化工学报 ›› 2023, Vol. 74 ›› Issue (10): 4330-4342.DOI: 10.11949/0438-1157.20230584
张鑫琦1,2( ), 张宸2, 张舵咏2, 宣涛2, 干桌臻2, 朱炫灿2, 王丽伟2(
), 张宸2, 张舵咏2, 宣涛2, 干桌臻2, 朱炫灿2, 王丽伟2( )
)
收稿日期:2023-06-19
修回日期:2023-09-15
出版日期:2023-10-25
发布日期:2023-12-22
通讯作者:
王丽伟
作者简介:张鑫琦(1997—),男,硕士研究生,550144979@sjtu.edu.cn
基金资助:
Xinqi ZHANG1,2( ), Chen ZHANG2, Duoyong ZHANG2, Tao XUAN2, Zhuozhen GAN2, Xuancan ZHU2, Liwei WANG2(
), Chen ZHANG2, Duoyong ZHANG2, Tao XUAN2, Zhuozhen GAN2, Xuancan ZHU2, Liwei WANG2( )
)
Received:2023-06-19
Revised:2023-09-15
Online:2023-10-25
Published:2023-12-22
Contact:
Liwei WANG
摘要:
以Zr基金属有机框架MOF-808为载体,采用聚乙烯亚胺(polyethylenimine, PEI)进行官能化,得到PEI@M-808复合材料,用于选择性捕集潮湿烟气(10% CO2+90% N2+10 kPa H2O)中的低浓度CO2。采用XRD、FT-IR、SEM以及BET等表征了吸附剂的物理化学性质,并评估改性方案的可行性。由于MOF-808表面PEI的负载引入了丰富胺基官能团,使复合吸附剂在低浓度CO2下的吸附性能大幅提升。PEI300-30@M-808在典型烟气温度(343 K)的低压CO2吸附量相比原材料由0.052 mmol/g上升到0.89 mmol/g,增加了约16倍。此外,CO2/N2吸附选择性在0.1 bar(1 bar=105 Pa)时能达到5524.65,相比原材料提升了约56倍。更重要的是,当吸附剂放置到5% RH工况中时吸附量由0.89 mmol/g提高到1.47 mmol/g,证明了水分对其CO2吸附起着良好的促进作用。经过5次循环后,PEI300-30@M-808的CO2饱和吸附量仍可维持在90.7%。
中图分类号:
张鑫琦, 张宸, 张舵咏, 宣涛, 干桌臻, 朱炫灿, 王丽伟. 高选择性PEI@MOF-808吸附剂在潮湿烟气中的碳捕集性能研究[J]. 化工学报, 2023, 74(10): 4330-4342.
Xinqi ZHANG, Chen ZHANG, Duoyong ZHANG, Tao XUAN, Zhuozhen GAN, Xuancan ZHU, Liwei WANG. Study on the carbon capture performance of highly selective PEI@MOF-808 adsorbent in humid flue gas[J]. CIESC Journal, 2023, 74(10): 4330-4342.
