化工学报 ›› 2022, Vol. 73 ›› Issue (9): 4122-4132.DOI: 10.11949/0438-1157.20220405
鲁统鹏1,2,3( ), 潘晓林1,2,3(
), 潘晓林1,2,3( ), 吴鸿飞1,2,3, 李煜1,2,3, 于海燕1,2,3(
), 吴鸿飞1,2,3, 李煜1,2,3, 于海燕1,2,3( )
)
收稿日期:2022-03-22
修回日期:2022-06-28
出版日期:2022-09-05
发布日期:2022-10-09
通讯作者:
潘晓林,于海燕
作者简介:鲁统鹏(1995—),男,硕士研究生,danubeblue@foxmail.com
基金资助:
Tongpeng LU1,2,3( ), Xiaolin PAN1,2,3(
), Xiaolin PAN1,2,3( ), Hongfei WU1,2,3, Yu LI1,2,3, Haiyan YU1,2,3(
), Hongfei WU1,2,3, Yu LI1,2,3, Haiyan YU1,2,3( )
)
Received:2022-03-22
Revised:2022-06-28
Online:2022-09-05
Published:2022-10-09
Contact:
Xiaolin PAN, Haiyan YU
摘要:
通过模拟拜耳法赤泥沉降过程研究了聚丙烯酸铵(PAAA)、阴离子聚丙烯酰胺(APAM)和氧肟酸絮凝剂(HPAM/HCPAM)对赤铁矿和针铁矿沉降性能的影响规律和絮凝后絮体的粒径分布及分形维数,并利用傅里叶变换红外光谱探讨了絮凝剂与铁矿相的吸附机理。在不同类型絮凝剂中,添加氧肟酸絮凝剂铁矿相沉降速度最快,且氧肟酸含量越高,沉降性能越好;聚丙烯酸铵和阴离子聚丙烯酰胺絮凝剂对铁矿相沉降性能影响较小;同等条件下赤铁矿沉降速度要远高于针铁矿,增加絮凝剂添加量有助于提高针铁矿沉降速度。在赤铁矿絮体中,添加PAAA絮体粒径最大,HPAM絮体分形维数最大、致密性最好;在针铁矿絮体中,添加APAM絮体粒径最大,HCPAM絮体分形维数最大、致密性最好。氧肟酸絮凝剂与铁矿相形成结构稳定、吸附能力强的五元环状螯合物,增强了赤铁矿和针铁矿的絮凝效果;PAAA通过双齿桥接配位与赤铁矿表面发生吸附,通过单齿配位与针铁矿表面发生吸附,其吸附能力弱于五元环;APAM与赤铁矿和针铁矿表面发生化学吸附,沉降性能差。
中图分类号:
鲁统鹏, 潘晓林, 吴鸿飞, 李煜, 于海燕. 有机絮凝剂对铁矿相沉降性能影响及其吸附机理[J]. 化工学报, 2022, 73(9): 4122-4132.
Tongpeng LU, Xiaolin PAN, Hongfei WU, Yu LI, Haiyan YU. Effect of organic flocculant on settling performance of iron-bearing minerals and its adsorption mechanism[J]. CIESC Journal, 2022, 73(9): 4122-4132.
