based resources[J]. Nature Communications, 2019, 10(1): 5060. |
| 63 | Xu J, Arkin M, Peng Y, et al. Enantiocomplementary decarboxylative hydroxylation combining photocatalysis and whole-cell biocatalysis in a one-pot cascade process[J]. Green Chemistry, 2019, 21(8): 1907-1911. |
| 64 | Cha H J, Hwang S Y, Lee D S, et al. Whole-cell photoenzymatic cascades to synthesize long-chain aliphatic amines and esters from renewable fatty acids[J]. Angewandte Chemie-International Edition, 2020, 59(18): 7024-7028. |
| 65 | Peng Y, Li D, Fan J, et al. Enantiocomplementary C—H bond hydroxylation combining photo-catalysis and whole-cell biocatalysis in a one-pot cascade process[J]. European Journal of Organic Chemistry, 2020, 2020(7): 821-825. |
| 66 | Sato H, Hummel W, Gröger H. Cooperative catalysis of noncompatible catalysts through compartmentalization: Wacker oxidation and enzymatic reduction in a one-pot process in aqueous media[J]. Angewandte Chemie-International Edition, 2015, 54(15): 4488-4492. |
| 67 | Schaaf P, Bayer T, Koley M, et al. Biocompatible metal-assisted C—C cross-coupling combined with biocatalytic chiral reductions in a concurrent tandem cascade[J]. Chemical Communications, 2018, 54(92): 12978-12981. |
| 68 | Zumbragel N, Groger H. Merging heterocyclic chemistry and biocatalysis in one-pot processes through compartmentalization of the reaction steps[J]. Bioengineering, 2018, 5(3): 60. |
| 69 | Wedde S, Rommelmann P, Scherkus C, et al. An alternative approach towards poly-ε-caprolactone through a chemoenzymatic synthesis: combined hydrogenation, bio-oxidations and polymerization without the isolation of intermediates[J]. Green Chemistry, 2017, 19(5): 1286-1290. |
| 70 | Verho O, Bäckvall J E. Chemoenzymatic dynamic kinetic resolution: a powerful tool for the preparation of enantiomerically pure alcohols and amines[J]. Journal of the American Chemical Society, 2015, 137(12): 3996-4009. |
| 71 | Miranda A S, Miranda L S, Souza R O. Lipases: valuable catalysts for dynamic kinetic resolutions[J]. Biotechnology Advances, 2015, 33(5): 372-393. |
| 72 | Engström K, Johnston E V, Verho O, et al. Co-immobilization of an enzyme and a metal into the compartments of mesoporous silica for cooperative tandem catalysis: an artificial metalloenzyme[J]. Angewandte Chemie-International Edition, 2013, 52(52): 14006-14010. |
| 73 | Li X, Cao X, Xiong J, et al. Enzyme-metal hybrid catalysts for chemoenzymatic reactions[J]. Small, 2020, 16(15): 1902751. |
| 74 | Warner M C, Bäckvall J E. Mechanistic aspects on cyclopentadienylruthenium complexes in catalytic racemization of alcohols[J]. Accounts of Chemical Research, 2013, 46(11): 2545-2555. |
| 75 | Fernandez-Salas J A, Manzini S, Nolan S P. A cationic ruthenium complex for the dynamic kinetic resolution of secondary alcohols[J]. Chemistry, 2014, 20(41): 13132-13135. |
| 76 | Akai S, Hanada R, Fujiwara N, et al. One-pot synthesis of optically active allyl esters via lipase-vanadium combo catalysis[J]. Organic Letters, 2010, 12(21): 4900-4903. |
| 77 | Akai S, Tanimoto K, Kanao Y, et al. A dynamic kinetic resolution of allyl alcohols by the combined use of lipases and [VO(OSiPh3)3][J]. Angewandte Chemie-International Edition, 2006, 45(16): 2592-2595. |