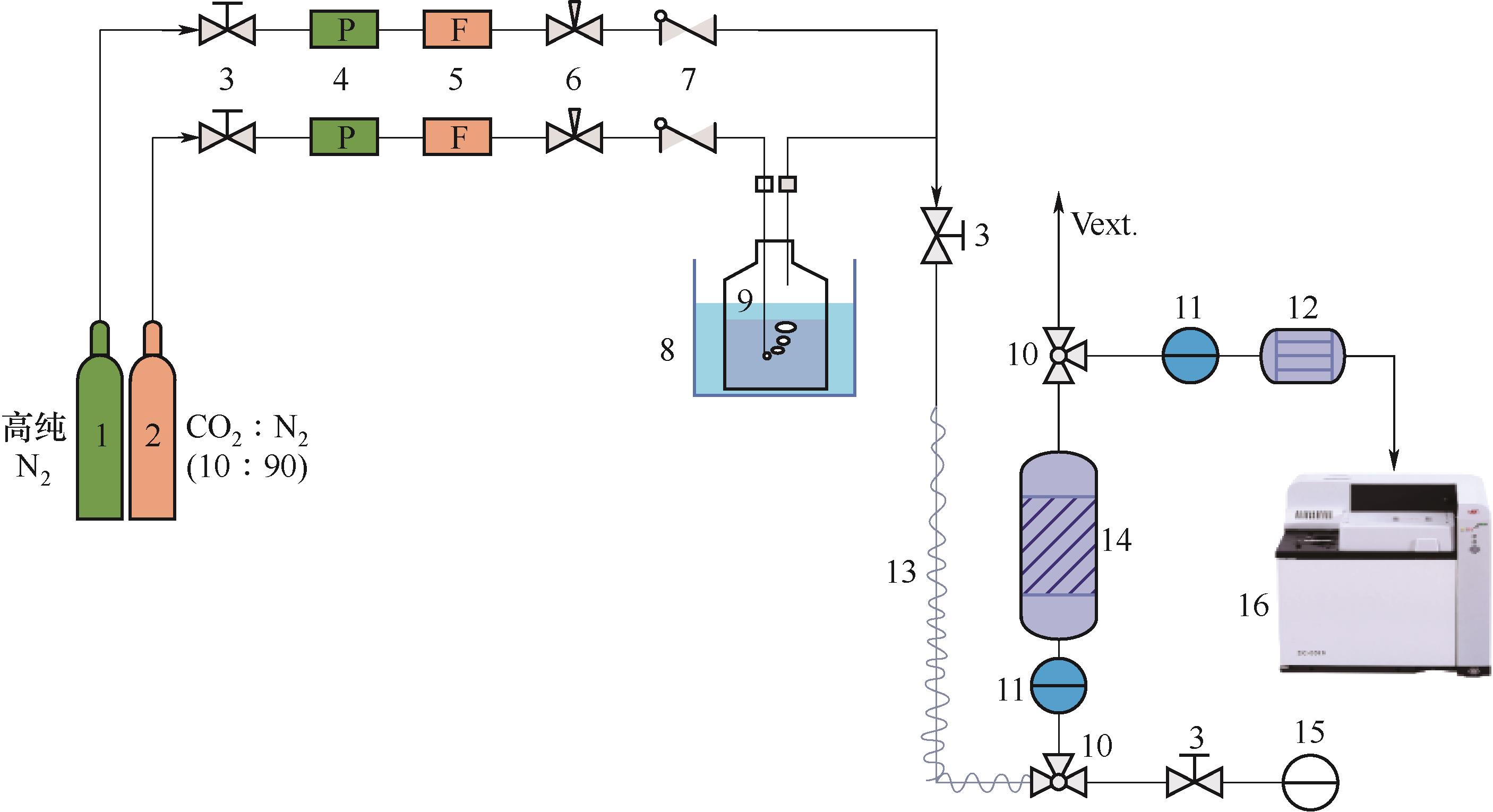
图2 CO2固定床吸附装置1—氮气;2—混合气;3—截止阀;4—压力调节器;5—质量流量控制器;6—针阀;7—单向阀;8—水浴锅;9—鼓泡发生器;10—三通阀;11—温度和湿度传感器;12—干燥管;13—热追踪;14—吸附柱;15—压力表;16—在线质谱仪
Fig.2 CO2 fixed bed sorption testing unit
| 样品名称 | 比表面积/ (m2/g) | 全孔体积/ (cm3/g) | 微孔体积/ (cm3/g) |
|---|---|---|---|
| M-808 | 1742.09 | 1.04 | 0.60 |
| PEI300-10@M-808 | 105.58 | 0.28 | 0.01 |
| PEI300-20@M-808 | 74.83 | 0.29 | 0 |
| PEI300-30@M-808 | 48.30 | 0.18 | 0 |
表1 PEI300-y@M-808孔结构参数
Table 1 Textural properties of PEI300-y@M-808
| 样品名称 | 比表面积/ (m2/g) | 全孔体积/ (cm3/g) | 微孔体积/ (cm3/g) |
|---|---|---|---|
| M-808 | 1742.09 | 1.04 | 0.60 |
| PEI300-10@M-808 | 105.58 | 0.28 | 0.01 |
| PEI300-20@M-808 | 74.83 | 0.29 | 0 |
| PEI300-30@M-808 | 48.30 | 0.18 | 0 |
| 样品名称 | PEI实际负载量/ %(质量) | 0.1 bar CO2吸附量/(mmol/g) | 1 bar CO2吸附量/(mmol/g) | 0.1 bar CO2/N2选择性 | 1 bar CO2/N2选择性 | 0.1 bar胺效率/(mol/mol) |
|---|---|---|---|---|---|---|
| M-808 | — | 0.052 | 0.51 | 96.69 | 110.29 | — |
| PEI300-10@M-808 | 6.81 | 0.63 | 1.06 | 2574.39 | 609.79 | 0.34 |
| PEI300-20@M-808 | 16.19 | 0.78 | 1.21 | 4480.93 | 851.27 | 0.22 |
| PEI300-30@M-808 | 24.25 | 0.89 | 1.29 | 5524.65 | 1043.86 | 0.16 |
表2 PEI300-y@M-808在343 K的CO2吸附量、CO2/N2选择性和胺效率
Table 2 CO2 sorption capacity, CO2/N2 selectivity and amine efficiency of PEI300-y@M-808 at 343 K
| 样品名称 | PEI实际负载量/ %(质量) | 0.1 bar CO2吸附量/(mmol/g) | 1 bar CO2吸附量/(mmol/g) | 0.1 bar CO2/N2选择性 | 1 bar CO2/N2选择性 | 0.1 bar胺效率/(mol/mol) |
|---|---|---|---|---|---|---|
| M-808 | — | 0.052 | 0.51 | 96.69 | 110.29 | — |
| PEI300-10@M-808 | 6.81 | 0.63 | 1.06 | 2574.39 | 609.79 | 0.34 |
| PEI300-20@M-808 | 16.19 | 0.78 | 1.21 | 4480.93 | 851.27 | 0.22 |
| PEI300-30@M-808 | 24.25 | 0.89 | 1.29 | 5524.65 | 1043.86 | 0.16 |
| 样品名称 | CO2拟合参数 | N2拟合参数 | ||||||
|---|---|---|---|---|---|---|---|---|
| qmax/(mmol/g) | KL-F | n | R2 | qmax/(mmol/g) | KL-F | n | R2 | |
| M-808 | 1.040 | 0.81 | 0.8009 | 0.9926 | 0.184 | 0.280 | 1.0001 | 0.9998 |