| Flocculant type | Functional group |
|---|---|
| ammonium polyacrylate (PAAA) | —COO- |
| anionic polyacrylamide (APAM) | —CONH2,—COO- |
| mixed hydroxamic acid (HPAM) | —CONHOH,—CONH2,—COO- |
| high content hydroxamic acid (HCPAM) | —CONHOH |
表1 不同絮凝剂的类型及官能团
Table 1 Types of different flocculants and functional groups
| Flocculant type | Functional group |
|---|---|
| ammonium polyacrylate (PAAA) | —COO- |
| anionic polyacrylamide (APAM) | —CONH2,—COO- |
| mixed hydroxamic acid (HPAM) | —CONHOH,—CONH2,—COO- |
| high content hydroxamic acid (HCPAM) | —CONHOH |
| Iron-bearing mineral | Flocculant | v1/(m·h-1) | v5/(m·h-1) | Compression ratio/% | Supernatant turbidity/NTU |
|---|---|---|---|---|---|
| hematite | no flocculant | 0.621 | 0.738 | 24.88 | 70.71 |
| PAAA | 0.771 | 0.842 | 25.71 | 136.59 | |
| APAM | 0.481 | 0.718 | 26.83 | 114.53 | |
| HPAM | 4.434 | 1.716 | 21.46 | 327.76 | |
| HCPAM | 7.397 | 1.759 | 19.51 | 292.47 | |
| goethite | no flocculant | 0.046 | 0.029 | 89.50 | 221.00 |
| PAAA | 0.068 | 0.041 | 88.00 | 160.71 | |
| APAM | 0.025 | 0.026 | 92.00 | 88.94 | |
| HPAM | 0.246 | 0.211 | 62.50 | 32.47 | |
| HCPAM | 0.305 | 0.508 | 53.00 | 30.41 |
表2 赤铁矿、针铁矿与不同絮凝剂作用下的沉降性能
Table 2 Settling performance of different flocculants with hematite and goethite
| Iron-bearing mineral | Flocculant | v1/(m·h-1) | v5/(m·h-1) | Compression ratio/% | Supernatant turbidity/NTU |
|---|---|---|---|---|---|
| hematite | no flocculant | 0.621 | 0.738 | 24.88 | 70.71 |
| PAAA | 0.771 | 0.842 | 25.71 | 136.59 | |
| APAM | 0.481 | 0.718 | 26.83 | 114.53 | |
| HPAM | 4.434 | 1.716 | 21.46 | 327.76 | |
| HCPAM | 7.397 | 1.759 | 19.51 | 292.47 | |
| goethite | no flocculant | 0.046 | 0.029 | 89.50 | 221.00 |
| PAAA | 0.068 | 0.041 | 88.00 | 160.71 | |
| APAM | 0.025 | 0.026 | 92.00 | 88.94 | |
| HPAM | 0.246 | 0.211 | 62.50 | 32.47 | |
| HCPAM | 0.305 | 0.508 | 53.00 | 30.41 |
| Iron-bearing mineral | Flocculant | D(0.1)/μm | D(0.5)/μm | D(0.9)/μm | Specific surface area/(m2‧kg-1) |
|---|---|---|---|---|---|
| hematite | no flocculant | 0.06 | 3.91 | 11.80 | 32390 |
| PAAA | 14.33 | 63.07 | 186.33 | 182.13 | |
| APAM | 11.40 | 42.38 | 186.00 | 223.73 | |
| HPAM | 13.00 | 36.63 | 80.87 | 236.47 | |
| HCPAM | 15.40 | 59.40 | 135.33 | 181.17 | |
| goethite | no flocculant | 0.11 | 6.25 | 29.73 | 12230 |
| PAAA | 13.10 | 43.60 | 125.00 | 83.27 | |
| APAM | 14.60 | 46.10 | 99.50 | 80.23 | |
| HPAM | 10.80 | 26.40 | 71.50 | 132.90 | |