| 78 | Hu L, Zhang Y, Ramström O. Lipase-catalyzed asymmetric synthesis of oxathiazinanones through dynamic covalent kinetic resolution[J]. Organic & Biomolecular Chemistry, 2014, 12(22): 3572-3575. |
| 79 | Zhang Y, Schaufelberger F, Sakulsombat M, et al. Asymmetric synthesis of 1,3-oxathiolan-5-one derivatives through dynamic covalent kinetic resolution[J]. Tetrahedron, 2014, 70(24): 3826-3831. |
| 80 | Schrittwieser J H, Coccia F, Kara S, et al. One-pot combination of enzyme and Pd nanoparticle catalysis for the synthesis of enantiomerically pure 1,2-amino alcohols[J]. Green Chemistry, 2013, 15(12): 3318. |
| 81 | González‐Martínez D, Gotor V, Gotor‐Fernández V. Stereoselective synthesis of 1‐arylpropan‐2‐amines from allylbenzenes through a Wacker‐Tsuji oxidation‐biotransamination sequential process[J]. Advanced Synthesis & Catalysis, 2019, 361: 2582-2593. |
| 82 | Kong X D, Yu H L, Yang S, et al. Chemoenzymatic synthesis of (R)- and (S)-propranolol using an engineered epoxide hydrolase with a high turnover number[J]. Journal of Molecular Catalysis B: Enzymatic, 2015, 122(2015): 275-281. |
| 83 | Marx L, Ríos-Lombardía N, Süss P, et al. Chemoenzymatic synthesis of sertraline[J]. European Journal of Organic Chemistry, 2020, 2020(4): 510-513. |
| 84 | Marx L, Ríos-Lombardía N, Farnberger J F, et al. Chemoenzymatic approaches to the synthesis of the calcimimetic agent cinacalcet employing transaminases and ketoreductases[J]. Advanced Synthesis & Catalysis, 2018, 360(11): 2157-2165. |
| 85 | Bornscheuer U T, Huisman G W, Kazlauskas R J, et al. Engineering the third wave of biocatalysis[J]. Nature, 2012, 485(7397): 185-194. |
| 86 | Simon R C, Mutti F G, Kroutil W. Biocatalytic synthesis of enantiopure building blocks for pharmaceuticals[J]. Drug Discovery Today: Technologies, 2013, 10(1): 37-44. |
| 87 | Plechkova N V, Seddon K R. Applications of ionic liquids in the chemical industry[J]. Chemical Society Reviews, 2008, 37(1): 123-150. |
| [1] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [2] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [3] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [4] | 孟令玎, 崇汝青, 孙菲雪, 孟子晖, 刘文芳. 改性聚乙烯膜和氧化硅固定化碳酸酐酶[J]. 化工学报, 2023, 74(8): 3472-3484. |
| [5] | 陈雅鑫, 袁航, 刘冠章, 毛磊, 杨纯, 张瑞芳, 张光亚. 蛋白质纳米笼介导的酶自固定化研究进展[J]. 化工学报, 2023, 74(7): 2773-2782. |
| [6] | 汤晓玲, 王嘉瑞, 朱玄烨, 郑仁朝. 基于Pickering乳液的卤醇脱卤酶催化合成手性环氧氯丙烷[J]. 化工学报, 2023, 74(7): 2926-2934. |
| [7] | 李彬, 徐正虎, 姜爽, 张天永. 双氧水催化氧化法清洁高效合成促进剂CBS[J]. 化工学报, 2023, 74(7): 2919-2925. |
| [8] | 葛加丽, 管图祥, 邱新民, 吴健, 沈丽明, 暴宁钟. 垂直多孔碳包覆的FeF3正极的构筑及储锂性能研究[J]. 化工学报, 2023, 74(7): 3058-3067. |
| [9] | 涂玉明, 邵高燕, 陈健杰, 刘凤, 田世超, 周智勇, 任钟旗. 钙基催化剂的设计合成及应用研究进展[J]. 化工学报, 2023, 74(7): 2717-2734. |
| [10] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [11] | 张谭, 刘光, 李晋平, 孙予罕. Ru基氮还原电催化剂性能调控策略[J]. 化工学报, 2023, 74(6): 2264-2280. |
| [12] | 葛泽峰, 吴雨青, 曾名迅, 查振婷, 马宇娜, 侯增辉, 张会岩. 灰化学成分对生物质气化特性的影响规律[J]. 化工学报, 2023, 74(5): 2136-2146. |
| [13] | 王锋, 陈钰, 裴鸿艳, 刘东东, 张静, 张立新. 1,2,4-𫫇二唑类衍生物的设计、合成及抗菌活性[J]. 化工学报, 2023, 74(3): 1390-1398. |
| [14] | 刘润竹, 储甜甜, 张孝阿, 王成忠, 张军营. α,ω-端羟基亚苯基氟硅聚合物的合成及性能[J]. 化工学报, 2023, 74(3): 1360-1369. |
| [15] | 袁海鸥, 叶方俊, 张硕, 罗祎青, 袁希钢. 考虑中间换热器的能量集成精馏序列合成[J]. 化工学报, 2023, 74(2): 796-806. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号