| PEI300-10@M-808 | 1.076 | 15.46 | 0.8938 | 0.9710 | 0.055 | 0.374 | 1.0038 | 0.9999 |
| PEI300-20@M-808 | 1.266 | 11.01 | 1.1954 | 0.9690 | 0.044 | 0.373 | 1.0043 | 0.9999 |
| PEI300-30@M-808 | 1.297 | 24.62 | 0.9557 | 0.9845 | 0.040 | 0.372 | 1.0043 | 0.9999 |
表3 Langmuir/Freundlich模型拟合PEI300-y@M-808等温线参数
Table 3 Parameters obtained by fitting the PEI300-y@M-808 isotherm with the Langmuir/Freundlich model
| 样品名称 | CO2拟合参数 | N2拟合参数 | ||||||
|---|---|---|---|---|---|---|---|---|
| qmax/(mmol/g) | KL-F | n | R2 | qmax/(mmol/g) | KL-F | n | R2 | |
| M-808 | 1.040 | 0.81 | 0.8009 | 0.9926 | 0.184 | 0.280 | 1.0001 | 0.9998 |
| PEI300-10@M-808 | 1.076 | 15.46 | 0.8938 | 0.9710 | 0.055 | 0.374 | 1.0038 | 0.9999 |
| PEI300-20@M-808 | 1.266 | 11.01 | 1.1954 | 0.9690 | 0.044 | 0.373 | 1.0043 | 0.9999 |
| PEI300-30@M-808 | 1.297 | 24.62 | 0.9557 | 0.9845 | 0.040 | 0.372 | 1.0043 | 0.9999 |
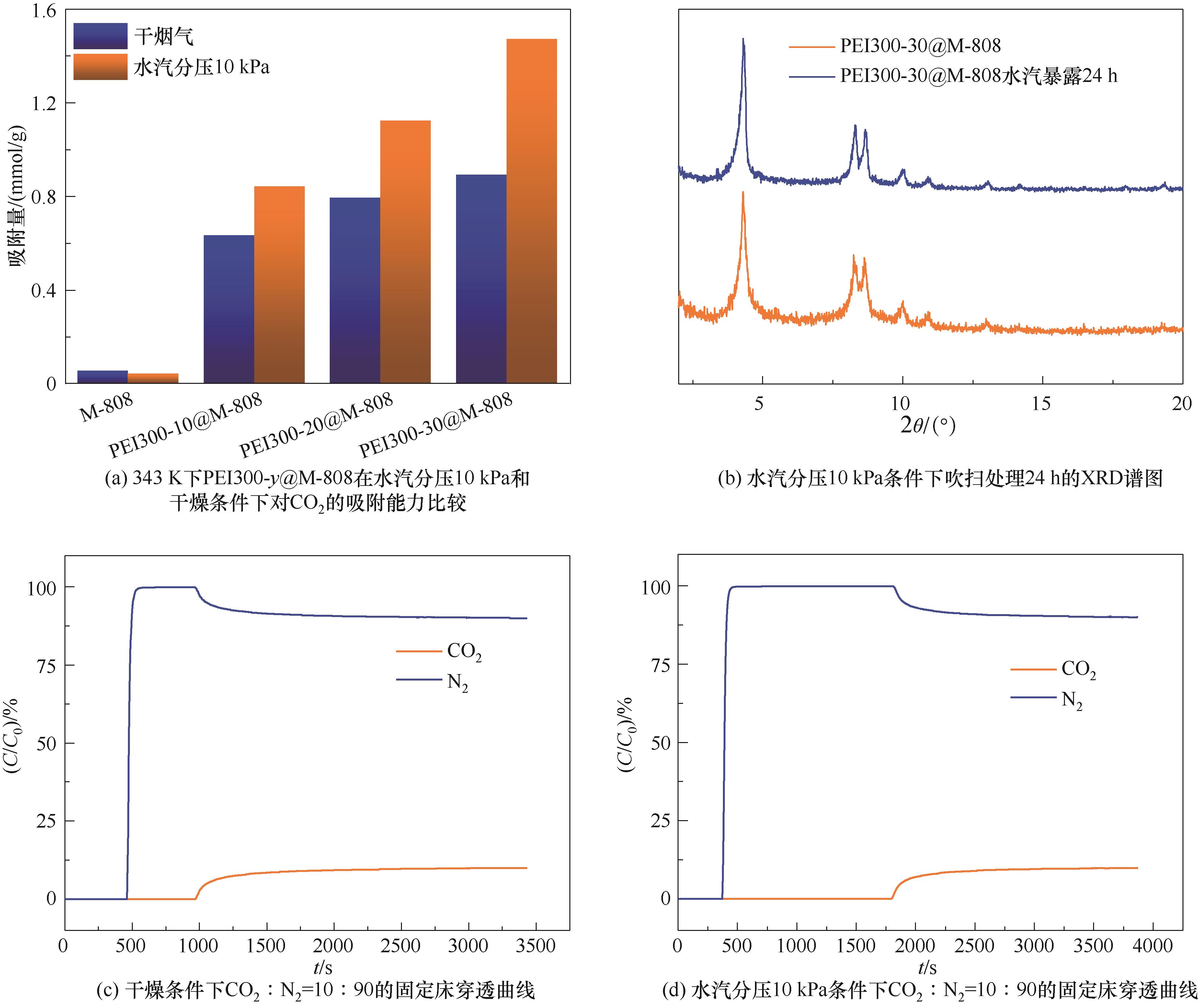
图9 343 K下PEI300-y@M-808在干燥和水汽分压10 kPa条件下对CO2的吸附能力及结构稳定性
Fig.9 Sorption capacity and structural stability of PEI300-y@M-808 for CO2 under dry and water vapor partial pressure of 10 kPa at 343 K
| 34 | Kang J H, Yoon T U, Kim S Y, et al. Extraordinarily selective adsorption of CO2 over N2 in a polyethyleneimine-impregnated NU-1000 material[J]. Microporous and Mesoporous Materials, 2019, 281: 84-91. |
| 35 | Zhu H J, Xue W J, Huang H L, et al. Water boosted CO2/C2H2 separation in L-arginine functionalized metal-organic framework[J]. Nano Research, 2023, 16(5): 6113-6119. |
| 36 | Lee J J, Chen C H, Shimon D, et al. Effect of humidity on the CO2 adsorption of tertiary amine grafted SBA-15[J]. The Journal of Physical Chemistry C, 2017, 121(42): 23480-23487. |
| 37 | Didas S A, Sakwa-Novak M A, Foo G S, et al. Effect of amine surface coverage on the co-adsorption of CO2 and water: spectral deconvolution of adsorbed species[J]. The Journal of Physical Chemistry Letters, 2014, 5(23): 4194-4200. |