| HCPAM | 12.40 | 33.50 | 106.00 | 87.70 |
表3 赤铁矿、针铁矿与不同絮凝剂形成絮体的粒度参数
Table 3 Particle size of hematite and goethite flocs formed with different flocculants
| Iron-bearing mineral | Flocculant | D(0.1)/μm | D(0.5)/μm | D(0.9)/μm | Specific surface area/(m2‧kg-1) |
|---|---|---|---|---|---|
| hematite | no flocculant | 0.06 | 3.91 | 11.80 | 32390 |
| PAAA | 14.33 | 63.07 | 186.33 | 182.13 | |
| APAM | 11.40 | 42.38 | 186.00 | 223.73 | |
| HPAM | 13.00 | 36.63 | 80.87 | 236.47 | |
| HCPAM | 15.40 | 59.40 | 135.33 | 181.17 | |
| goethite | no flocculant | 0.11 | 6.25 | 29.73 | 12230 |
| PAAA | 13.10 | 43.60 | 125.00 | 83.27 | |
| APAM | 14.60 | 46.10 | 99.50 | 80.23 | |
| HPAM | 10.80 | 26.40 | 71.50 | 132.90 | |
| HCPAM | 12.40 | 33.50 | 106.00 | 87.70 |
| Iron-bearing mineral | Flocculant | Df | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | Average | ||
| hematite | PAAA | 2.493 | 2.488 | 2.492 | 2.491 |
| APAM | 2.436 | 2.443 | 2.447 | 2.442 | |
| HPAM | 2.537 | 2.541 | 2.547 | 2.542 | |
| HCPAM | 2.373 | 2.377 | 2.381 | 2.377 | |
| goethite | PAAA | 2.224 | 2.238 | 2.248 | 2.237 |
| APAM | 2.157 | 2.156 | 2.155 | 2.156 | |
| HPAM | 2.324 | 2.331 | 2.338 | 2.331 | |
| HCPAM | 2.433 | 2.434 | 2.433 | 2.433 | |
表4 赤铁矿和针铁矿的分形维数
Table 4 Fractal dimension of hematite and goethite
| Iron-bearing mineral | Flocculant | Df | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | Average | ||
| hematite | PAAA | 2.493 | 2.488 | 2.492 | 2.491 |
| APAM | 2.436 | 2.443 | 2.447 | 2.442 | |
| HPAM | 2.537 | 2.541 | 2.547 | 2.542 | |
| HCPAM | 2.373 | 2.377 | 2.381 | 2.377 | |
| goethite | PAAA | 2.224 | 2.238 | 2.248 | 2.237 |
| APAM | 2.157 | 2.156 | 2.155 | 2.156 | |
| HPAM | 2.324 | 2.331 | 2.338 | 2.331 | |
| HCPAM | 2.433 | 2.434 | 2.433 | 2.433 | |
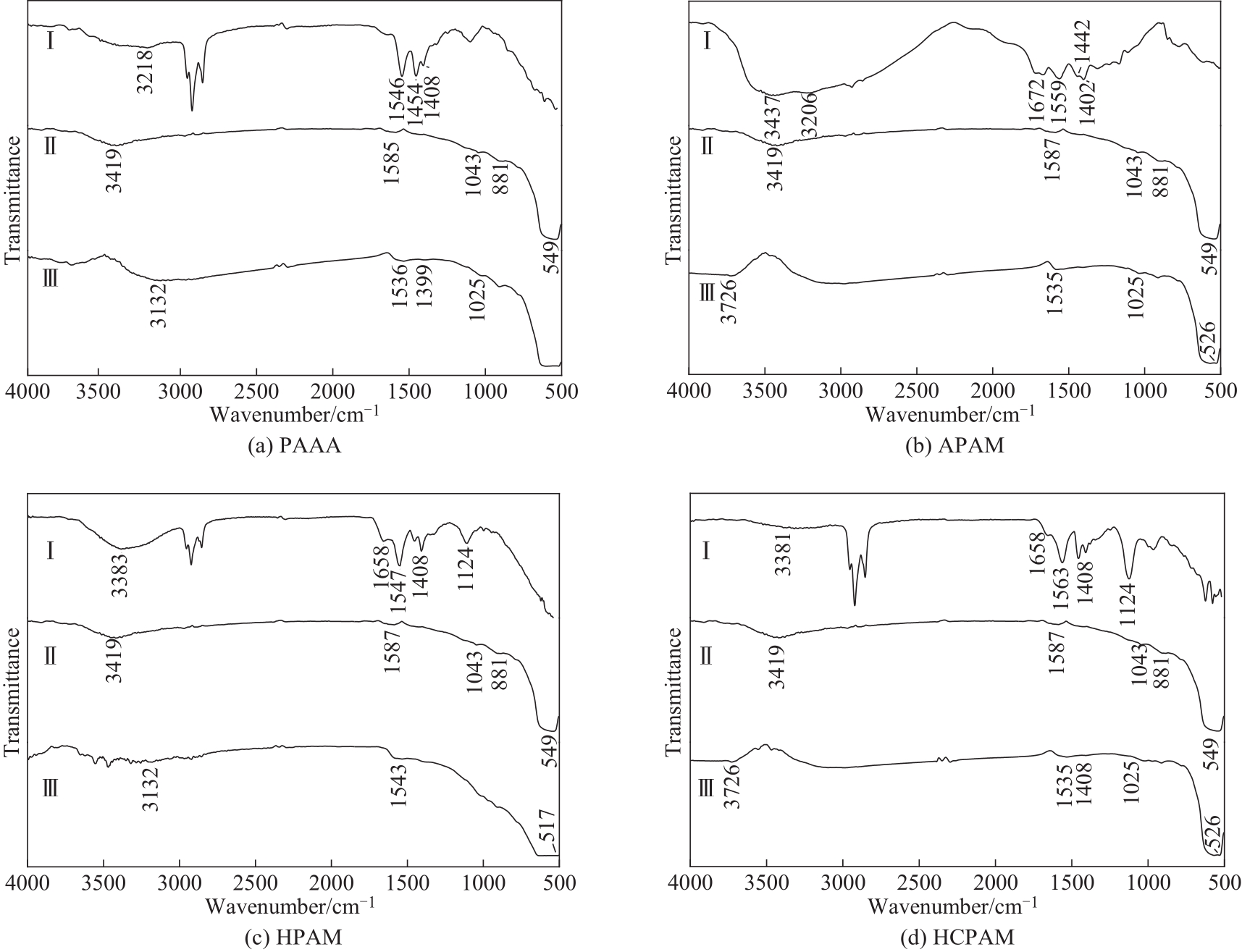
图6 赤铁矿、絮凝剂和絮凝后的赤铁矿絮体的FT-IR图(Ⅰ—絮凝剂;Ⅱ—赤铁矿;Ⅲ—絮凝后的赤铁矿)
Fig.6 FT-IR spectra of flocculants, hematite and flocculant-treated hematite(Ⅰ—flocculant;Ⅱ—hematite;Ⅲ—flocculant-treated hematite)

图8 针铁矿、絮凝剂和絮凝后的针铁矿絮体的FT-IR图(Ⅰ—絮凝剂;Ⅱ—针铁矿;Ⅲ—絮凝后的针铁矿)
Fig.8 FT-IR spectra of flocculants, goethite and flocculant-treated goethite(Ⅰ—flocculant;Ⅱ—goethite;Ⅲ—flocculant-treated goethite)
| 1 | 毕诗文, 于海燕, 杨毅宏. 拜耳法生产氧化铝[M]. 北京: 冶金工业出版社, 2007. |
| Bi S W, Yu H Y, Yang Y H. Production Technology of Alumina[M]. Beijing: Metallurgical Industry Press, 2007. | |
| 2 | 费祥俊. 浆体与粒状物料输送水力学[M]. 北京: 清华大学出版社, 1994. |
| Fei X J. Hydraulics of Transporting Slurry and Granular Material[M]. Beijing: Tsinghua University Press, 1994. | |