| 38 | Zhang G J, Zhao P Y, Hao L X, et al. A novel amine double functionalized adsorbent for carbon dioxide capture using original mesoporous silica molecular sieves as support[J]. Separation and Purification Technology, 2019, 209: 516-527. |
| 1 | Quan C, Chu H, Zhou Y Y, et al. Amine-modified silica zeolite from coal gangue for CO2 capture[J]. Fuel, 2022, 322: 124184. |
| 2 | Ochedi F O, Yu J L, Yu H, et al. Carbon dioxide capture using liquid absorption methods: a review[J]. Environmental Chemistry Letters, 2021, 19(1): 77-109. |
| 3 | Dutcher B, Fan M H, Russell A G. Amine-based CO2 capture technology development from the beginning of 2013—a review[J]. ACS Applied Materials & Interfaces, 2015, 7(4): 2137-2148. |
| 4 | Wang M H, Joel A S, Ramshaw C, et al. Process intensification for post-combustion CO2 capture with chemical absorption: a critical review[J]. Applied Energy, 2015, 158: 275-291. |
| 5 | Samanta A, Zhao A, Shimizu G K H, et al. Post-combustion CO2 capture using solid sorbents: a review[J]. Industrial & Engineering Chemistry Research, 2012, 51(4): 1438-1463. |
| 6 | Zhang C, Zhang Y H, Su T Y, et al. Molecular simulation on carbon dioxide capture performance for carbons doped with various elements[J]. Energy Storage and Saving, 2023, 2(2): 435-441. |
| 7 | Wurzbacher J A, Gebald C, Steinfeld A. Separation of CO2 from air by temperature-vacuum swing adsorption using diamine-functionalized silica gel[J]. Energy & Environmental Science, 2011, 4(9): 3584-3592. |
| 8 | Hudson M R, Queen W L, Mason J A, et al. Unconventional, highly selective CO2 adsorption in zeolite SSZ-13[J]. Journal of the American Chemical Society, 2012, 134(4): 1970-1973. |
| 9 | Boyd P G, Chidambaram A, García-Díez E, et al. Data-driven design of metal-organic frameworks for wet flue gas CO2 capture[J]. Nature, 2019, 576(7786): 253-256. |
| 10 | González-Zamora E, Ibarra I A. CO2 capture under humid conditions in metal-organic frameworks[J]. Materials Chemistry Frontiers, 2017, 1(8): 1471-1484. |