| 3 | 潘晓林, 于海燕, 涂赣峰, 等. 石灰对三水铝石型铝土矿低温溶出行为的影响[J]. 东北大学学报(自然科学版), 2013, 34(4): 551-555. |
| Pan X L, Yu H Y, Tu G F, et al. Effect of lime on digestion of gibbsitic bauxites at low temperature[J]. Journal of Northeastern University (Natural Science), 2013, 34(4): 551-555. | |
| 4 | James D F, Blakey B. Comparison of the rheologies of laterite and goethite suspensions[J]. Korea-Australia Rheology Journal, 2004, 16: 109-115. |
| 5 | Shrimali K, Jin J Q, Hassas B V, et al. The surface state of hematite and its wetting characteristics[J]. Journal of Colloid and Interface Science, 2016, 477: 16-24. |
| 6 | Li L Y. A study of iron mineral transformation to reduce red mud tailings[J]. Waste Management, 2001, 21(6): 525-534. |
| 7 | Zhou G T, Wang Y L, Qi T G, et al. Low-temperature thermal conversion of Al-substituted goethite in gibbsitic bauxite for maximum alumina extraction[J]. RSC Advances, 2022, 12(7): 4162-4174. |
| 8 | 孙茜, 黄红岗. 拜耳法赤泥分离沉降槽跑浑的原因及解决方法[J]. 中国有色冶金, 2004, 33(2): 9-10, 21. |
| Sun Q, Huang H G. Method for sovling the problem about too much solid in the solution in red mud separation thickener of Bayer process[J]. China Nonferrous Metallurgy, 2004, 33(2): 9-10, 21. | |
| 9 | 方乘, 杨盛, 吴云, 等. 絮体表面形态对膜污染预测的影响[J]. 化工学报, 2020, 71(2): 715-723. |
| Fang C, Yang S, Wu Y, et al. Effect of floc surface morphology on membrane pollution prediction[J]. CIESC Journal, 2020, 71(2): 715-723. | |
| 10 | 李警阳, 张忠国, 孙春宝, 等. 基于分形学的絮凝理论研究进展[J]. 化工进展, 2012, 31(12): 2609-2614, 2625. |
| Li J Y, Zhang Z G, Sun C B, et al. A review of flocculation theories incorporating fractal geometry[J]. Chemical Industry and Engineering Progress, 2012, 31(12): 2609-2614, 2625. | |
| 11 | Kirwan L J, Fawell P D, Bronswijk W. An in situ FTIR-ATR study of polyacrylate adsorbed onto hematite at high pH and high ionic strength[J]. Langmuir: the ACS Journal of Surfaces and Colloids, 2004, 20(10): 4093-4100. |
| 12 | Cheng K, Wu X Q, Tang H H, et al. The flotation of fine hematite by selective flocculation using sodium polyacrylate[J]. Minerals Engineering, 2022, 176: 107273. |
| 13 | Huang C B, Wang Y H. Removal of aluminosilicates from diasporic-bauxite by selective flocculation using sodium polyacrylate[J]. Separation and Purification Technology, 2008, 59(3): 299-303. |
| 14 | 冯永宁, 金鹏康, 王晓昌. 典型pH条件下水中离子对腐殖酸絮凝体构造的影响[J]. 环境化学, 2013, 32(6): 1088-1093. |
| Feng Y N, Jin P K, Wang X C. Effects of ion in water on humic acid floc structure under typical pH conditions[J]. Environmental Chemistry, 2013, 32(6): 1088-1093. | |
| 15 | 苏宇峰, 王兴军, 于广锁, 等. 高岭土颗粒在聚丙烯酰胺作用下的动态絮凝过程[J]. 华东理工大学学报(自然科学版), 2016, 42(4): 439-445. |
| Su Y F, Wang X J, Yu G S, et al. Dynamic flocculation process of Kaolin particles under the effect of polyacrylamide[J]. Journal of East China University of Science and Technology (Natural Science Edition), 2016, 42(4): 439-445. | |
| 16 | Zhang S X, Zheng H L, Tang X M, et al. Evaluation a self-assembled anionic polyacrylamide flocculant for the treatment of hematite wastewater: role of microblock structure[J]. Journal of the Taiwan Institute of Chemical Engineers, 2019, 95: 11-20. |
| 17 | Ferretti R, Zhang J W, Buffle J. Flocculation of hematite with polyacrylic acid: fractal structures in the reaction- and diffusion-limited aggregation regimes[J]. Journal of Colloid and Interface Science, 1998, 208(2): 509-517. |
| 18 | Atkinson R J, Posner A M, Quirk J P. Adsorption of potential-determining ions at the ferric oxide-aqueous electrolyte interface[J]. The Journal of Physical Chemistry, 1967, 71(3): 550-558. |
| 19 | 杜倩倩, 谷景华, 默广, 等. TiO2-ZrO2聚合溶胶形成过程的小角X射线散射研究[J]. 化工学报, 2018, 69(4): 1731-1740. |
| Du Q Q, Gu J H, Mo G, et al. Study on formation of polymeric TiO2-ZrO2 sols by small angle X-ray scattering[J]. CIESC Journal, 2018, 69(4): 1731-1740. | |
| 20 | Gmachowski L. Calculation of the fractal dimension of aggregates[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2002, 211(2/3): 197-203. |
| 21 | Logan B E, Kilps J R. Fractal dimensions of aggregates formed in different fluid mechanical environments[J]. Water Research, 1995, 29(2): 443-453. |
| 22 | 梁高杰, 陈文汨, 范尚. 赤泥沉降用新型氧肟酸絮凝剂的合成与应用[J]. 中南大学学报(自然科学版), 2017, 48(2): 295-301. |
| Liang G J, Chen W M, Fan S. Preparation of new hydroxamic acid flocculant and application for red mud settlement[J]. Journal of Central South University (Science and Technology), 2017, 48(2): 295-301. | |
| 23 | 张钦礼, 王石, 王新民. 絮凝剂单耗对全尾砂浆浑液面沉速的影响规律[J]. 中国有色金属学报, 2017, 27(2): 318-324. |
| Zhang Q L, Wang S, Wang X M. Influence rules of unit consumptions of flocculants on interface sedimentation velocity of unclassified tailings slurry[J]. The Chinese Journal of Nonferrous Metals, 2017, 27(2): 318-324. | |
| 24 | 梁高杰, 王丹丹, 谢巧玲, 等. 氧肟酸型聚合物制备及其在铜氨废水处理中的应用研究[J]. 应用化工, 2021, 50(12): 3304-3308. |
| Liang G J, Wang D D, Xie Q L, et al. Synthesis of hydroxamic polymer and application on the copper ammonia wastewater treatment[J]. Applied Chemical Industry, 2021, 50(12): 3304-3308. | |