| 11 | Trickett C A, Helal A, Al-Maythalony B A, et al. The chemistry of metal-organic frameworks for CO2 capture, regeneration and conversion[J]. Nature Reviews Materials, 2017, 2: 17045. |
| 12 | Lei L, Cheng Y, Chen C W, et al. Taming structure and modulating carbon dioxide (CO2) adsorption isosteric heat of nickel-based metal organic framework (MOF-74(Ni)) for remarkable CO2 capture[J]. Journal of Colloid and Interface Science, 2022, 612: 132-145. |
| 13 | Zheng W J, Ding R, Dai Y, et al. Regulating the pore engineering of MOFs by the confined dissolving of PSA template to improve CO2 capture[J]. Journal of Membrane Science, 2023, 670: 121373. |
| 14 | Jun H J, Yoo D K, Jhung S H. Metal-organic framework (MOF-808) functionalized with ethyleneamines: selective adsorbent to capture CO2 under low pressure[J]. Journal of CO2 Utilization, 2022, 58: 101932. |
| 15 | Xian S K, Wu Y, Wu J L, et al. Enhanced dynamic CO2 adsorption capacity and CO2/CH4 selectivity on polyethylenimine-impregnated UiO-66[J]. Industrial & Engineering Chemistry Research, 2015, 54(44): 11151-11158. |
| 16 | Su X A, Bromberg L, Martis V, et al. Postsynthetic functionalization of Mg-MOF-74 with tetraethylenepentamine: structural characterization and enhanced CO2 adsorption[J]. ACS Applied Materials & Interfaces, 2017, 9(12): 11299-11306. |
| 17 | Sanjit G, Yeonhee K, Ranjit G, et al. Enhanced CO2 capture capacity of amine-functionalized MOF-177 metal organic framework[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105523. |
| 18 | Miao Y H, Wang Y Z, Ge B Y, et al. Mixed diethanolamine and polyethyleneimine with enhanced CO2 capture capacity from air[J]. Advanced Science, 2023, 10(16): 2207253. |
| 19 | Zhu J J, Wu L B, Bu Z Y, et al. Polyethyleneimine-modified UiO-66-NH2(Zr) metal-organic frameworks: preparation and enhanced CO2 selective adsorption[J]. ACS Omega, 2019, 4(2): 3188-3197. |
| 20 | Xian S K, Xu F, Ma C, et al. Vapor-enhanced CO2 adsorption mechanism of composite PEI@ZIF-8 modified by polyethyleneimine for CO2/N2 separation[J]. Chemical Engineering Journal, 2015, 280: 363-369. |