| 25 | Liang G J, Nguyen A V, Chen W M, et al. Interaction forces between goethite and polymeric flocculants and their effect on the flocculation of fine goethite particles[J]. Chemical Engineering Journal, 2018, 334: 1034-1045. |
| 26 | Ponou J, Ide T, Suzuki A, et al. Evaluation of the flocculation and de-flocculation performance and mechanism of polymer flocculants[J]. Water Science and Technology: a Journal of the International Association on Water Pollution Research, 2014, 69(6): 1249-1258. |
| 27 | Dash M, Dwari R K, Biswal S K, et al. Studies on the effect of flocculant adsorption on the dewatering of iron ore tailings[J]. Chemical Engineering Journal, 2011, 173(2): 318-325. |
| 28 | Zhang H L, Xu Z J, Chen D X, et al. Adsorption mechanism of water molecules on hematite (1 0 4) surface and the hydration microstructure[J]. Applied Surface Science, 2021, 550: 149328. |
| 29 | 赵文林, 何洁冰. 羧酸金属盐β晶型成核剂合成及应用研究[J]. 化工学报, 2019, 70(S1): 211-216, 260. |
| Zhao W L, He J B. Synthesis and application of carboxylic acid metal salt beta crystal nucleating agent[J]. CIESC Journal, 2019, 70(S1): 211-216, 260. | |
| 30 | Baigorri R, García-Mina J M, González-Gaitano G. Supramolecular association induced by Fe(Ⅲ) in low molecular weight sodium polyacrylate[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2007, 292(2/3): 212-216. |
| 31 | Lee D H, Condrate R A, Reed J S. Infrared spectral investigation of polyacrylate adsorption on alumina[J]. Journal of Materials Science, 1996, 31(2): 471-478. |
| 32 | Liu J W, Hu H P, Wang M, et al. Adsorption mechanism of the simulated red mud from diaspore with high levels of silicon and iron[J]. The Canadian Journal of Chemical Engineering, 2016, 94(9): 1700-1709. |
| 33 | Güngör N, Karaoğlan S. Interactions of polyacrylamide polymer with bentonite in aqueous systems[J]. Materials Letters, 2001, 48(3/4): 168-175. |
| 34 | AlKhatib H S, Taha M O, Aiedeh K M, et al. Synthesis and in vitro behavior of iron-crosslinked N-methyl and N-benzyl hydroxamated derivatives of alginic acid as controlled release carriers[J]. European Polymer Journal, 2006, 42(10): 2464-2474. |
| 35 | Adiguzel E, Yilmaz F, Emirik M, et al. Synthesis and characterization of two new hydroxamic acids derivatives and their metal complexes. An investigation on the keto/enol, E/Z and hydroxamate/hydroximate forms[J]. Journal of Molecular Structure, 2017, 1127: 403-412. |