| 21 | Pander M, Gil-San-Millan R, Delgado P, et al. MOF/polymer hybrids through in situ free radical polymerization in metal-organic frameworks[J]. Materials Horizons, 2023, 10(4): 1301-1308. |
| 22 | Lam I T Y, Choi S, Lu D, et al. Functionalized metal-organic frameworks for heavy metal ion removal from water[J]. Nanoscale, 2023, 15(24): 10189-10205. |
| 23 | Park J M, Yoo D K, Jhung S H. Selective CO2 adsorption over functionalized Zr-based metal organic framework under atmospheric or lower pressure: contribution of functional groups to adsorption[J]. Chemical Engineering Journal, 2020, 402: 126254. |
| 24 | Lyu H, Chen O I F, Hanikel N, et al. Carbon dioxide capture chemistry of amino acid functionalized metal-organic frameworks in humid flue gas[J]. Journal of the American Chemical Society, 2022, 144(5): 2387-2396. |
| 25 | Ahmed S, Ramli A, Yusup S, et al. Adsorption behavior of tetraethylenepentamine-functionalized Si-MCM-41 for CO2 adsorption[J]. Chemical Engineering Research and Design, 2017, 122: 33-42. |
| 26 | Hiroyasu F, Felipe G, Zhang Y B, et al. Water adsorption in porous metal-organic frameworks and related materials[J]. Journal of the American Chemical Society, 2014, 136(11): 4369-4381. |
| 27 | Rojas-Buzo S, García-García P, Corma A. Zr-MOF-808@MCM-41 catalyzed phosgene-free synthesis of polyurethane precursors[J]. Catalysis Science & Technology, 2019, 9(1): 146-156. |
| 28 | Hao Y X, Li J S, Yang X J, et al. Preparation of ZrO2-Al2O3 composite membranes by sol-gel process and their characterization[J]. Materials Science and Engineering: A, 2004, 367(1/2): 243-247. |
| 29 | Elvira M R, Mazo M A, Tamayo A, et al. Study and characterization of organically modified silica-zirconia anti-graffiti coatings obtained by sol-gel[J]. Journal of Chemistry and Chemical Engineering, 2013, 7(2): 120. |