| 36 | Jones F, Farrow J B, van Bronswijk W. An infrared study of a polyacrylate flocculant adsorbed on hematite[J]. Langmuir, 1998, 14(22): 6512-6517. |
| 37 | 翟丽军. 基于芳香五羧酸配位聚合物的合成、结构及性能研究[D]. 太原: 中北大学, 2020. |
| Zhai L J. Syntheses, structures and properties of coordination polymers based on aromatic pentacarboxylic acids[D]. Taiyuan: North University of China, 2020. | |
| 38 | Wang M, Hu H P, Chen X P, et al. Flocculation mechanism of synthetic goethite suspension using hydroxamated polymer and sodium polyacrylate[J]. The Chinese Journal of Process Engineering, 2016(3): 452-462. |
| 39 | Ahmed A A, Gypser S, Leinweber P, et al. Infrared spectroscopic characterization of phosphate binding at the goethite-water interface[J]. Physical Chemistry Chemical Physics, 2019, 21(8): 4421-4434. |
| 40 | Villalobos M, Cheney M A, Alcaraz-Cienfuegos J. Goethite surface reactivity(Ⅱ): A microscopic site-density model that describes its surface area-normalized variability[J]. Journal of Colloid and Interface Science, 2009, 336(2): 412-422. |
| 41 | Kubicki J D, Paul K W, Kabalan L, et al. ATR-FTIR and density functional theory study of the structures, energetics, and vibrational spectra of phosphate adsorbed onto goethite[J]. Langmuir: the ACS Journal of Surfaces and Colloids, 2012, 28(41): 14573-14587. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [3] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [4] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [5] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [6] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [7] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [8] | 王新悦, 王俊杰, 曹思贤, 王翠, 李灵坤, 吴宏宇, 韩静, 吴昊. 玻璃内包材界面修饰对机械应力诱导的单克隆抗体聚集体形成的影响[J]. 化工学报, 2023, 74(6): 2580-2588. |
| [9] | 陈韶云, 徐东, 陈龙, 张禹, 张远方, 尤庆亮, 胡成龙, 陈建. 单层聚苯胺微球阵列结构的制备及其吸附性能[J]. 化工学报, 2023, 74(5): 2228-2238. |
| [10] | 蔺彩虹, 王丽, 吴瑜, 刘鹏, 杨江峰, 李晋平. 沸石中碱金属阳离子对CO2/N2O吸附分离性能的影响[J]. 化工学报, 2023, 74(5): 2013-2021. |
| [11] | 李辰鑫, 潘艳秋, 何流, 牛亚宾, 俞路. 基于碳微晶结构的炭膜模型及其气体分离模拟[J]. 化工学报, 2023, 74(5): 2057-2066. |
| [12] | 王蕾, 王磊, 白云龙, 何柳柳. SA膜状锂离子筛的制备及其锂吸附性能[J]. 化工学报, 2023, 74(5): 2046-2056. |
| [13] | 肖川宝, 李林洋, 刘武锋, 钟年丙, 解泉华, 钟登杰, 常海星. 光催化与离子交换吸附耦合有效去除2,4,6-三氯苯酚[J]. 化工学报, 2023, 74(4): 1587-1597. |
| [14] | 王瑞恒, 何品晶, 吕凡, 章骅. 垃圾焚烧飞灰水洗后三种固液分离方法参数比较及优化[J]. 化工学报, 2023, 74(4): 1712-1723. |
| [15] | 吴选军, 王超, 曹子健, 蔡卫权. 数据与物理信息混合驱动的固定床吸附穿透深度学习模型[J]. 化工学报, 2023, 74(3): 1145-1160. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号