| 30 | DeCoste J B, Peterson G W, Jasuja H, et al. Stability and degradation mechanisms of metal-organic frameworks containing the Zr6O4(OH)4 secondary building unit[J]. Journal of Materials Chemistry A, 2013, 1(18): 5642-5650. |
| 31 | Lin Y C, Lin H, Wang H M, et al. Enhanced selective CO2 adsorption on polyamine/MIL-101(Cr) composites[J]. Journal of Materials Chemistry A, 2014, 2(35): 14658-14665. |
| 32 | Li Z, Chen H F, Chen C, et al. High dispersion of polyethyleneimine within mesoporous UiO-66s through pore size engineering for selective CO2 capture[J]. Chemical Engineering Journal, 2019, 375: 121962. |
| 33 | Russel W W. The adsorption of gases and vapors(Ⅰ): Physical adsorption (Brunauer, Stephen)[J]. Journal of Chemical Education, 1944, 21(1): 52. |
| [1] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [2] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [3] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [4] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| [5] | 朱兴驰, 郭志远, 纪志永, 汪婧, 张盼盼, 刘杰, 赵颖颖, 袁俊生. 选择性电渗析镁锂分离过程模拟优化[J]. 化工学报, 2023, 74(6): 2477-2485. |
| [6] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [7] | 顾浩, 张福建, 刘珍, 周文轩, 张鹏, 张忠强. 力电耦合作用下多孔石墨烯膜时间维度的脱盐性能及机理研究[J]. 化工学报, 2023, 74(5): 2067-2074. |
| [8] | 张正, 何永平, 孙海东, 张荣子, 孙正平, 陈金兰, 郑一璇, 杜晓, 郝晓刚. 蛇形流场电控离子交换装置用于选择性提锂[J]. 化工学报, 2023, 74(5): 2022-2033. |
| [9] | 王皓, 唐思扬, 钟山, 梁斌. MEA吸收CO2富液解吸过程中固体颗粒表面的强化作用分析[J]. 化工学报, 2023, 74(4): 1539-1548. |
| [10] | 时国华, 何林珅, 赵玺灵, 张世钢. 余热回收喷淋塔的烟气颗粒物脱除特性研究[J]. 化工学报, 2023, 74(4): 1735-1745. |
| [11] | 王子健, 柯明, 李佳涵, 李舒婷, 孙巾茹, 童燕兵, 赵治平, 刘加英, 任璐. 短b轴ZSM-5分子筛制备方法及应用研究进展[J]. 化工学报, 2023, 74(4): 1457-1473. |
| [12] | 肖川宝, 李林洋, 刘武锋, 钟年丙, 解泉华, 钟登杰, 常海星. 光催化与离子交换吸附耦合有效去除2,4,6-三氯苯酚[J]. 化工学报, 2023, 74(4): 1587-1597. |
| [13] | 潘煜, 王子航, 王佳韵, 王如竹, 张华. 基于可得然-氯化锂复合吸附剂的除湿换热器热湿性能研究[J]. 化工学报, 2023, 74(3): 1352-1359. |
| [14] | 李敏, 阎雪茹, 刘新磊. 苯并咪唑连接聚合物吸附剂和膜研究进展[J]. 化工学报, 2023, 74(2): 599-616. |
| [15] | 许万, 陈振斌, 张慧娟, 牛昉昉, 火婷, 刘兴盛. 线性温敏性聚合物嵌段调控的 |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号